Isolation and Comparative Study on the Characterization of Guanidine Hydrochloride Soluble Collagen and Pepsin Soluble Collagen from the Body of Surf Clam Shell (Coelomactra antiquata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals Reagents
2.3. Extraction of Guanidine Hydrochloride Soluble Collagen (GSC)
2.4. Extraction of Pepsin Soluble Collagen (PSC)
2.5. Biochemical Compositions Analysis and the Collagen Yield of Surf Clam Shell
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.7. Amino Acid Analysis
2.8. Analysis of Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Scanning Electron Microscopy (SEM)
2.10. Determination of Denaturation Temperature
3. Results and Discussion
3.1. Biochemical Compositions
3.2. The Yields of GSC and PSC from Surf Clam Shell
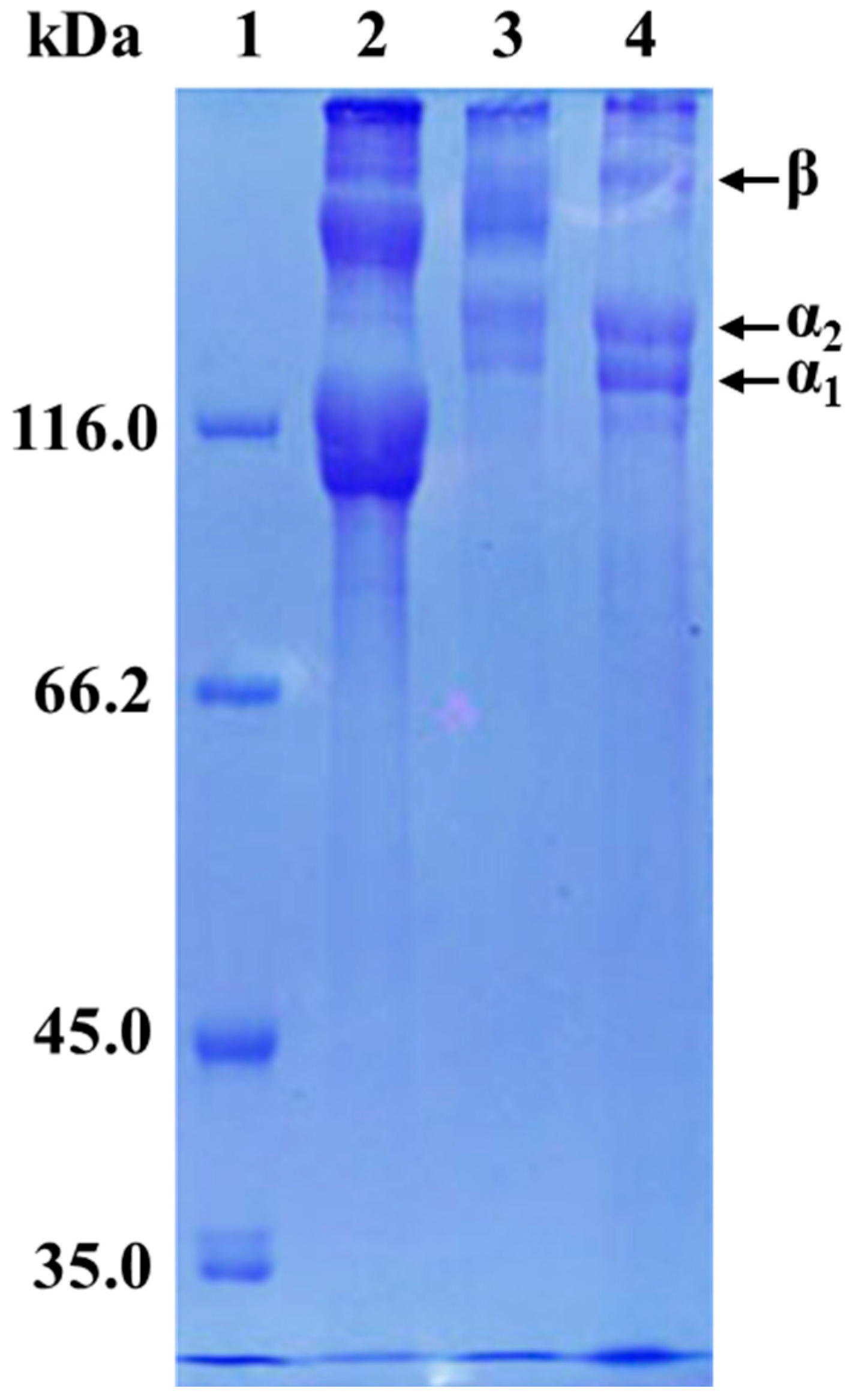
3.3. Protein Patterns
3.4. Amino Acid Composition
3.5. Fourier Transforms Infrared (FTIR) Spectra of Collagen
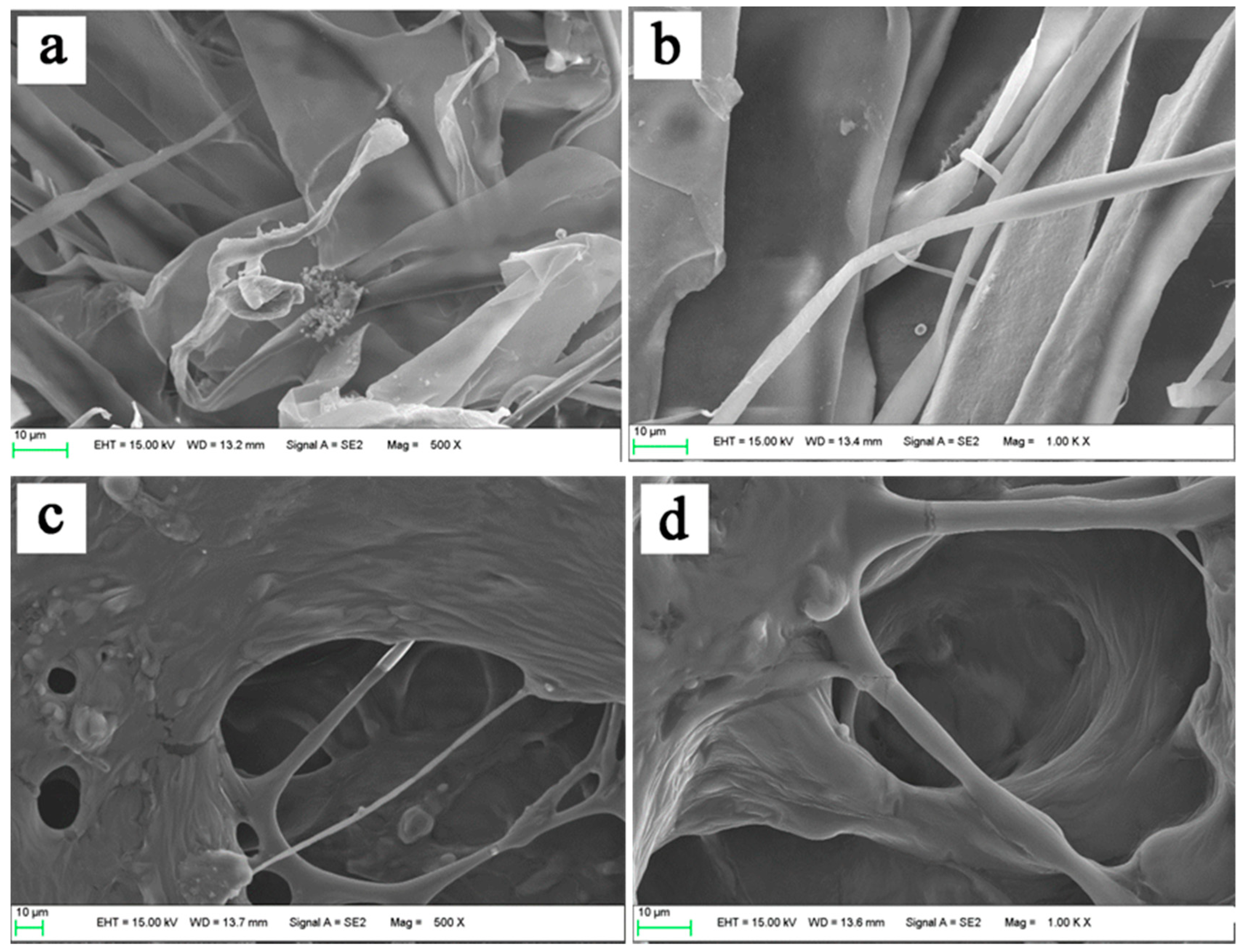
3.6. Morphological Characterization of Collagens
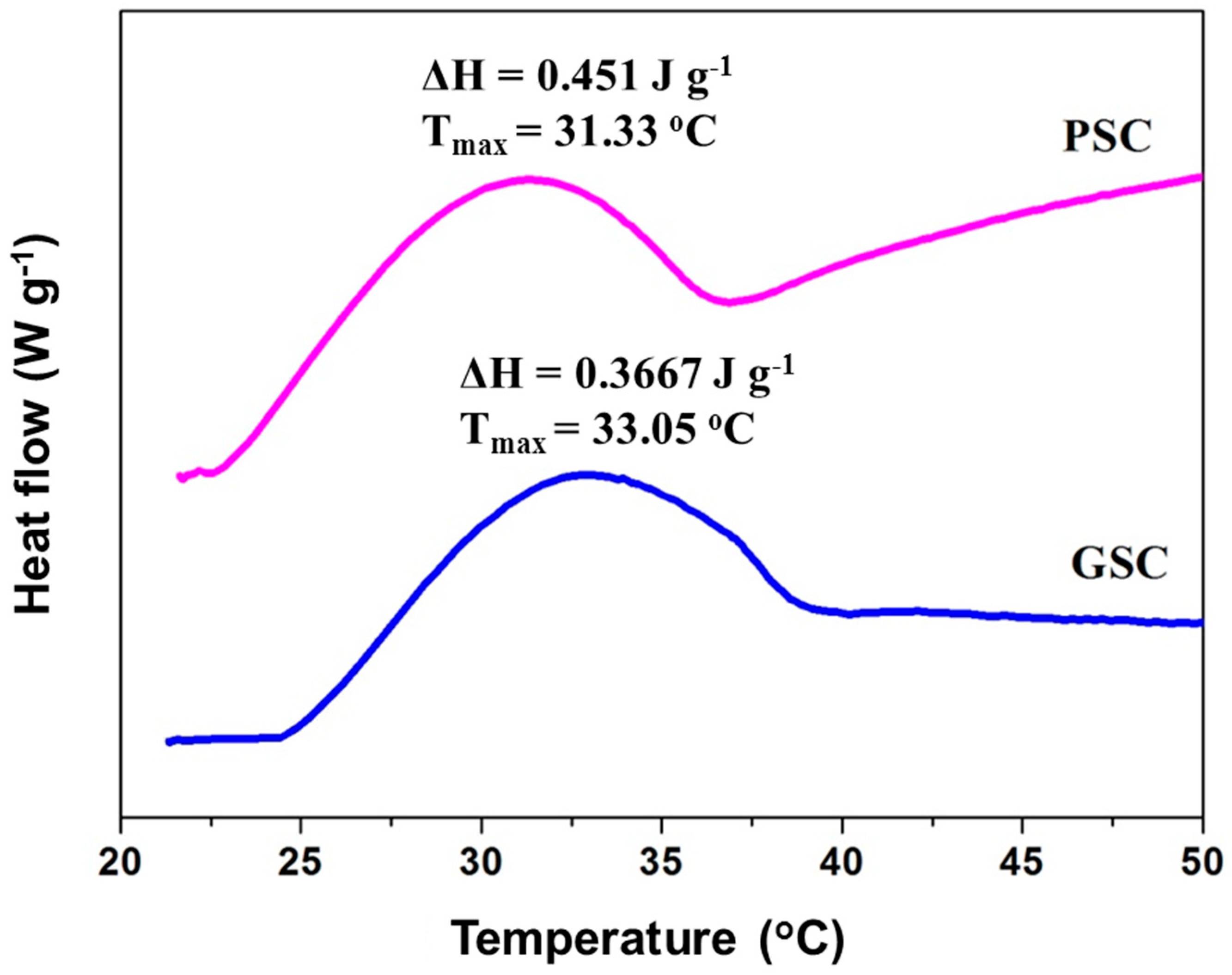
3.7. Thermal Transition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suleria, H.A.R. Marine Processing Waste—In Search of Bioactive Molecules. Nat. Prod. Chem. Res. 2016, 4, e118. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-Based Nutraceuticals: An Innovative Trend in the Food and Supplement Industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Masci, P.; Gobe, G.; Osborne, S. Current and potential uses of bioactive molecules from marine processing waste. J. Sci. Food Agric. 2016, 96, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. 2017, 57, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the Marine Sponges Axinella cannabina and Suberites carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Chi, C.F.; Zhao, Y.Q.; Wang, B. Preparation, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Swim Bladders of Miiuy Croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.L.; Qi, W.Y.; Xu, H.M. