Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Leaves Material
2.2. Preparation of Powder and Olive Leaves Extract
2.3. Estimation of the Total Phenolic Compounds in the Olive Leaves Extract
2.4. Analysis of Phenolic Compounds in sylv.OLE
2.5. In Vitro Antioxidant Activity
- -
- Abs (DPPH) = absorbance value at 517 nm of the methanolic solution of DPPH.
- -
- Abs (sylv.OLE) = absorbance value at 517 nm for the sylv.OLE.
- -
- AAI < 0.5 (poor antioxidant activity).
- -
- 0.5 < AAI < 1.0 (moderate antioxidant activity).
- -
- 1.0 < AAI < 2.0 (strong antioxidant activity).
- -
- AAI > 2.0 (very strong activity).
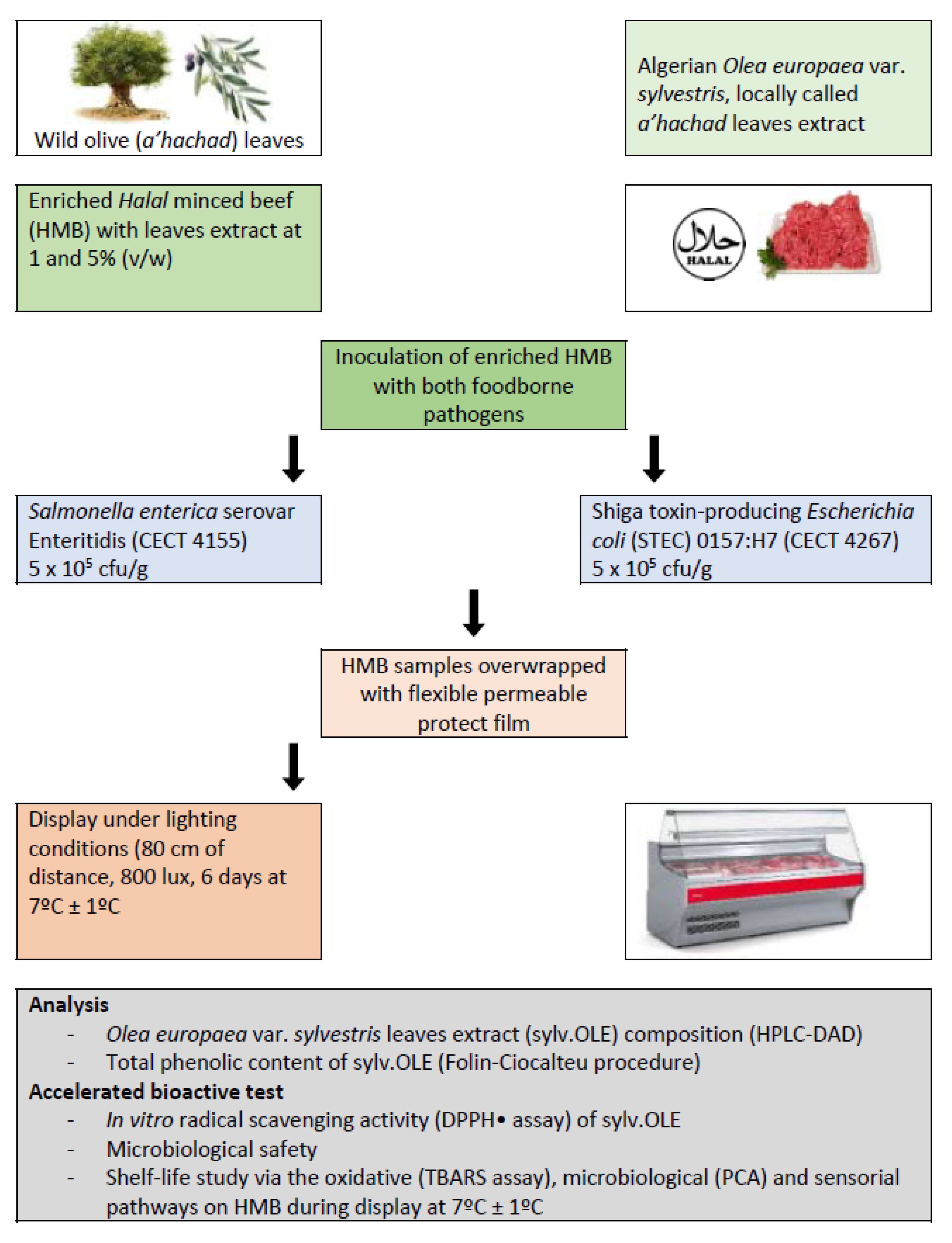
2.6. Application in Halal Minced Beef
2.6.1. Microorganisms and Standardization of Inoculum
2.6.2. Halal Minced Beef and Treatments
2.6.3. Microbiological Analysis
2.6.4. Malonaldehyde Compounds as Lipid Oxidation Biomarkers in HMB
2.6.5. Sensory Evaluation of Halal Minced Beef
2.6.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Contents
3.2. HPLC Analysis
3.3. In Vitro Antioxidant Activity
3.4. Microbiological Analysis
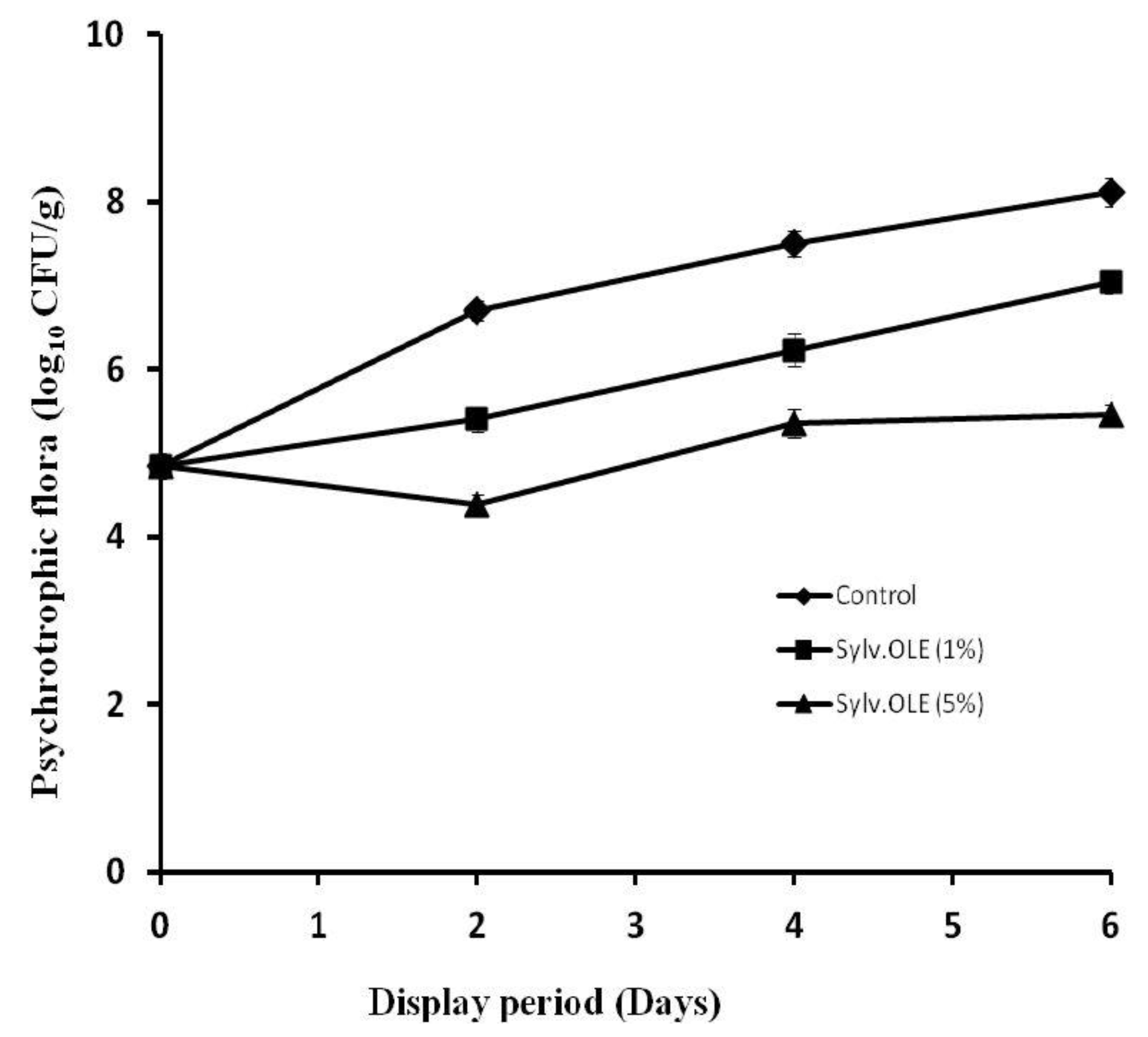
3.4.1. Plate Count Agar (Psychrotrophic Microbiota)
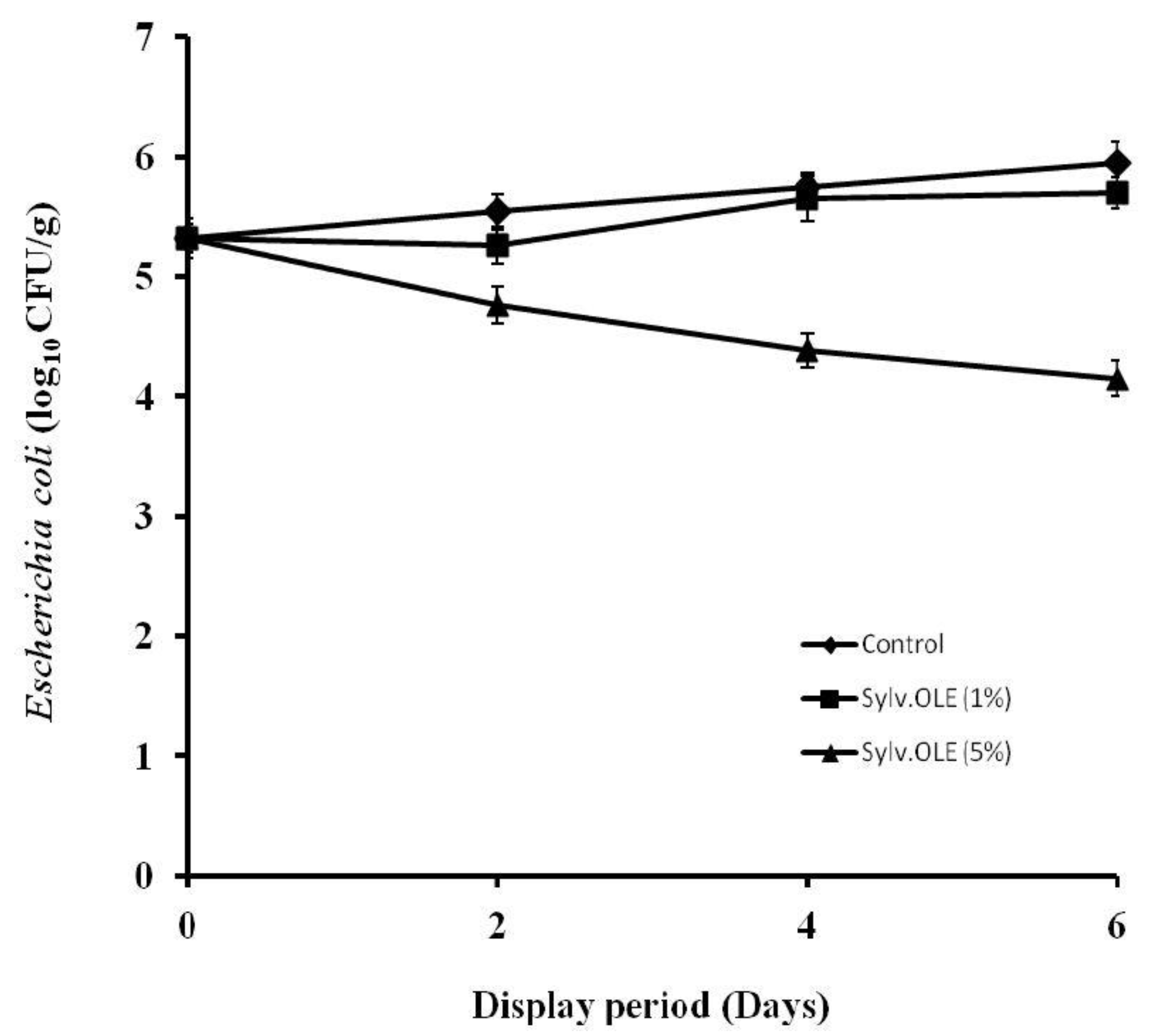
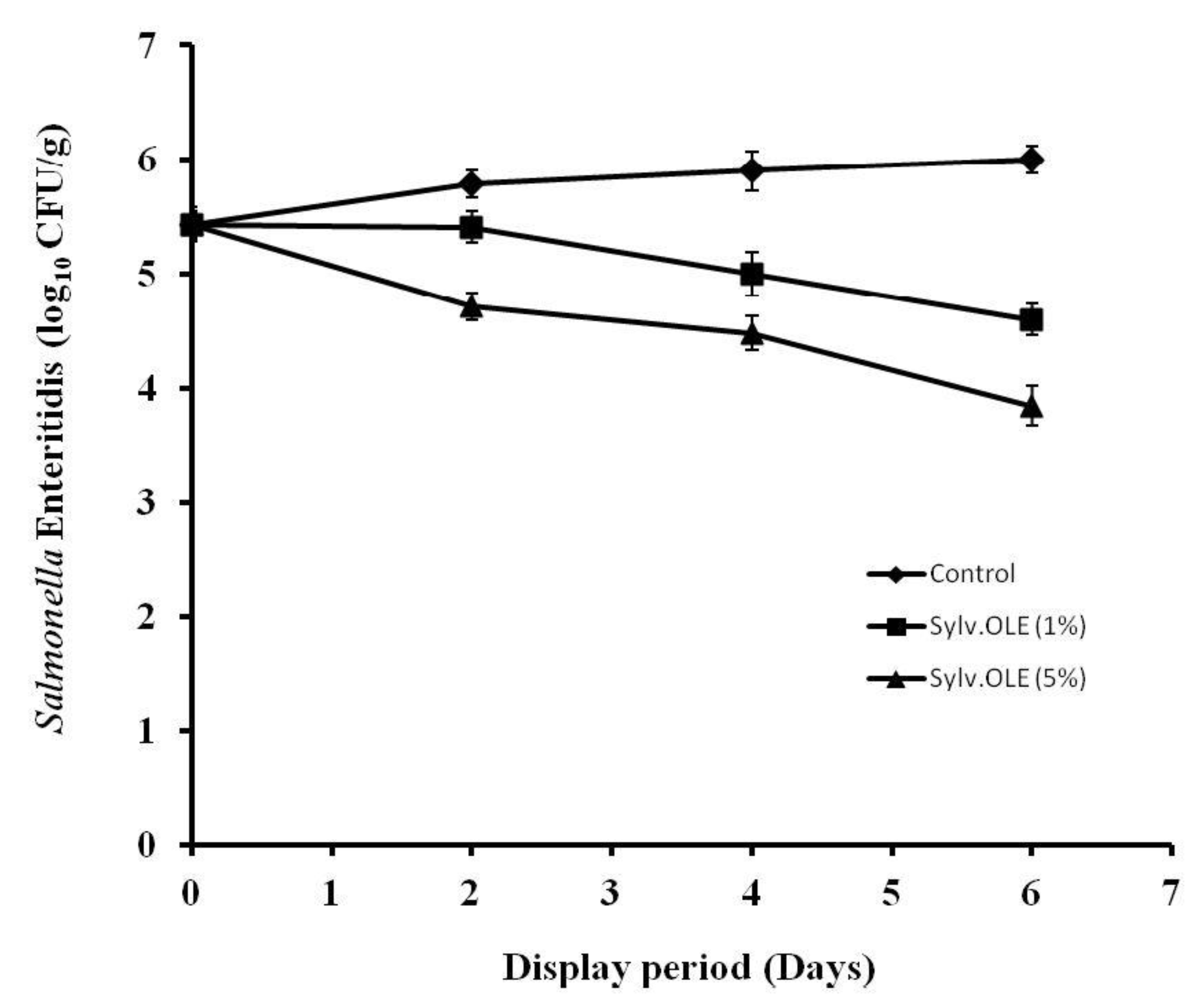
3.4.2. Pathogenic Microbiota
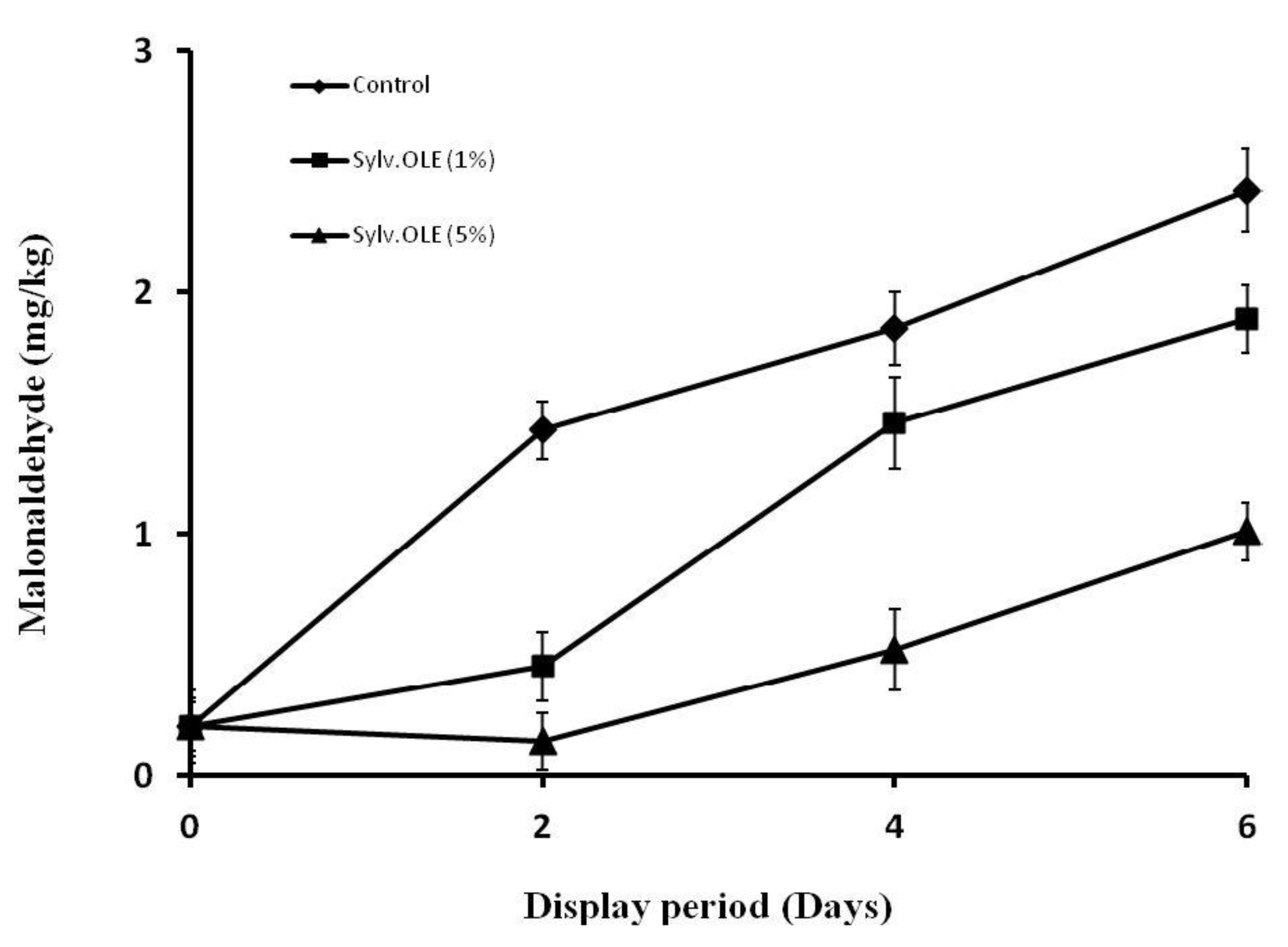
3.5. Lipid Oxidation
