Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Generation of Milk-Derived Powders
2.3. Milk Oligosaccharides Analysis
2.4. Bacterial Strains and Culture
2.5. Mammalian Cell Culture
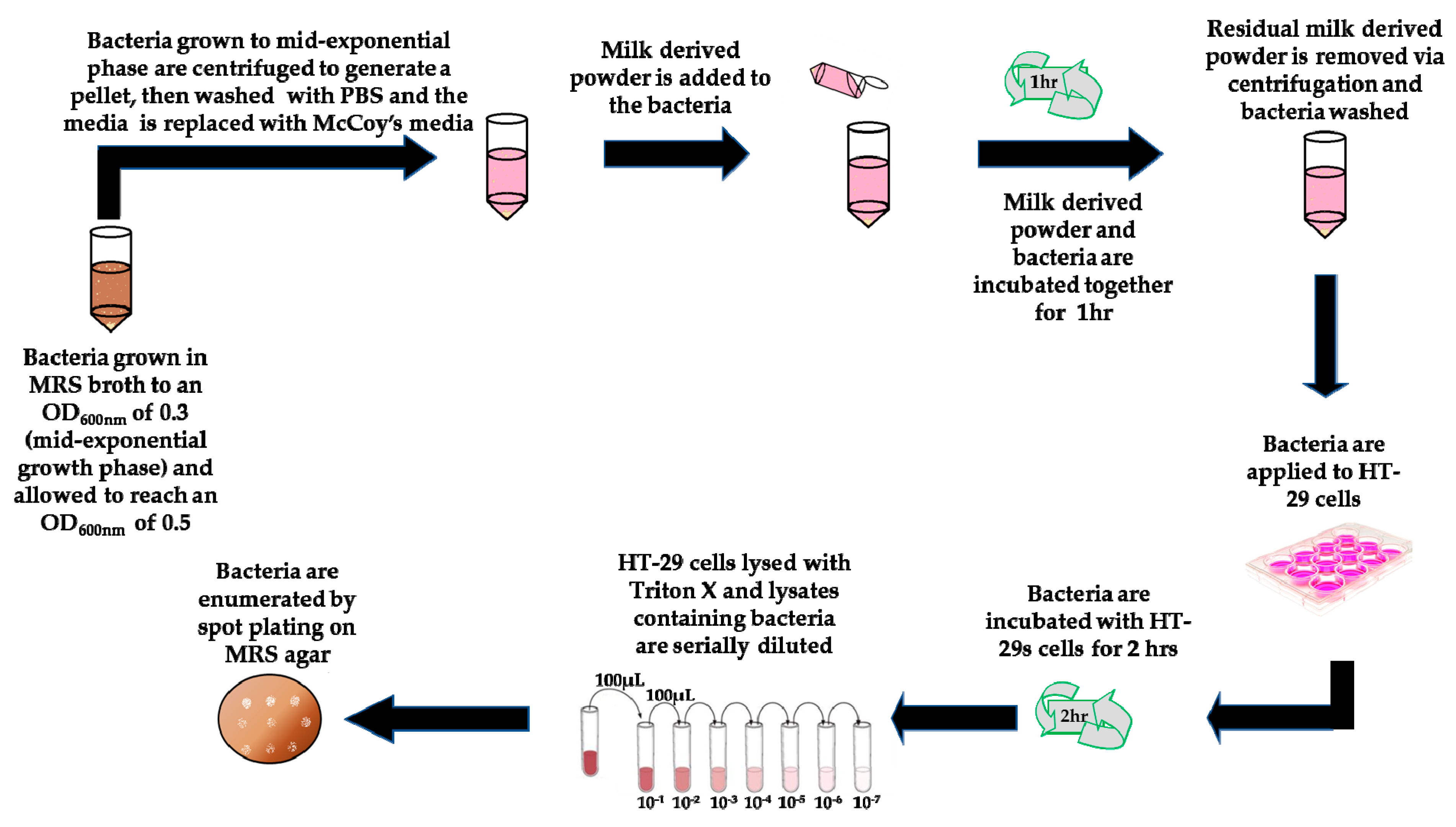
2.6. Exposure of Bacteria to Milk-Derived Powders
2.7. Adhesion Assays
3. Results and Discussion
3.1. Comparison of Conventional and Miniaturised Assays
