Feeding of 1-Kestose Induces Glutathione-S-Transferase Expression in Mouse Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Analysis for Blood Components
2.3. Preparation of Total RNA
2.4. Microarray Analyses
2.5. Quantification of the Gene Expression Level Using qRT-PCR
2.6. Hepatic GST Activity Assay
2.7. Statistics Analysis
3. Results
3.1. Body Weight, Food Intake and Tissue Weights
3.2. Concentrations of Blood Components
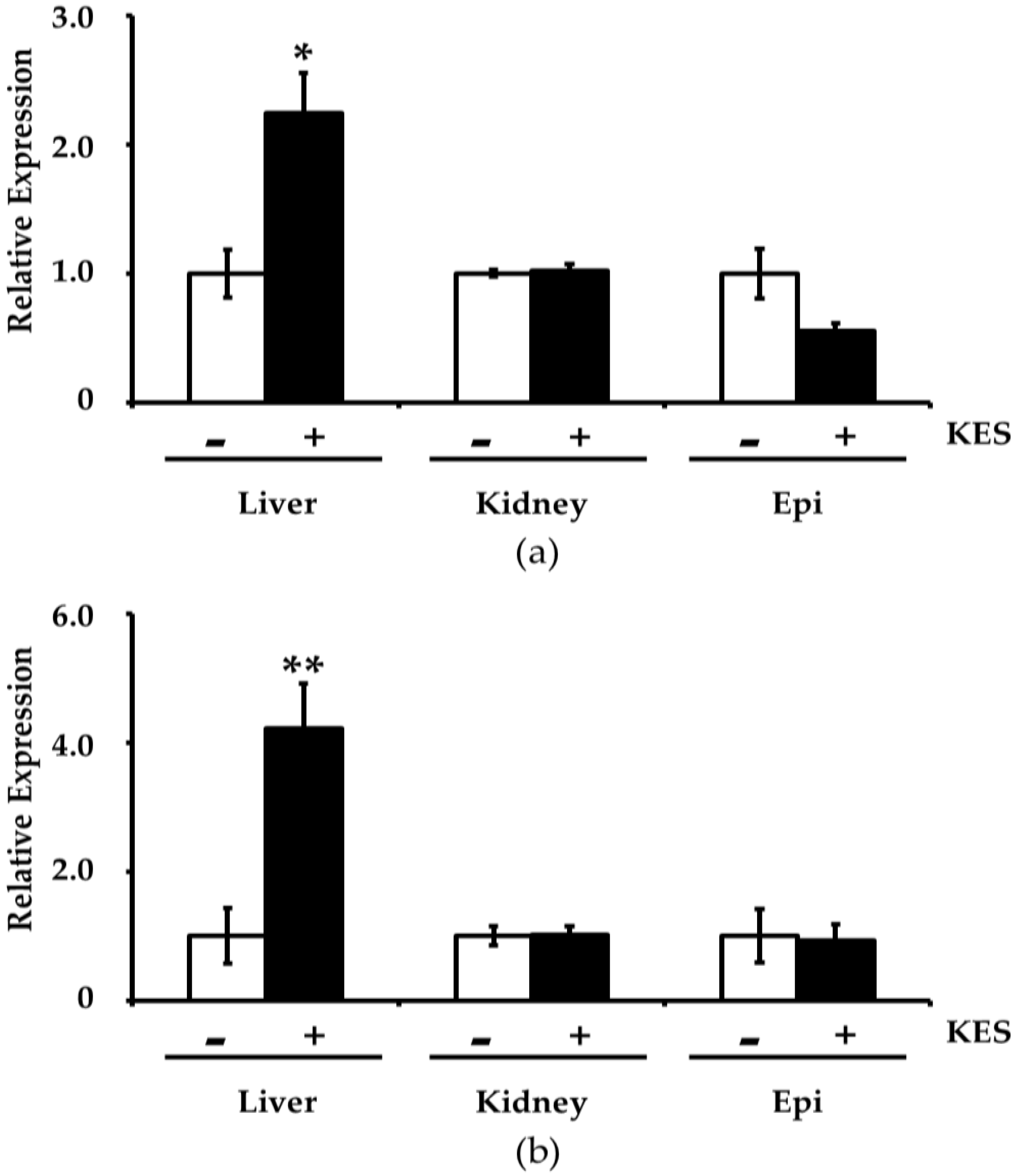
3.3. Effects of Dietary Intake of 1-Kestose on Gene Expression Related to Oxidative Stress in the Tissues
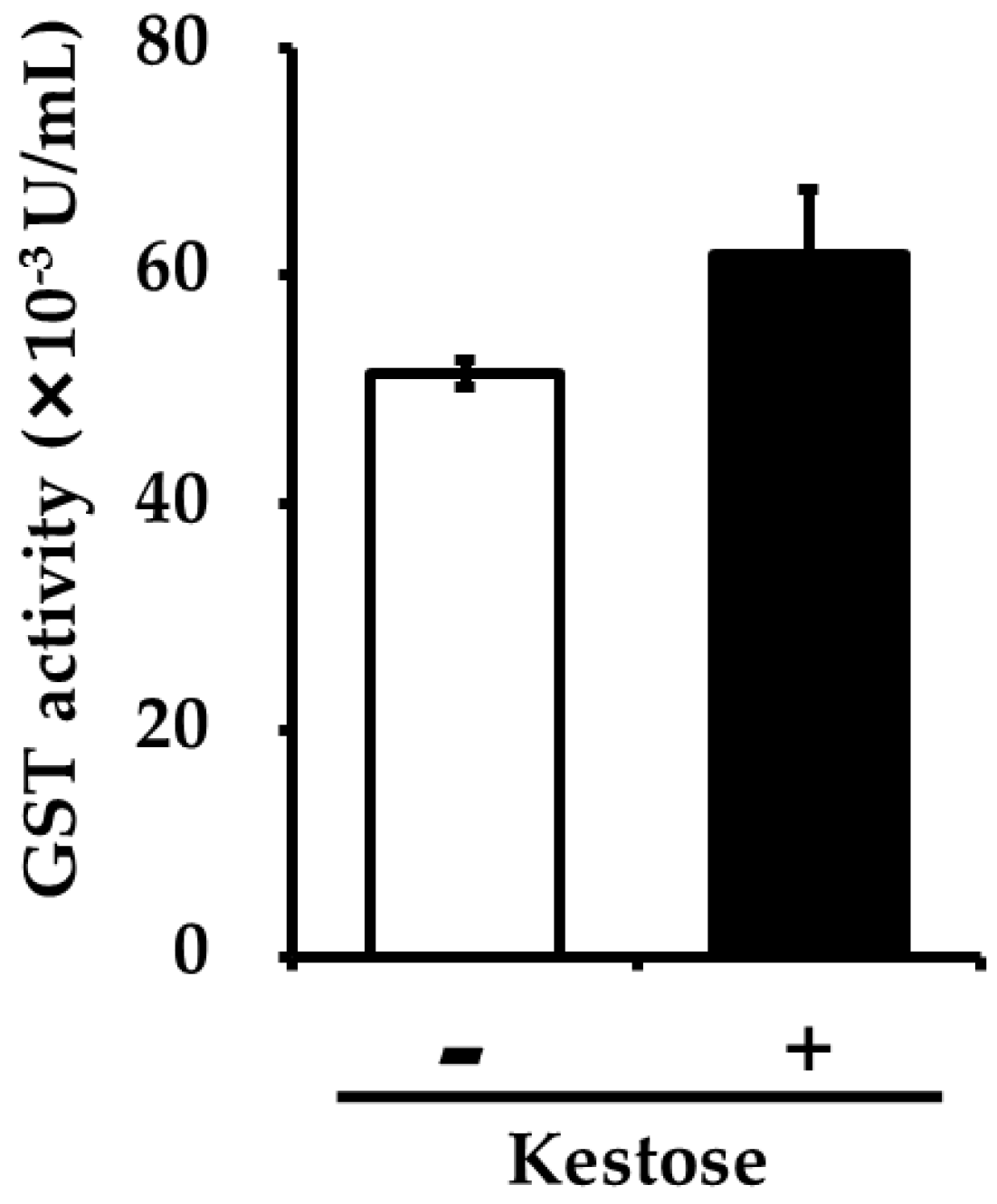
3.4. Hepatic GST Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [PubMed]
- Paineau, D.; Respondek, F.; Menet, V.; Sauvage, R.; Bornet, F.; Wagner, A. Effects of short-chain fructooligosaccharideson faecal bifidobacteria and specific immune response in formula-fed term infants: A randomized, double-blind, placebo-controlled trial. J. Nutr. Sci. Vitaminol. 2014, 60, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kondo, N.; Yamaguchi, Y.; Hashiguchi, M.; Tanabe, K.; Ushiroda, C.; Kawahashi-Tokuhisa, M.; Yui, K.; Miyakoda, M.; Oku, T. Daily feeding of fructooligosaccharide or glucomannan delays onset of senescence in SAMP8 mice. Gastroenterol. Res. Pract. 2014, 2014, 303184. [Google Scholar] [CrossRef] [PubMed]
- Gomides, A.F.F.; de Paula, S.O.; GoncËalves, R.V.; de Oliveira, L.L.; Ferreira, C.L.; Comastri, D.S.; Peluzio Mdo, C. Prebiotics prevent the appearance of aberrant crypt foci (ACF) in the colon of BALB/c mice for increasing the gene expression of p16 protein. Nutr Hosp. 2014, 30, 883–890. [Google Scholar] [CrossRef] [PubMed]