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Panel on biological hazards (BIOHAZ) scientific opinion on the public health risks related to the maintenance of the cold chain during storage and transport of meat. Part 2 (minced meat from all species). EFSA J. 2014, 12, 3783. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive tree (Olea europaea L.) leaves: Importance and advances in the analysis of phenolic compounds: Review. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Gonzalez, M.P.; Ramírez-Exposito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Navarro, M.; Morales, F.J. Evaluation of an olive leaf extract as a natural source of antiglycative compounds. Food Res. Int. 2017, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health benefits and use as functional ingredient in meat: Review. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and analysis of alternatives for the valorisation of agro-industrial olive oil by-products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. The importance and potential uses of olive leaves. Food Rev. Int. 2010, 26, 319–334. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Taoudiat, A.; Djenane, D.; Ferhat, Z.; Spigno, G. The effect of Laurus nobilis L. essential oil and different packaging systems on the photo-oxidative stability of Chemlal extra-virgin olive oil. J. Food Sci. Technol. 2018, 55, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Djenane, D.; Beltrán, J.A.; Camo, J.; Roncalés, P. Influence of vacuum ageing times of whole beef on retail shelf-life of steaks packaged with oregano (Origanum vulgare L.) active film under high O2. J. Food Sci. Technol. 2016, 53, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Fresh Meat; American Meat Science Association and the National Livestock and Meat Board: Chicago, IL, USA, 1995. [Google Scholar]