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kozlowska, J.; Skorupska, M.; Michalska, M. Isolation and characterization of collagen from the skin of Brama australis. Int. J. Biol. Macromol. 2015, 80, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Kamath, S.; Chuang, L.L.; Kasapis, S.; Lopata, A.L. Biochemical and thermo-mechanical analysis of collagen from the skin of Asian Sea bass (Lates calcarifer) and Australasian Snapper (Pagrus auratus), an alternative for mammalian collagen. Eur. Food Res. Technol. 2013, 236, 873–882. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Mizuta, S.; Isobe, S.; Yoshinaka, R. Existence of two molecular species of collagen in the muscle layer of the ascidian (Halocynthia roretzi). Food Chem. 2002, 79, 9–13. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010, 120, 817–824. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008, 108, 49–54. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Extraction and characterisation of pepsin-solubilised collagens from the skin of bigeye snapper (Priacanthus tayenus and Priacanthus macracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Characteristics of collagen from the skin of clown featherback (Chitala ornata). Int. J. Food Sci. Technol. 2015, 50, 1972–1978. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.F.; Chen, T.T.; Wang, Z.B.; Xu, J.M.; Ma, H.L. Characterization of collagen from the skin of Amur sturgeon (Acipenser schrenckii). Food Hydrocoll. 2014, 38, 104–109. [Google Scholar] [CrossRef]
- Waite, J.H.; Hansen, D.C.; Little, K.T. The Glue Protein of Ribbed Mussels (Geukensia-Demissa)—A Natural Adhesive with Some Features of Collagen. J. Comp. Physiol. B 1989, 159, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Miyagi, T.; Nishimiya, T.; Yoshinaka, R. Partial characterization of collagen in mantle and adductor of pearl oyster (Pinctada fucata). Food Chem. 2002, 79, 319–325. [Google Scholar] [CrossRef]
