Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized Gellan Gum
2.3. Carboxyl Content Determination
2.4. Carbonyl Content Determination
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
2.7. Gel Properties of the Oxidized Gellan Gum in the Presence of Ions
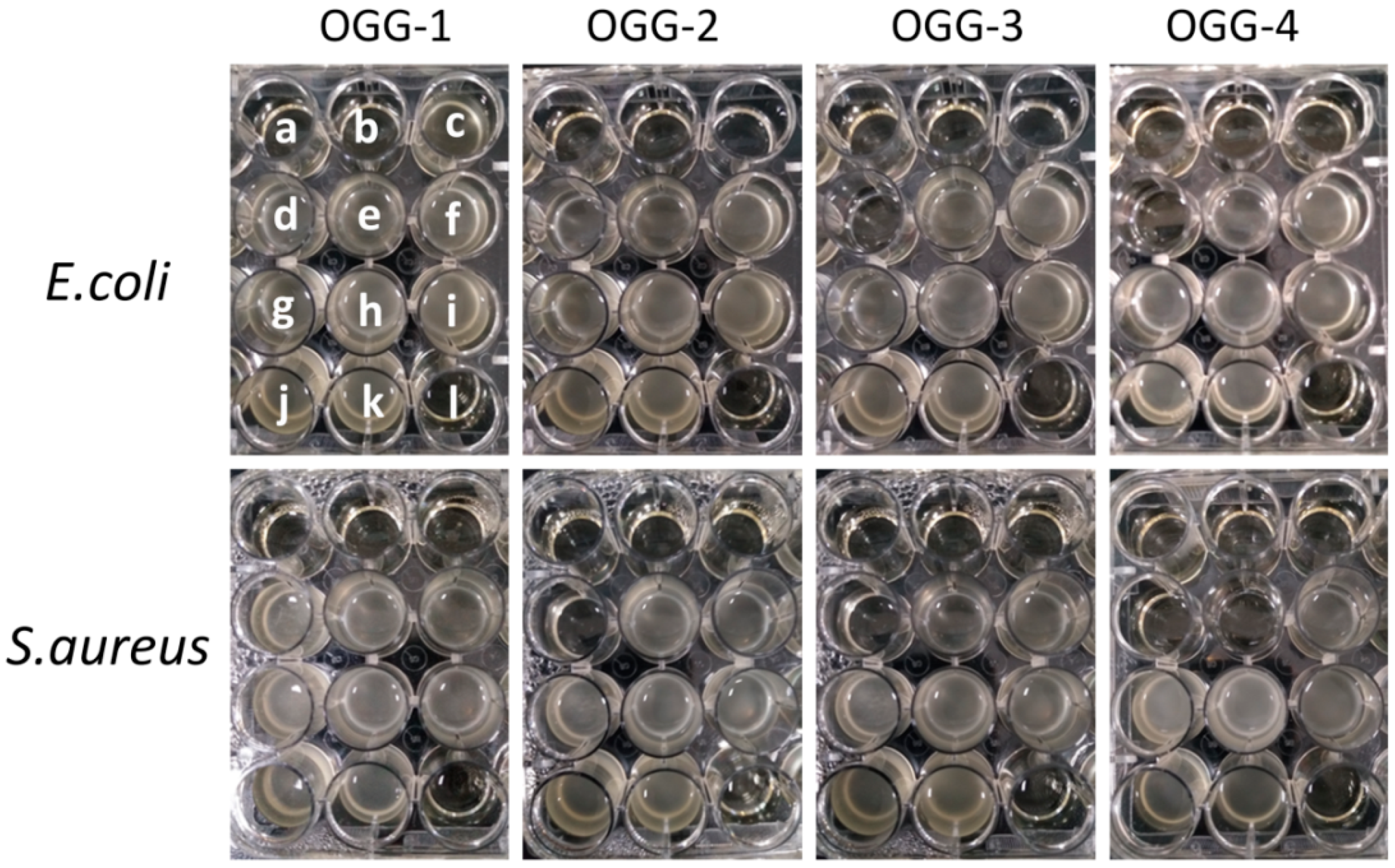
2.8. Antibacterial Activity of the Oxidized Gellan Gum
2.9. Antifungal Activity of the Oxidized Gellan Gum
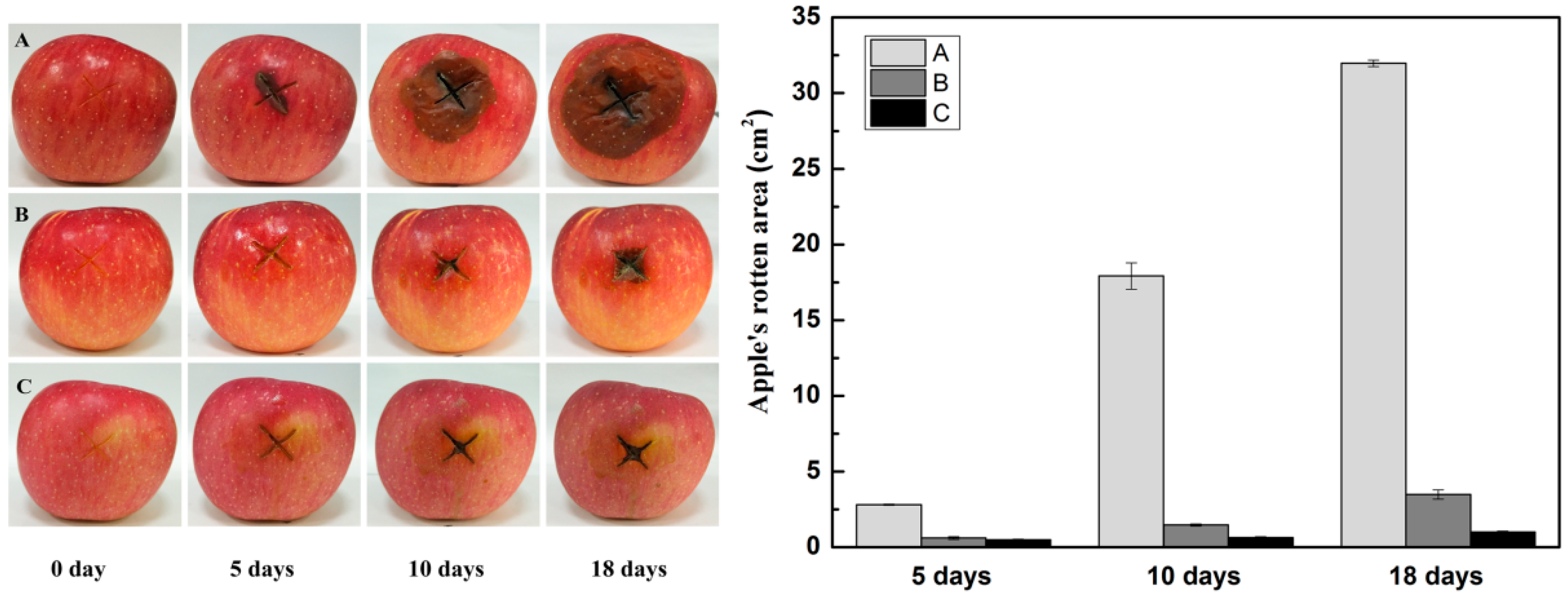
2.10. Effect of the Oxidized Gellan Gum on Apple Preservation
3. Results and Discussions
3.1. Characterizations
3.1.1. Carboxyl and Carbonyl Contents of the Oxidized Gellan Gums
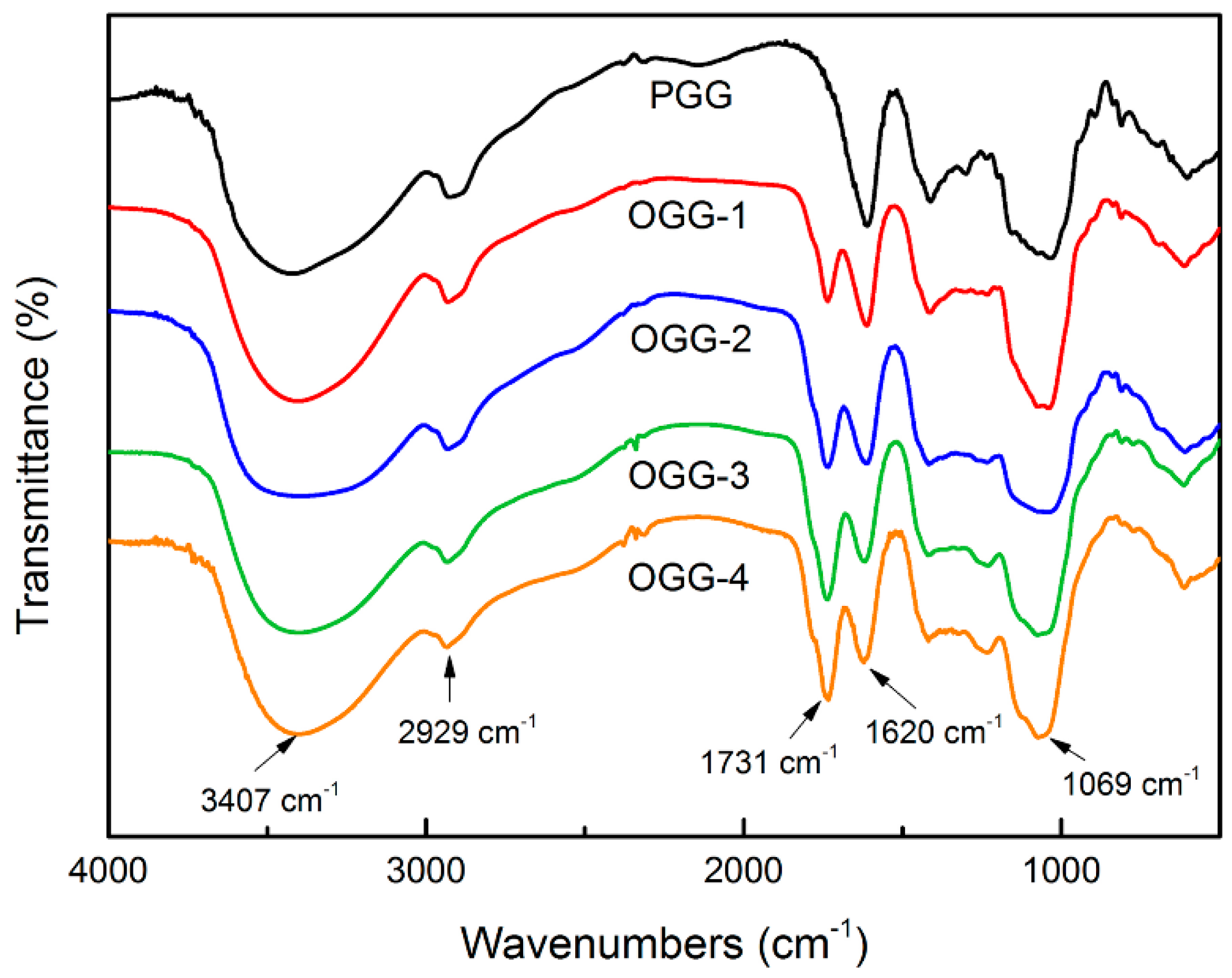
3.1.2. Fourier Transform Infrared Spectroscopy Analysis
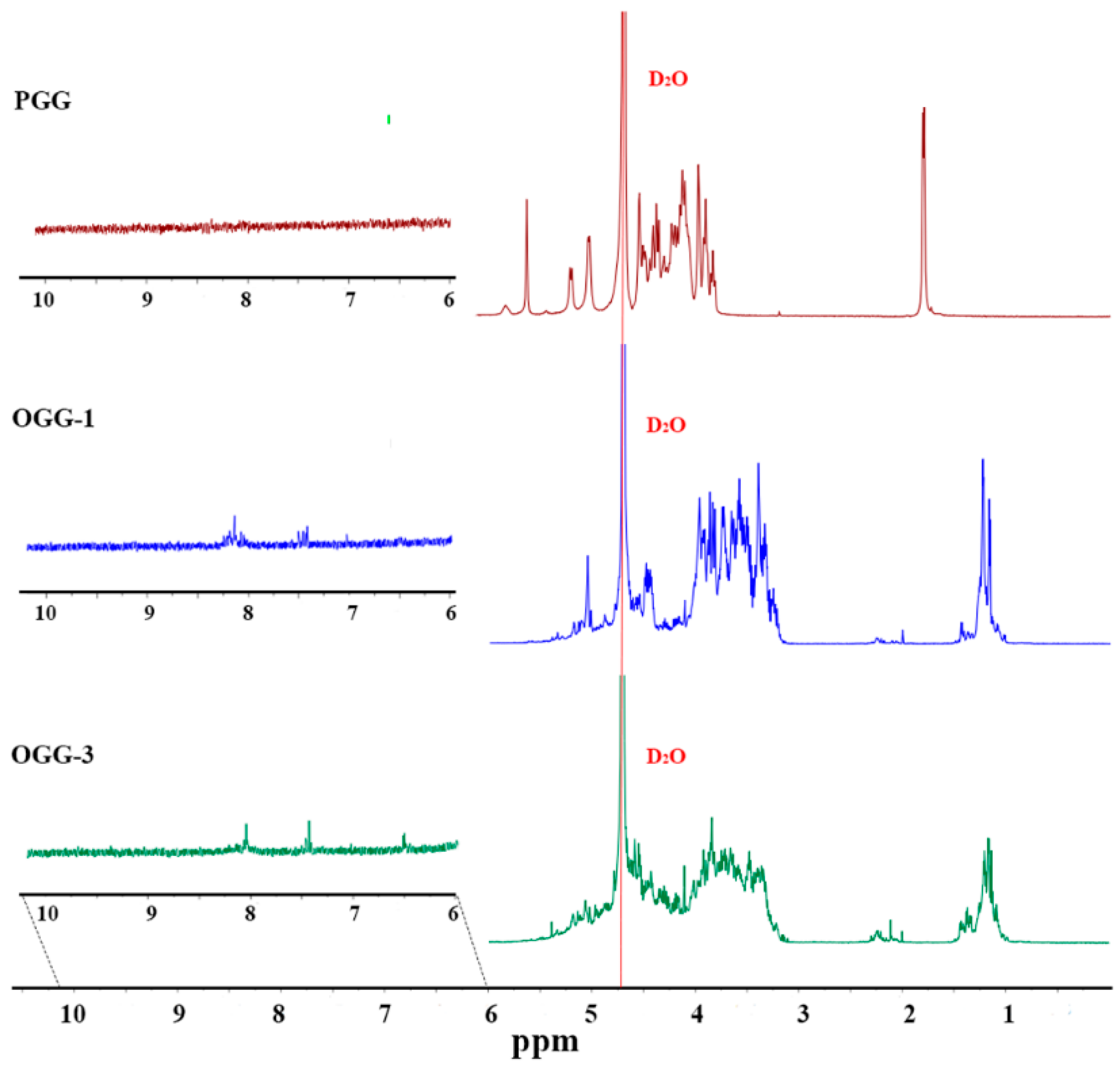
3.1.3. Nuclear Magnetic Resonance Spectrum
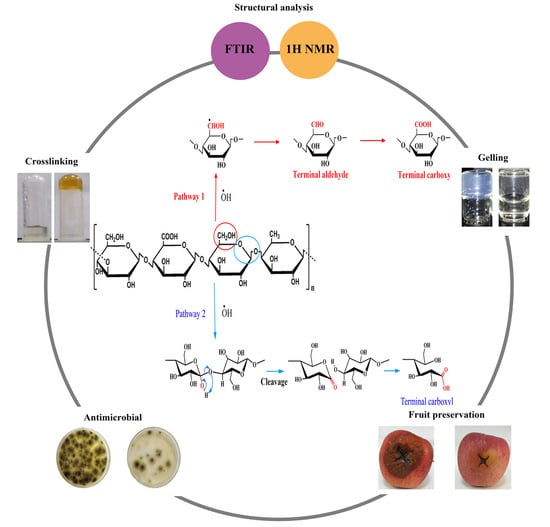
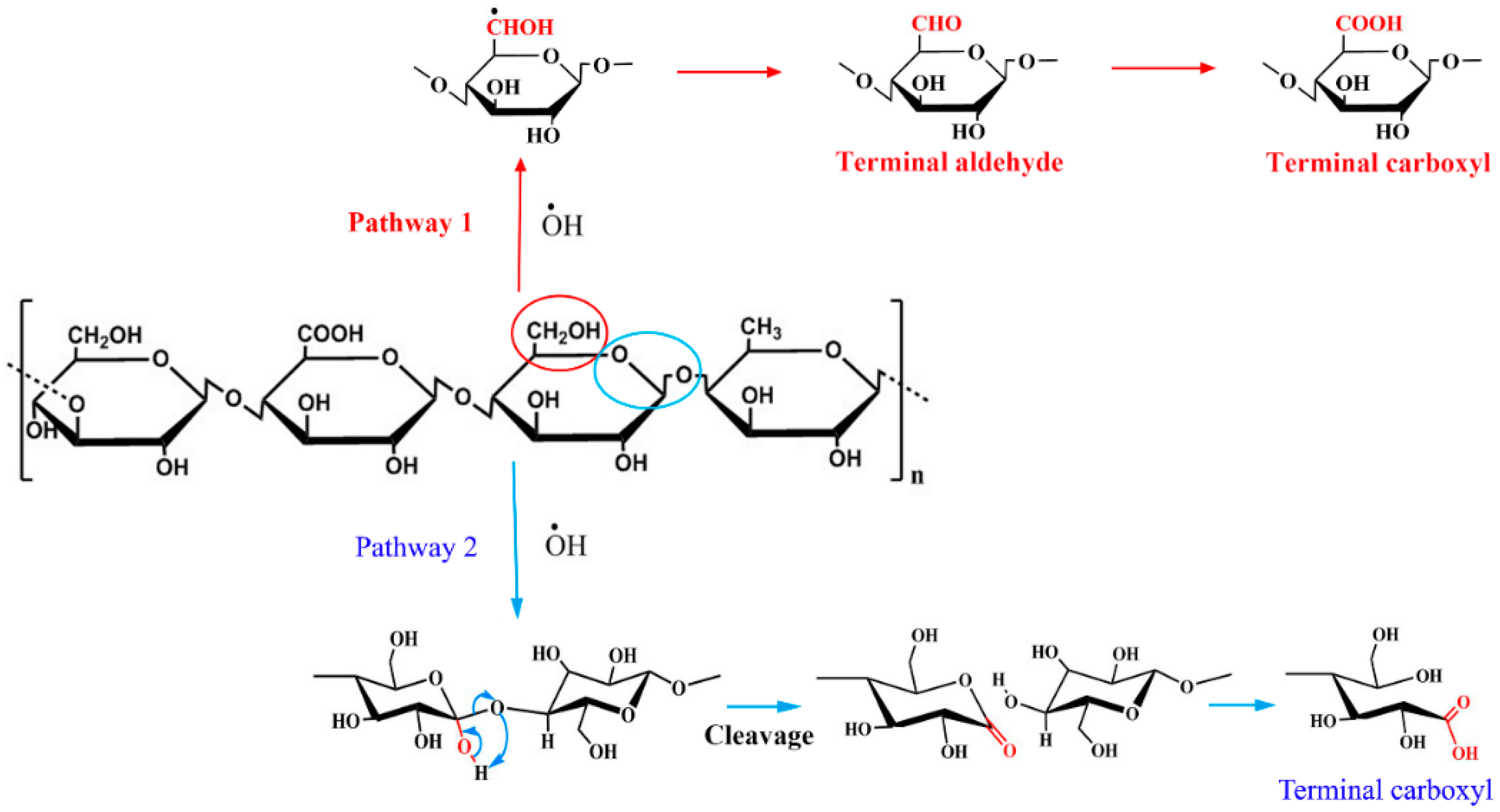
3.2. Oxidation Mechanisms
3.3. Gelation Properties
Gelling Properties of the Oxidized Gellan Gums
3.4. Antimicrobial Activities and Food Coating Applications
