1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans
Abstract
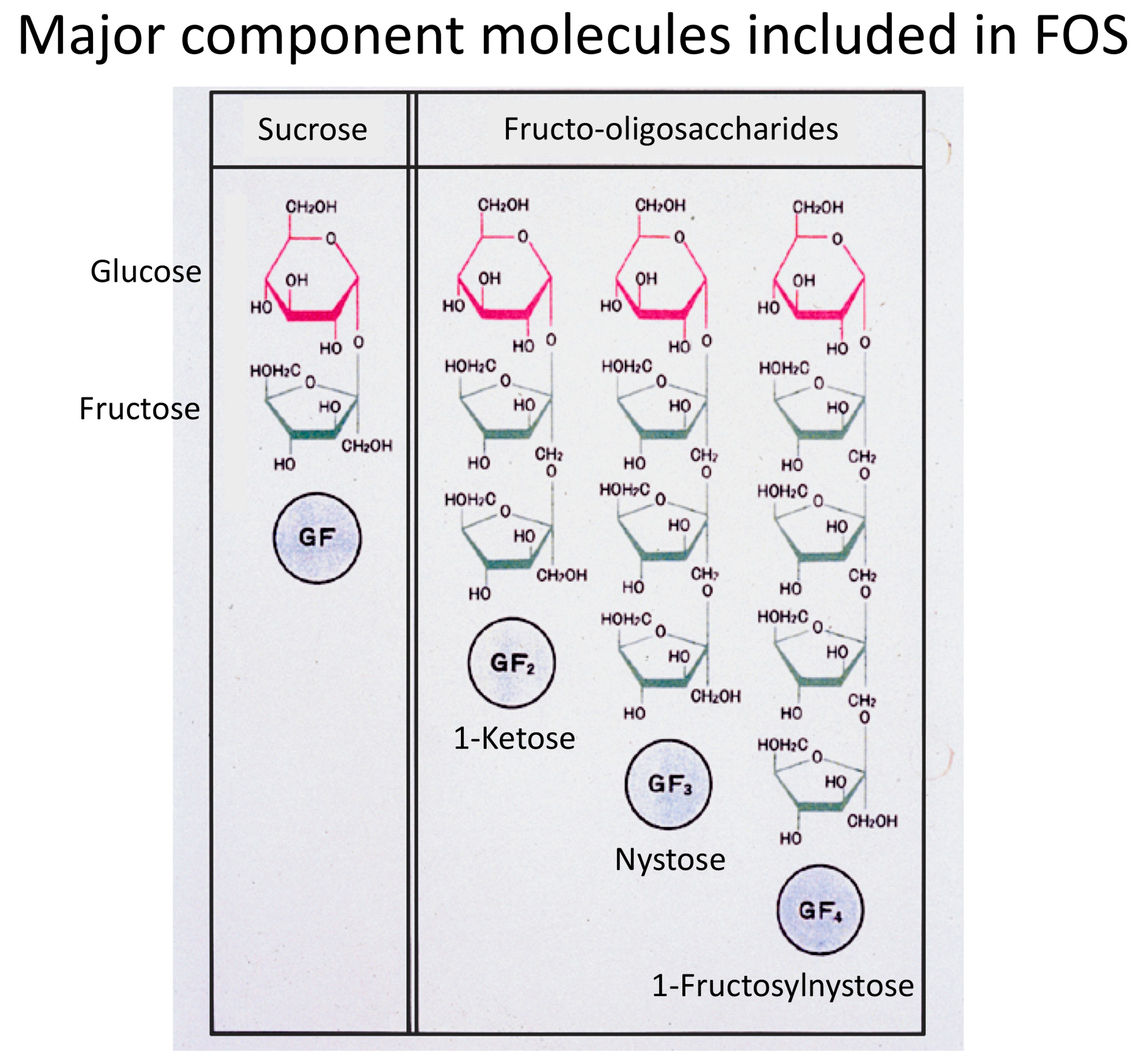
1. Fructooligosaccharides Have Been the Major Prebiotics
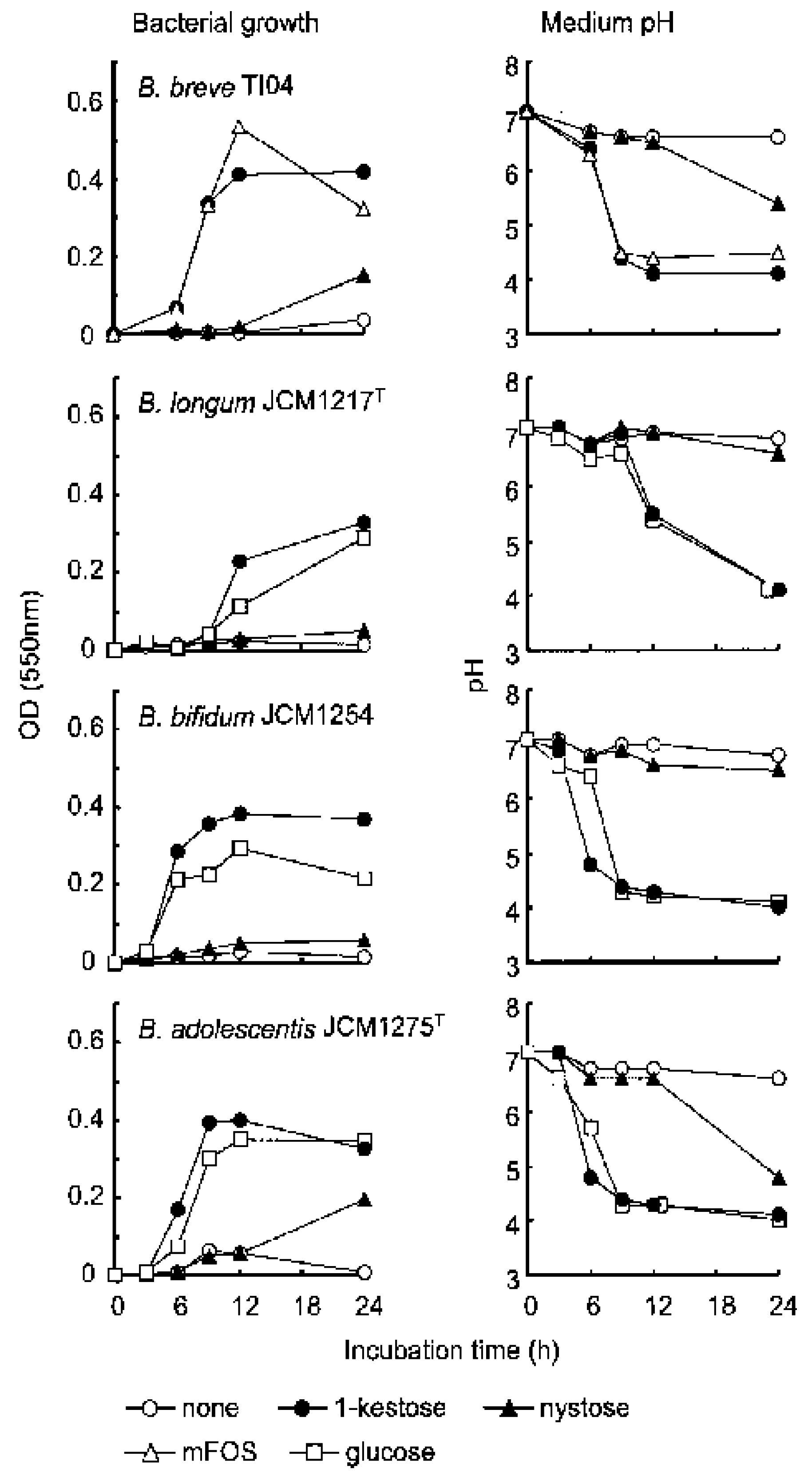
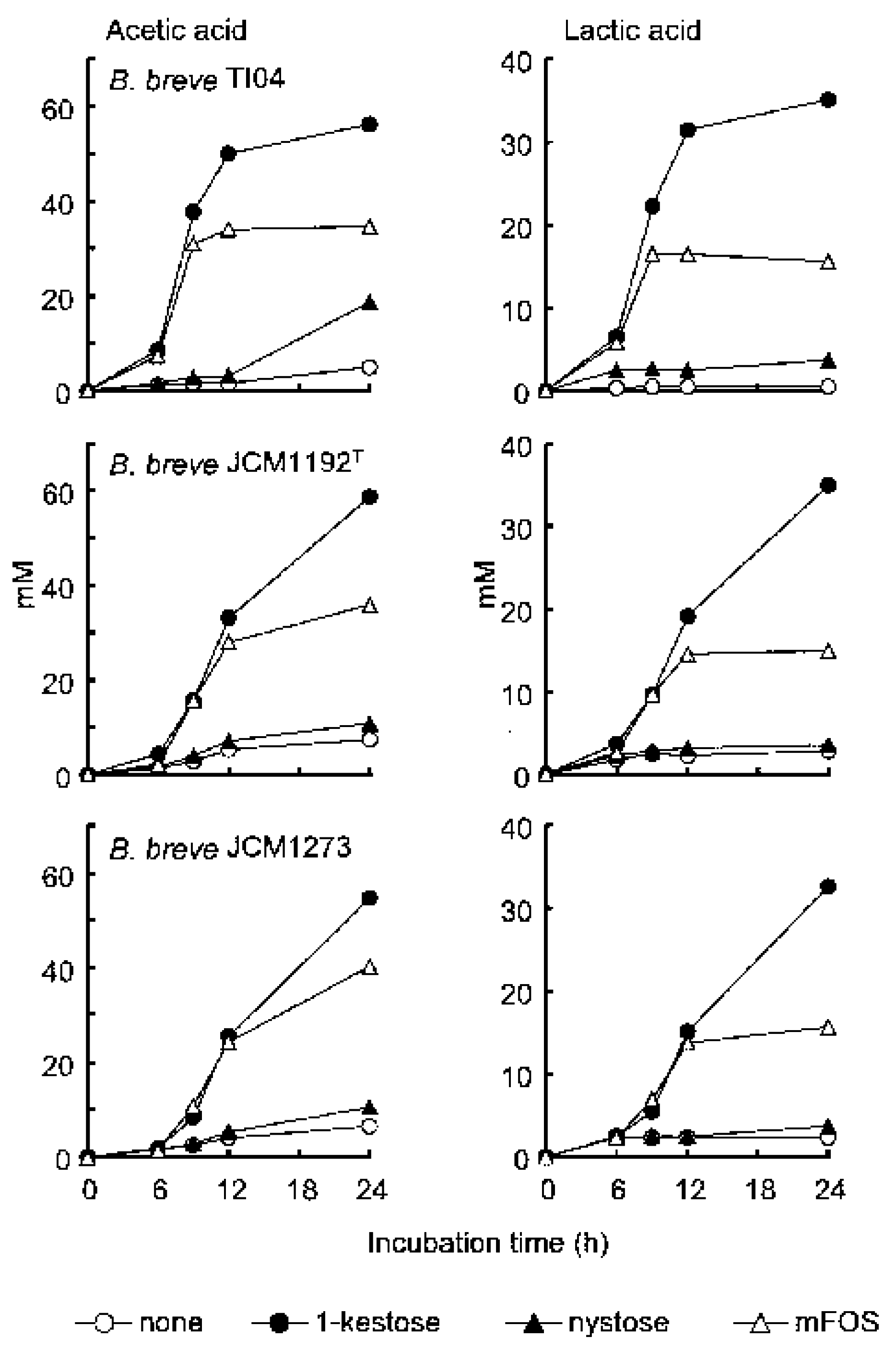
2. Superiority of 1-Kestose to the Longer-Chain FOS Components Such as Nystose with Regard to Bifidogenic Activity
3. Faecalibacterium prausnitzii as a Next-Generation Probiotic Capable of Producing Butyrate
4. Biological Effects of Butyrate Secreted by Butyrate-Producing Bacteria Including F. prausnitzii in the Intestine
5. Difficulty Using F. prausnitzii as a Probiotic
6. Evidence of the Prebiotic Effects of 1-Kestose on Both Atopic Dermatitis and Beneficial Bacteria
6.1. Evidence of the Prebiotic Effect of 1-Kestose on Beneficial Bacteria Other Than Bifidobacteria in Humans
6.2. Increasing the Number of F. prausnitzii in the Intestine of Infants Treated with 1-Kestose
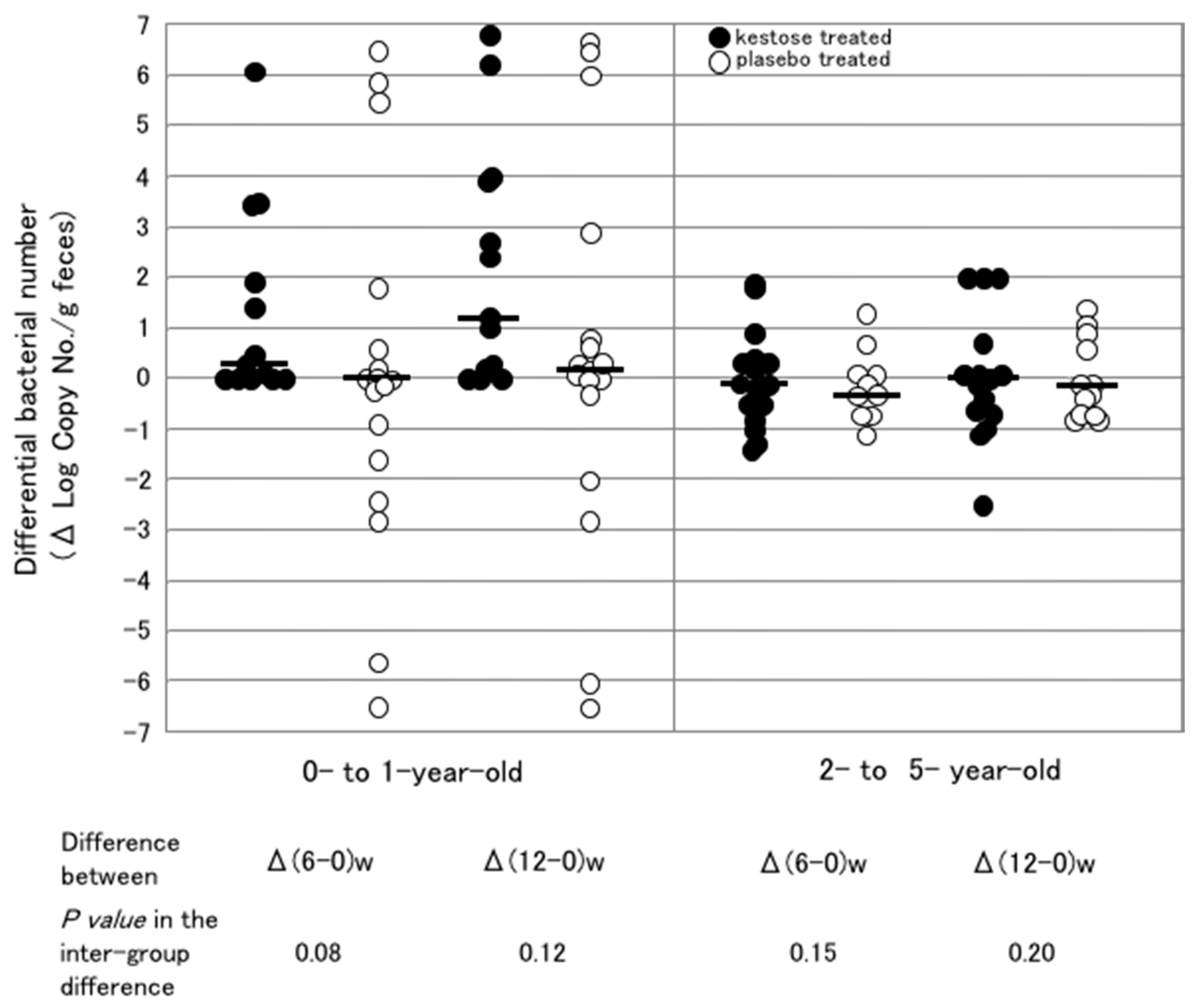
6.3. Difference in the Stimulating Effect of 1-Kestose on F. prausnitzii in Infantile Hosts with Different Ages
6.4. Effects of 1-Kestose on the F. prausnitzii Count in the Intestine of Adults
7. Effects of 1-Kestose on the Butyrate Level
8. F. prausnitzii-Targeting Prebiotics