- Mizuta, S.; Miyagi, T.; Nishimiya, T.; Yoshinaka, R. Partial characterization of collagen in several bivalve molluscs. Food Chem. 2004, 87, 83–88. [Google Scholar] [CrossRef]
- Dong, X.P.; Yuan, Q.X.; Qi, H.; Yang, J.F.; Zhu, B.W.; Zhou, D.Y.; Murata, Y.; Ye, W.X. Isolation and Characterization of Pepsin-Soluble Collagen from Abalone (Haliotis discus hannai) Gastropod Muscle Part II. Food Sci. Technol. Res. 2012, 18, 271–278. [Google Scholar] [CrossRef]
- Sivakumar, P.; Suguna, L.; Chandrakasan, G. Molecular species of collagen in the intramuscular connective tissues of the marine crab, Scylla serrata. Comp. Biochem. Phys. B 2000, 125, 555–562. [Google Scholar] [CrossRef]
- Mizuta, S.; Yoshinaka, R.; Sato, M.; Sakaguchi, M. Biochemical and immunochemical characterization of guanidine hydrochloride-soluble collagen in the mantle muscle of squid Todarodes pacificus. Fish. Sci. 1997, 63, 291–296. [Google Scholar] [CrossRef]
- Meng, X.P.; Gao, R.C.; Dong, Z.G.; Cheng, H.L.; Yan, B.L. Analysis and evaluation of nutritive composition in edible part of Coelomactra antiquate. Mar. Sci. 2007, 31, 17–22. [Google Scholar]
- Wu, J.L.; Ge, S.Y.; Cai, Z.X.; Liu, H.; Liu, Y.X.; Wang, J.H.; Zhang, Q.Q. Purification and characterization of a gelatinolytic matrix metalloproteinase from the skeletal muscle of grass carp (Ctenopharyngodon idellus). Food Chem. 2014, 145, 632–638. [Google Scholar] [CrossRef]
- Lin, S.J.; Xue, Y.P.; San, E.L.; Keong, T.C.; Chen, L.F.; Zheng, Y.G. Extraction and Characterization of Pepsin Soluble Collagen from the Body Wall of Sea Cucumber Acaudina leucoprocta. J. Aquat. Food Prod. Trans. 2017, 26, 502–515. [Google Scholar] [CrossRef]