3.4.1. Antimicrobial Activities of the Oxidized Gellan Gums
3.4.2. Effect of the Oxidized Gellan Gums on Fruit Preservation as Antimicrobial Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hilal, Y.; Neva, K. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Recent advances in endophytic exopolysaccharides: Production, structural characterization, physiological role and biological activity. Carbohydr. Polym. 2016, 157, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of pb(ii) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzyme Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef]
- Ahuja, M.; Singh, S.; Kumar, A. Evaluation of carboxymethyl gellan gum as a mucoadhesive polymer. Int. J. Biol. Macromol. 2013, 53, 114–121. [Google Scholar] [CrossRef]
- Matricardi, P.; Cencetti, C.; Ria, R.; Alhaique, F.; Coviello, T. Preparation and characterization of novel gellan gum hydrogels suitable for modified drug release. Molecules 2009, 14, 3376–3391. [Google Scholar] [CrossRef]
- Redouan, E.; Emmanuel, P.; Christine, B.; Bernard, C.; Josiane, C.; Cédric, D. Development of new ulvan-like polymer by regioselective oxidation of gellan exopolysaccharide using TEMPO reagent. Carbohydr. Polym. 2010, 80, 485–490. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2011, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Ye, Y.X.; Zhang, W.W.; Li, S.L.; Chen, J.; Wang, S.T.; Li, D.F.; Mu, C.D. Oxidized amylose with high carboxyl content: A promising solubilizer and carrier of linalool for antimicrobial activity. Carbohydr. Polym. 2016, 154, 13–19. [Google Scholar]
- Zhu, M.J.; Ge, L.M.; Lyu, Y.; Zi, Y.X.; Li, X.Y.; Li, D.F.; Mu, C.D. Preparation, characterization and antibacterial activity of oxidized κ-carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Ye, Y.; Ren, H.; Zhu, S.; Tan, H.; Li, X.; Li, D.; Mu, C. Synthesis of oxidized β-cyclodextrin with high aqueous solubility and broad-spectrum antimicrobial activity. Carbohydr. Polym. 2017, 177, 97–104. [Google Scholar] [CrossRef]