9. Future Prospects of 1-Kestose Concerning Clinical Application
Funding
Conflicts of Interest
References
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [PubMed]
- Kawase, K.; Suzuki, T.; Kiyosawa, I.; Okonogi, S.; Kawashima, T.; Kuboyama, M. Effects of composition infant’s formulas on the intestinel microflora of infants. Bifidobact. Microflora 1981, 2, 25–31. [Google Scholar] [CrossRef]
- Suzuki, N.; Aiba, Y.; Takeda, H.; Fukumori, Y.; Koga, Y. Superiority of 1-kestose, the smallest fructooligosaccharide, to a synthetic mixture of fructooligosaccharides in the selective stimulating activity on bifidobacteria. Biosci. Microflora 2006, 25, 109–116. [Google Scholar] [CrossRef]
- Rossi, M.; Corradini, C.; Amaretti, A.; Nicolini, M.; Pompei, A.; Zanoni, S.; Matteuzzi, D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 2005, 71, 6150–6158. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Cummings, J.H.; Macfarlane, G.T. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other carbohydrate sources. J. Appl. Microbiol. 1998, 85, 381–386. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Klaenhammer, T.R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Ann. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Scweizer, A.; Aminov, R.I.; Blaut, M.; Flint, H.J. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 2003, 69, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watteriot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, J.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Turovskaya, O.; Kim, G.; Madan, R.; Karp, C.L.; Cheroutre, H.; Kronenberg, M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009, 10, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogensis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and proposal to reclassify it as Faecalibacterium prausnitzii gen. nov.; comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robert-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Kimura, M.; Takahashi, H.; Mikami, K.; Aiba, Y.; Takeda, H.; Koga, Y. Clinical effect of 1-kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin. Exp. Allergy 2009, 39, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery, S.; Boldinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Tokunaga, S.; Nagano, J.; Sato, F.; Konishi, K.; Tochio, T.; Murakami, Y.; Masumoto, N.; Tezuka, J.; Sudo, N.; et al. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr. Res. 2016, 80, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokunaga, T.; Tasiro, Y. Effects of fructooligosaccharides on intestinal flora and human health. Bifidobact. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bauerlein, M.; Frohberg, C.; Landschutze, V.; Gibson, G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Tochio, T.; Kitaura, Y.; Nakamura, S.; Sugawa, C.; Takahashi, M.; Endo, A.; Shimomura, Y. An alteration in the cecal microbiota composition by feeding of 1-kestose results in a marked increase in the cecal butyrate content in rats. PLoS ONE 2016, 11, e0166850. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, E.; Sakoda, C.; Toramaru, Y. Caecal fermentation and energy accumulation in the rat fed on indigestible oligosaccharides. Br. J. Nutr. 1998, 80, 469–476. [Google Scholar] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, S.; Vitali, B.; Klinder, A.; Kolida, S.; Ndagijimana, M.; Laghi, L.; Calnni, F.; Brigidi, P.; Gibson, G.R.; Costabile, A. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: An in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 2010, 65, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Vaneechoutte, M.; Doerffel, Y. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel Dis. 2008, 14, 147–161. [Google Scholar] [CrossRef] [PubMed]
| Indigenous Bacteria Treated with a | Log (No./g feces) of Bacteria | ||
|---|---|---|---|
| Before Treatment | After Treatment | ||
| Bifidobacterium spp. | Maltose | 11.4 b (11.1–11.4) c | 11.3 (11.0–11.5) |
| 1-kestose | 11.5 (11.0–11.6) | 12.0 (11.8–12.1) * | |
| Clostridium cluster XlVa | Maltose | 11.9 (11.5–12.0) | 12.1 (11.8–12.1) |
| 1-kestose | 12.0 (11.8–12.1) | 12.0 (12.0–12.3) | |
| F. prausnitzii | Maltose | 10.3 (10.0–10.8) | 10.7 (9.1–11.2) |
| 1-kestose | 10.4 (10.2–10.8) | 11.1 (11.1–11.1) * | |
| Lactobacillus spp. | Maltose | 8.2 (7.9–9.0) | 8.5 (7.9–8.6) |
| 1-kestose | 8.8 (8.5–9.7) | 10.8 (10.0–10.8) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. https://doi.org/10.3390/foods7090140
Tochio T, Kadota Y, Tanaka T, Koga Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans. Foods. 2018; 7(9):140. https://doi.org/10.3390/foods7090140
Chicago/Turabian StyleTochio, Takumi, Yoshihiro Kadota, Toshio Tanaka, and Yasuhiro Koga. 2018. "1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans" Foods 7, no. 9: 140. https://doi.org/10.3390/foods7090140
APA StyleTochio, T., Kadota, Y., Tanaka, T., & Koga, Y. (2018). 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans. Foods, 7(9), 140. https://doi.org/10.3390/foods7090140