- Ben Miri, Y.; Ariño, A.; Djenane, D. Study of antifungal, anti-aflatoxigenic, antioxidant activity and phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia essential oils. J. Essent. Oil Bear. Plants 2018, 21, 345–361. [Google Scholar] [CrossRef]
- Altemimi, A.B. A study of the protective properties of Iraqi olive leaves against oxidation and pathogenic bacteria in food applications. Antioxidants 2017, 6, 34. [Google Scholar] [CrossRef]
- Altıok, E.; Bayçin, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Lafka, I.T.; Lazou, E.A.; Sinanoglou, J.V.; Lazos, E. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, S.; Kiassos, E.; Makris, D.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–998. [Google Scholar] [CrossRef] [PubMed]
- Boscaiu, M.; Sánchez, M.; Bautista, I.; Donat, P.; Lidón, A.; Llinares, J.; Llul, C.; Mayoral, O.; Vicente, O. Phenolic compounds as stress markers in plants from Gypsum habitats. Bull. UASVM Hortic. 2010, 67, 1843–5254. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, J.Y.; Park, C.S.; Shetty, K.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Zhang, L.; Lin Huang, P.; Chang, Y.-T.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Fletouris, D.; Botsoglou, N. Lipid oxidation of stored eggs enriched with very long chain n-3 fatty acids, as affected by dietary olive leaves (Olea europaea L.) or a-tocopheryl acetate supplementation. Food Chem. 2012, 134, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Talhaoui, N.; Vezza, T.