- Moura, F.A.D.; Pereira, J.M.; Silva, D.O.D.; Zavareze, E.D.R.; Moreira, A.D.S.; Helbig, E.; Dias, A.R.G. Effects of oxidative treatment on the physicochemical, rheological and functional properties of oat β-glucan. Food Chem. 2011, 128, 982–987. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and Characterization of Chitosan-Collagen Peptide/Oxidized Konjac Glucomannan Hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Rosík, J.; Kardošová, A.; Kubala, J. Infrared spectroscopy of peach-gum polysaccharides of Prunus persica (L.) Batsch. Carbohydr. Res. 1971, 18, 151–156. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, X.L.; Zhao, G.M.; Wang, Y.Z. Preparation and properties of oxidized starch with high degree of oxidation. Carbohydr. Polym. 2012, 87, 2554–2562. [Google Scholar] [CrossRef]
- Liang, X.; Du, L.; Su, F.; Parekh, H.S.; Su, W. The application of quantitative NMR for the facile, rapid and reliable determination of clindamycin phosphate in a conventional tablet formulation. Magn. Reson. Chem. 2014, 52, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Ren, H.; Yu, M.C.; Li, X.Y.; Li, D.F.; Mu, C.D. Using oxidized amylose as carrier of linalool for the development of antibacterial wound dressing. Carbohydr. Polym. 2017, 174, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Vanier, N.L.; El Halal, S.L.; Dias, A.R.; Dias, R.Z.E.; Zavareze, E.D.R. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, N.; Chen, X.; Su, W. Asymmetric a-oxyamination of aldehydes by synergistic catalysis of imidazolethiones and metal salts. RSC Adv. 2014, 4, 44039–44042. [Google Scholar] [CrossRef]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Radha, A. Molecular architectures and functional properties of gellan gum and related polysaccharides. Trends Food Sci. Technol. 1995, 6, 143–148. [Google Scholar] [CrossRef]
- Upstill, C.; Atkins, E.D.T.; Attwool, P.T. Helical conformations of gellan gum. Int. J. Biol. Macromol. 1986, 8, 275–288. [Google Scholar] [CrossRef]