- Yang, Y.N.; Li, C.Y.; Song, W.; Wang, W.; Qian, G.Y. Purification, optimization and physicochemical properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2016, 89, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Tabakaeva, O.V.; Tabakaev, A.V.; Piekoszewski, W. Nutritional composition and total collagen content of two commercially important edible bivalve molluscs from the Sea of Japan coast. J. Food Sci. Technol. 2018, 55, 4877–4886. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- Zhang, J.J.; Duan, R.; Ye, C.; Konno, K. Isolation and Characterization of Collagens from Scale of Silver Carp (Hypophthalmichthys molitrix). J. Food Biochem. 2010, 34, 1343–1354. [Google Scholar] [CrossRef]
- Olaechea, R.P.; Ushio, H.; Watabe, S.; Takada, K.; Hatae, K. Toughness and Collagen Content of Abalone Muscles. Biosci. Biotechnol. Biochem. 1993, 57, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT-Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Bu, Y.S.; Elango, J.; Zhang, J.Y.; Bao, B.; Guo, R.H.; Palaniyandi, K.; Robinson, J.S.; Geevaretnam, J.; Regenstein, J.M.; Wu, W.H. Immunological effects of collagen and collagen peptide from blue shark cartilage on 6T-CEM cells. Process Biochem. 2017, 57, 219–227. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204. [Google Scholar] [CrossRef]
- Astre, G.; Deleruyelle, S.; Dortignac, A.; Bonnet, C.; Valet, P.; Dray, C. Diet-induced obesity and associated disorders are prevented by natural bioactive type 1 fish collagen peptides (Naticol (R)) treatment. J. Physiol. Biochem. 2018, 74, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.B.; Chi, C.F.; Yang, F.; Zhao, Y.Q.; Wang, B. Physicochemical properties of acid- and pepsin-soluble collagens from the cartilage of Siberian sturgeon. Environ. Sci. Pollut. Res. 2018, 25, 31427–31438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.T.; Li, G.Y.; Shi, B.; Miao, Y.Q.; Wu, X.H. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Rigby, B.J. Amino-acid Composition and Thermal Stability of the Skin Collagen of the Antarctic Ice-fish. Nature 1968, 219, 166–167. [Google Scholar] [CrossRef] [PubMed]