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Gálvez, J.; Segura-Carretero, A. Phenolic compounds and in vitro immunomodulatory properties of three Andalusian olive leaf extracts. J. Funct. Foods 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque de Castro, M.D. Superheated liquid extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1136, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Qasem, M.H.; Ahmad-Qasem, B.H.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Drying and storage of olive leaf extracts. Influence on polyphenols stability. Ind. Crops Prod. 2016, 79, 232–239. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Yoon, S.K. Book Chapter: Oxidative Stress and Dietary Antioxidants—The Liver; Chapter 27—Oleuropein as an Antioxidant and Liver Protect; Patel, V.B., Rajendram, R., Preedy, V.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 323–335. ISBN 978-0-12-803951-9. [Google Scholar]
- Koutsoumanis, K.; Tassou, C.C.; Taoukis, P.S.; Nychas, G.-J.E. Modelling the effectiveness of a natural antimicrobial on Salmonella Enteritidis as a function of concentration, temperature and pH, using conductance measurements. J. Appl. Microbiol. 1998, 84, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]
- Bouaziz, M.; Fki, I.; Jemai, H.; Ayadi, M.; Sayadi, S. Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008, 108, 253–262. [Google Scholar] [CrossRef]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2008, 114, 806–812. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Altern. Med. 2011, 2011, 431021. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.; Henis, Y.; Jacoby, B. Studies on the mechanism of the antimicrobial action of oleuropein. J. Appl. Microbiol. 1972, 35, 559–567. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Pei, D.; Qu, L.; Li, Y.; Chen, J.; Di, D.; Gao, K. Antibacterial activity of hydroxytyrosol acetate from olive leaves (Olea Europaea L.). Nat. Prod. Res. 2018, 32, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, E.; Govaris, A.; Christaki, E.; Botsoglou, N. Effect of dietary olive leaves and/or α-tocopheryl acetate supplementation on microbial growth and lipid oxidation of turkey breast fillets during refrigerated storage. Food Chem. 2010, 121, 17–22. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Brenes, M.; de Castro, A. Antimicrobial activity of olive Oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; O’Brien, N.M.; Kerry, J.P. The effect of lutein, sesamol, ellagic acid and olive leaf extract on lipid oxidation and oxymyoglobin oxidation in bovine and porcine muscle model Systems. Meat Sci. 2009, 83, 201–208. [Google Scholar] [CrossRef]
- Radford, S.A.; Tassou, C.C.; Nychas, G.J.E.; Board, R.G. The influence of different oils on the death rate of Salmonella Enteritidis in homemade mayonnaise. Lett. Appl. Microbiol. 1991, 12, 125–128. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Islam, N.; Ng, J.