- Jayakumar, G.C.; Kanth, S.V.; Chandrasekaran, B.; Rao, J.R.; Nair, B.U. Preparation and antimicrobial activity of scleraldehyde from schizophyllum commune. Carbohydr. Res. 2010, 345, 2213–2219. [Google Scholar] [CrossRef]
- Abaev, Y.; Kaputsky, V.; Adarchenko, A.A.; Sobeshchuk, O.P. Mechanism of antibacterial effects of monocarboxyl cellulose and other ion exchange derivatives of cellulose. Antibiot. Med. Biotekhnol. 1986, 31, 624–628. [Google Scholar] [PubMed]
- Zi, Y.X.; Zhu, M.J.; Li, X.Y.; Xu, Y.B.; Wei, H.; Li, D.F.; Mu, C.D. Effects of carboxyl and aldehyde groups on the antibacterial activity of oxidized amylose. Carbohydr. Polym. 2018, 192, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.; Rothenburger, S.; Nguyen, K.; Jampani, H.; Weiss, S.; Bhende, S. In vitro antimicrobial activity of oxidized regenerated cellulose against antibiotic-resistant microorganisms. Surg. Infect. 2003, 4, 255–262. [Google Scholar] [CrossRef] [PubMed]

| Sample | Carboxyl Content (%) | Carbonyl Content (%) |
|---|---|---|
| PGG | - | - |
| OGG-1 | 6.11 ± 0.05 | 0.36 ± 0.01 |