| Amino Acids | GSC | PSC |
|---|---|---|
| Alanine | 70 | 67 |
| Cystine | 1 | 2 |
| Aspartic acid | 81 | 76 |
| Glutamic acid | 118 | 119 |
| Phenylalanine | 17 | 16 |
| Glycine | 244 | 254 |
| Histidine | 11 | 9 |
| Isoleucine | 26 | 24 |
| Lysine | 35 | 22 |
| Leucine | 42 | 44 |
| Methionine | 14 | 15 |
| Proline | 85 | 86 |
| Arginine | 52 | 65 |
| Serine | 55 | 50 |
| Threonine | 35 | 34 |
| Valine | 32 | 32 |
| Tyrosine | 17 | 16 |
| Hydroxyproline | 65 | 69 |
| Total | 1000 | 1000 |
| Imino acid | 150 | 155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Guo, X.; Liu, H.; Chen, L. Isolation and Comparative Study on the Characterization of Guanidine Hydrochloride Soluble Collagen and Pepsin Soluble Collagen from the Body of Surf Clam Shell (Coelomactra antiquata). Foods 2019, 8, 11. https://doi.org/10.3390/foods8010011
Wu J, Guo X, Liu H, Chen L. Isolation and Comparative Study on the Characterization of Guanidine Hydrochloride Soluble Collagen and Pepsin Soluble Collagen from the Body of Surf Clam Shell (Coelomactra antiquata). Foods. 2019; 8(1):11. https://doi.org/10.3390/foods8010011
Chicago/Turabian StyleWu, Jiulin, Xiaoban Guo, Hui Liu, and Li Chen. 2019. "Isolation and Comparative Study on the Characterization of Guanidine Hydrochloride Soluble Collagen and Pepsin Soluble Collagen from the Body of Surf Clam Shell (Coelomactra antiquata)" Foods 8, no. 1: 11. https://doi.org/10.3390/foods8010011
APA StyleWu, J., Guo, X., Liu, H., & Chen, L. (2019). Isolation and Comparative Study on the Characterization of Guanidine Hydrochloride Soluble Collagen and Pepsin Soluble Collagen from the Body of Surf Clam Shell (Coelomactra antiquata). Foods, 8(1), 11. https://doi.org/10.3390/foods8010011