- CapitaÂn-Cañadas, F.; Ortega-Gonzalez, M.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 2014, 58, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Diane, E.; Hunter, D.E. Selected fructooligosaccharide (1-kestose, nystose, and 1F-β-fructofuranosylnystose) composition of foods and feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Vahedi, K.; Achour, L.; Attar, A.; Salfati, J.; Pochart, P.; Marteau, P.; Flourié, B.; Bornet, F.; Rambaud, J.C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 1999, 129, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Nakamura, S.; Konishi, K.; Nakagawa, J.; Tochio, T. Variations in prebiotic oligosaccharide fermentation by intestinal lactic acid bacteria. Int. J. Food Sci. Nutr. 2016, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tochio, T.; Kitaura, Y.; Nakamura, S.; Sugawa, C.; Takahashi, M.; Endo, A.; Shimomura, Y. An Alteration in the Cecal Microbiota Composition by Feeding of 1-Kestose Results in a Marked Increase in the Cecal Butyrate Content in Rats. PLoS ONE 2016, 11, e0166850. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Advances in mechanisms of anti-oxidation. Discov. Med. 2014, 17, 121–130. [Google Scholar] [PubMed]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Westhuyzen, J. The oxidation hypothesis of atherosclerosis: An update. Ann. Clin. Lab. Sci. 1997, 27, 1–10. [Google Scholar] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [PubMed]
- Kostner, K.; Hornykewycz, S.; Yang, P.; Neunteufl, T.; Glogar, D.; Weidinger, F.; Maurer, G.; Huber, K. Is oxidative stress causally linked to unstable angina pectoris? A study in 100 CAD patients and matched controls. Cardiovasc. Res. 1997, 36, 330–336. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Abargouei, A.; Ghiasvand, R.; Hariri, M. Prebiotics, Prosynbiotics and Synbiotics: Can They Reduce Plasma Oxidative Stress Parameters? A Systematic Review. Probiotics Antimicrob. Proteins 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Ebert, M.N.; Beyer-Sehlmeyer, G.; Liegibel, U.M.; Kautenburger, T.; Becker, T.W.; Pool-Zobel, B.L. Butyrate induces glutathione S-transferase in human colon cells and protects from genetic damage by 4-hydroxy-2-nonenal. Nutr. Cancer 2001, 41, 156–164. [Google Scholar] [CrossRef]