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.X.; Rico, D.; McHugh, T.H. Antimicrobial olive leaf gelatin films for enhancing the quality of cold smoked salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Sahan, Y.; Yigit, A.; Tumay Ozer, E.; Gücer, S. Antibacterial activity and chemical constitutions of Olea europaea L. leaf extracts. J. Food Process. Preserv. 2010, 34, 383–396. [Google Scholar] [CrossRef]
- Medina-Martínez, M.S.; Truchado, P.; Castro-Ibáñez, I.; Allende, A. Antimicrobial activity of hydroxytyrosol: A current controversy. Biosci. Biotechnol. Biochem. 2016, 80, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Tranter, H.S.; Tassou, C.C.; Nychas, G.J.E. Effect of the olive phenolic compound, oleuropein on enterotoxin B production by Staphylococcus aureus S-6. J. Appl. Bacteriol. 1993, 74, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Nychas, G.J.E. Inhibition of Salmonella Enteritidis by oleuropein in broth and in a model food system. Lett. Appl. Microbiol. 1995, 20, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.P.; Walter, W.M., Jr.; Etchells, J.L. Antimicrobial properties of oleuropein and products of its hydrolysis from green olives. Appl. Microbiol. 1973, 26, 777–782. [Google Scholar] [PubMed]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Roncalés, P. Carbon monoxide in meat and fish packaging: Advantages and limits. Foods 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D.; Botsoglou, N. Effect of olive leaf (Olea europea L.) extracts on protein and lipid oxidation of long-term frozen n-3 fatty acids-enriched pork patties. Meat Sci. 2014, 98, 150–157. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- DeJong, S.; Lanari, M.C. Extracts of olive polyphenols improve lipid stability in cooked beef and pork: Contribution of individual phenolics to the antioxidant activity of the extract. Food Chem. 2009, 116, 892–897. [Google Scholar] [CrossRef]
- Bianchi, G. Review Article: Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Zoidou, E.; Magiatis, P.; Melliou, E.; Constantinou, M.; Haroutounian, S.; Skaltsounis, A.L. Oleuropein as a bioactive constituent added in milk and yogurt. Food Chem. 2014, 158, 319–324. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion: Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA J. 2009, 7, 1249. [Google Scholar]
| Method | Parameters | Sylv.OLE | Standard BHT |
|---|---|---|---|
| Free-radical scavenging activity 1 |
| 19.2 ± 1.6 4.2 ± 0.1 Very strong | 11.4 ± 0.5 7.1 ± 0.2 Very strong |
| Folin-Ciocalteu |
| 198.7 ± 3.6 |
| Phenol Compounds | Composition (%) |
|---|---|
| 1. Oleuropein | 43.25 ±1.26 |
| 2. Verbascoside | 13.12 ± 0.96 |
| 3. Apigenin-7-glucoside | 9.82 ± 1.06 |
| 4. Hydroxytyrosol | 7.32 ± 0.58 |
| 5. Tyrosol | 3.15 ± 0.09 |
| 6. Luteolin-7-glucoside | 1.34 ± 0.08 |
| 7. Luteolin | 0.89 ± 0.03 |
| 8. Caffeic acid | 0.79 ± 0.04 |
| 9. Rutin | 0.76 ± 0.01 |
| 10. Vanillic acid | 0.65 ± 0.01 |