| OGG-2 | 7.96 ± 0.04 | 0.44 ± 0.03 |
| OGG-3 | 11.82 ± 0.01 | 0.75 ± 0.04 |
| OGG-4 | 14.51 ± 0.03 | 0.87 ± 0.02 |
| Bacteria | Sample | MIC (mg/mL) |
|---|---|---|
| E. coli | PGG | -- |
| OGG-1 | 40 | |
| OGG-2 | 20 | |
| OGG-3 | 20 | |
| OGG-4 | 10 | |
| S. aureus | PGG | -- |
| OGG-1 | 20 | |
| OGG-2 | 20 | |
| OGG-3 | 10 | |
| OGG-4 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhao, X.; Fang, S. Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide. Foods 2019, 8, 31. https://doi.org/10.3390/foods8010031
Lu Y, Zhao X, Fang S. Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide. Foods. 2019; 8(1):31. https://doi.org/10.3390/foods8010031
Chicago/Turabian StyleLu, Yushuang, Xiaojian Zhao, and Sheng Fang. 2019. "Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide" Foods 8, no. 1: 31. https://doi.org/10.3390/foods8010031
APA StyleLu, Y., Zhao, X., & Fang, S. (2019). Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide. Foods, 8(1), 31. https://doi.org/10.3390/foods8010031