- De Vos, W.M.; de Vos, E.A. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr. Rev. 2012, 70, S45–S56. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Fuentes, S.; van den Bogert, B.; Honkanen, H.; de Vos, W.M.; Welling, G.W.; Hyöty, H.; Harmsen, H.J. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 2014, 57, 1569–1577. [Google Scholar] [CrossRef]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Control Diet | 1-Kestose Diet |
|---|---|---|
| (g/100 g Diet) | ||
| Corn starch | 39.7 | 39.7 |
| Maltodextrin 10 | 13.2 | 13.2 |
| Sucrose | 10.0 | 5.0 |
| Casein | 20.0 | 20.0 |
| Soybean oil | 7.0 | 7.0 |
| Cellulose | 5.0 | 5.0 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 |
| L-Cysteine | 0.3 | 0.3 |
| Choline bitartrate | 0.3 | 0.3 |
| 1-Kestose | 0 | 5.0 |
| Total | 100.0 | 100.0 |
| Target | GenBank Acc | Oligonucleotide Sequence |
|---|---|---|
| β-Actin | NM007393.4 | CATCCGTAAAGACCTCTATGCCAAC |
| ATGGAGCCACCGATCCACA | ||
| Gstp1 | NM_0103541.1 | CGGCAAATATGTCACCCTCATCTA |
| TCTGGGACAGCAGGGTCTCA | ||
| Gsta4 | NM_010357.3 | TGACACAGACCAGGGCCATC |
| ATCAGGTCCTGGGTGCCATC |
| KES (−) | KES (+) | |
|---|---|---|
| Means ± SE | ||
| Body weight (g) | 33.7 ± 1.4 | 32.0 ± 1.3 |
| Food intake (g/day) | 3.30 ± 0.09 | 3.53 ± 0.12 |
| Liver (g) | 1.45 ± 0.09 | 1.31 ± 0.08 |
| Kidney (g) | 0.32 ± 0.01 | 0.31 ± 0.01 |
| Epidermal adipose tissue (g) | 1.52 ± 0.14 | 1.23 ± 0.16 |
| Cecum (g) | 0.09 ± 0.00 | 0.16 ± 0.01 * |
| Cecum content (g) | 0.27 ± 0.02 | 0.43 ± 0.03 * |
| Serum triglycerides (mg/dL) | 155 ± 23 | 133 ± 13 |
| Serum total cholesterol (mg/dL) | 155 ± 1 | 138 ± 4 |
| Serum free fatty acids (mEq/L) | 0.57 ± 0.01 | 0.51 ± 0.02 |
| Gene | Expression Ratio KES (+)/KES (−) |
|---|---|