| Parameters | Treatments | Days of Display | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| Bitterness | Control HMB (1%) HMB (5%) | 1.00(0.00) 1.88(0.35) 2.63(0.52) | 1.00(0.00) 1.75(0.46) 2.63(0.52) | 1.00(0.00) 1.63(0.52) 2.38(0.52) | 1.00(0.00) 1.75(0.46) 2.00(0.53) |
| Off-odor | Control HMB (1%) HMB (5%) | 1.00(0.00) 1.00(0.00) 1.00(0.00) | 2.63(0.52) 2.38(0.52) 2.13(0.35) | 3.25(0.46) 2.63(0.52) 2.25(0.46) | 4.00(0.53) 3.13(0.35) 2.25(0.46) |
| Overall acceptability | Control HMB (1%) HMB (5%) | 1.00(0.00) 1.00(0.00) 1.25(0.46) | 2.63 (0.52) 2.00 (0.53) 2.00 (0.53) | 3.38(0.52) 2.63(0.52) 2.38(0.52) | 4.25(0.46) 3.63(0.52) 2.50(0.53) |
| Sample Type | Shelf-Life Period (days) at 7 °C |
|---|---|
| Control | 2 |
| Sylv.OLE (1%) | 4 |
| Sylv.OLE (5%) | >6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenane, D.; Gómez, D.; Yangüela, J.; Roncalés, P.; Ariño, A. Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display. Foods 2019, 8, 10. https://doi.org/10.3390/foods8010010
Djenane D, Gómez D, Yangüela J, Roncalés P, Ariño A. Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display. Foods. 2019; 8(1):10. https://doi.org/10.3390/foods8010010
Chicago/Turabian StyleDjenane, Djamel, Diego Gómez, Javier Yangüela, Pedro Roncalés, and Agustín Ariño. 2019. "Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display" Foods 8, no. 1: 10. https://doi.org/10.3390/foods8010010
APA StyleDjenane, D., Gómez, D., Yangüela, J., Roncalés, P., & Ariño, A. (2019). Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display. Foods, 8(1), 10. https://doi.org/10.3390/foods8010010