| Gstp1 (glutathione S-transferase pi 1) | 2.13 |
| Tgfb3 (transforming growth factor beta 3) | 1.61 |
| Gsta4 (glutathione S-transferase alpha 4) | 1.50 |
| Cyp4a10 (cytochrome P450, family 4, subfamily a, polypeptide 10) | 0.75 |
| Srebf1 (sterol regulatory element-binding transcription factor 1) | 0.75 |
| Col18a1 (collagen type XVIII alpha 1 chain) | 0.70 |
| Cyp4a12a (cytochrome P450, family 4, subfamily a, polypeptide 12a) | 0.70 |
| Ldlr (low-density lipoprotein receptor) | 0.69 |
| Keap1 (Kelch-like ECH-associated protein 1) | 0.69 |
| Gck (glucokinase) | 0.69 |
| Gstt3 (glutathione S-transferase theta 3) | 0.64 |
| Fasn (fatty acid synthase) | 0.47 |
| Mt3 (metallothionein 3) | 0.45 |
| Apoa4 (apolipoprotein A4) | 0.40 |
| Cyp7a1 (cytochrome P450, family 7, subfamily a, polypeptide 1) | 0.34 |
| Mt1 (metallothionein 1) | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tochio, T.; Ueno, Y.; Kitaura, Y.; Shinohara, M.; Kadota, Y.; Minoda, K.; Shimomura, Y.; Osawa, T. Feeding of 1-Kestose Induces Glutathione-S-Transferase Expression in Mouse Liver. Foods 2019, 8, 69. https://doi.org/10.3390/foods8020069
Tochio T, Ueno Y, Kitaura Y, Shinohara M, Kadota Y, Minoda K, Shimomura Y, Osawa T. Feeding of 1-Kestose Induces Glutathione-S-Transferase Expression in Mouse Liver. Foods. 2019; 8(2):69. https://doi.org/10.3390/foods8020069
Chicago/Turabian StyleTochio, Takumi, Yuki Ueno, Yasuyuki Kitaura, Mikako Shinohara, Yoshihiro Kadota, Kanako Minoda, Yoshiharu Shimomura, and Toshihiko Osawa. 2019. "Feeding of 1-Kestose Induces Glutathione-S-Transferase Expression in Mouse Liver" Foods 8, no. 2: 69. https://doi.org/10.3390/foods8020069
APA StyleTochio, T., Ueno, Y., Kitaura, Y., Shinohara, M., Kadota, Y., Minoda, K., Shimomura, Y., & Osawa, T. (2019). Feeding of 1-Kestose Induces Glutathione-S-Transferase Expression in Mouse Liver. Foods, 8(2), 69. https://doi.org/10.3390/foods8020069