3.2. Effect of Milk Components on Growth of B. Longum subsp. Infantis ATCC 15697
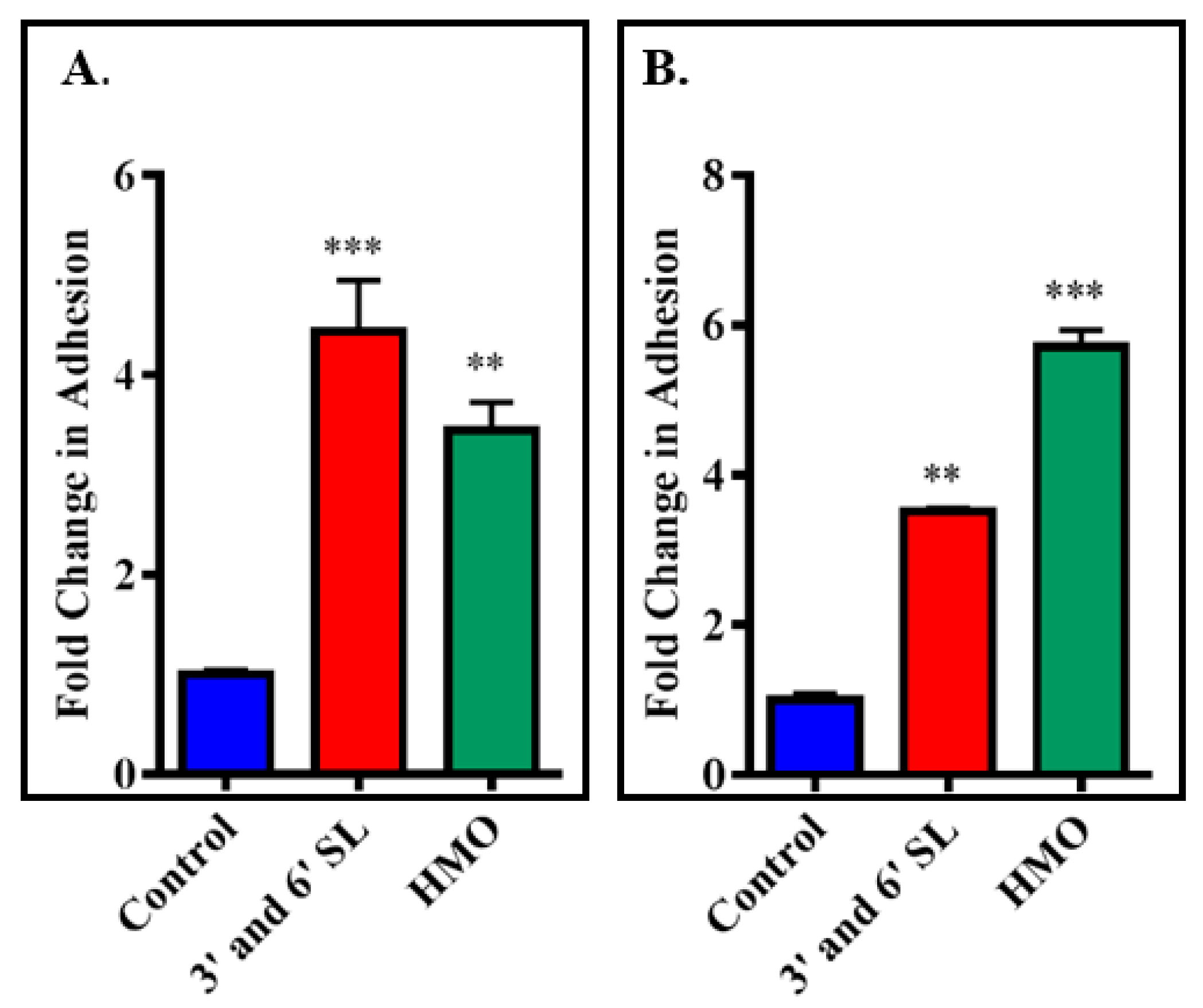
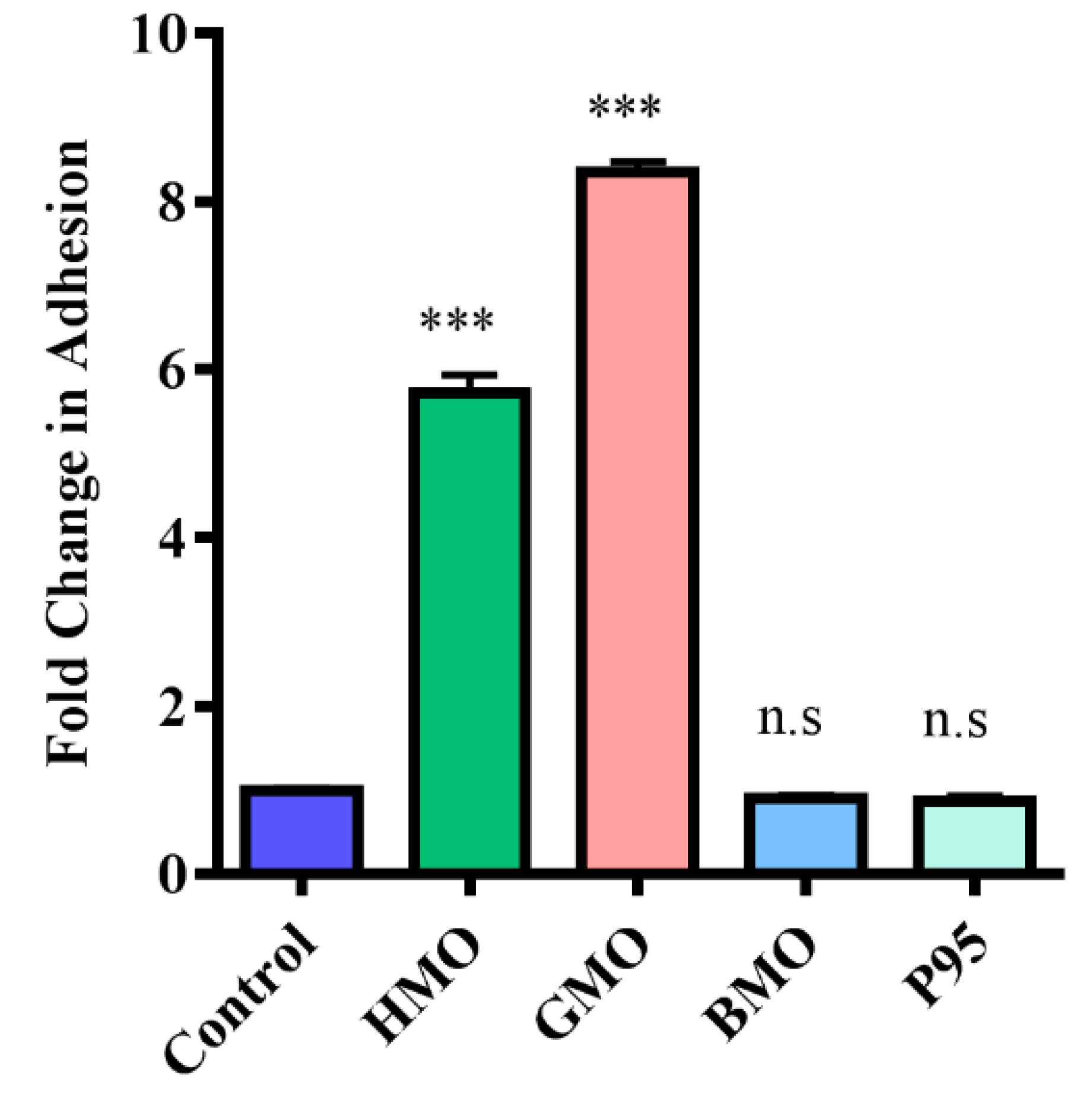
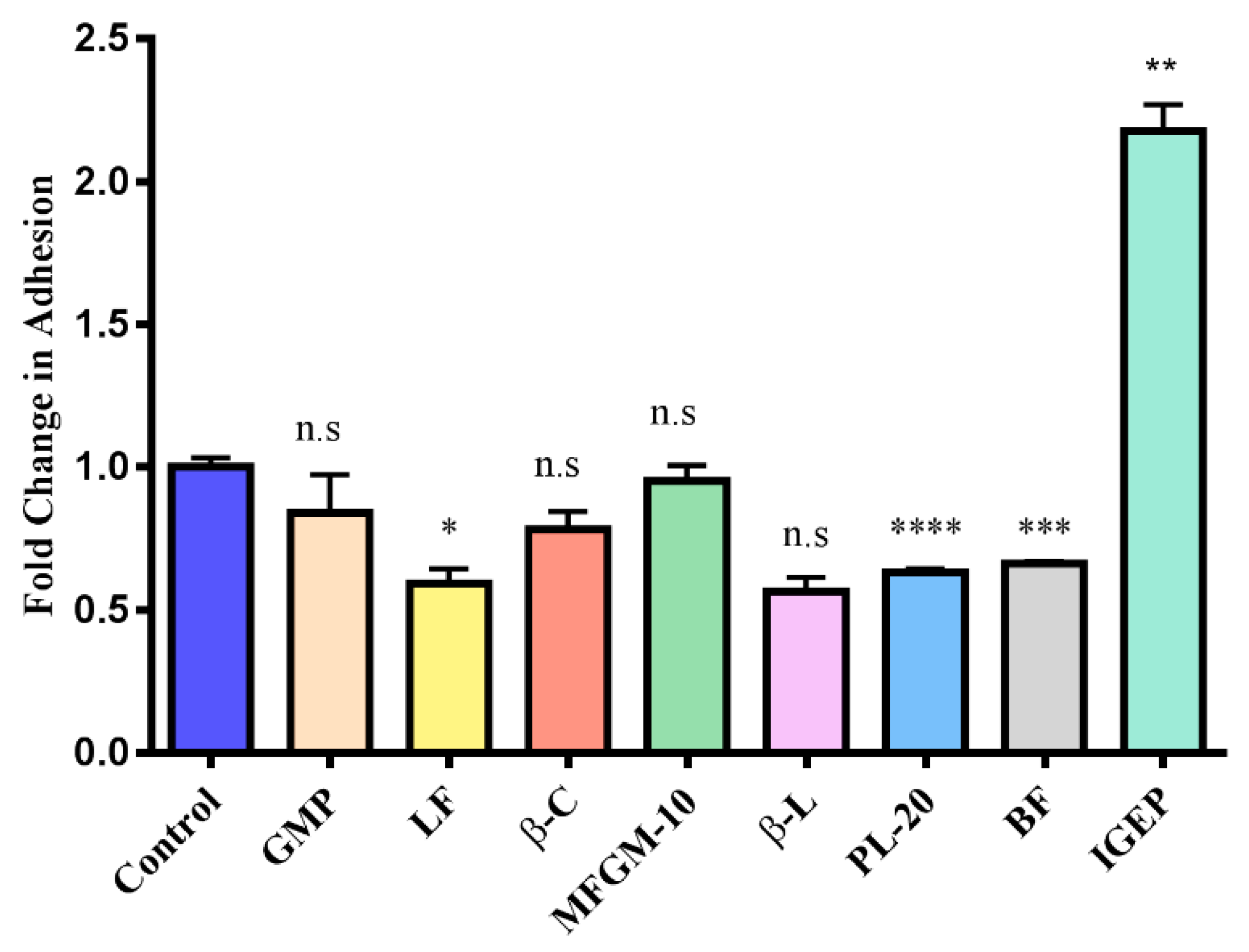
3.3. Screening Milk Components for Increased Adhesion of B. longum subsp. Infantis ATCC 15697 to HT-29 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pacheco, A.R.; Barile, D.; Underwood, M.A.; Mills, D.A. The Impact of the Milk Glycobiome on the Neonate Gut Microbiota. Annu. Rev. Anim. Biosci. 2015, 3, 419–445. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of Bifidobacteria within the Infant Gut Microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Front. Microbiol. 2016, 7, 1220. [Google Scholar] [CrossRef]
- Boehm, G.; Stahl, B. Oligosaccharides from Milk. J. Nutr. 2007, 137, 847S–849S. [Google Scholar] [CrossRef] [Green Version]
- González, R.; Klaassens, E.S.; Malinen, E.; de Vos, W.M.; Vaughan, E.E. Differential transcriptional response of Bifidobacterium longum to human milk, formula milk, and galactooligosaccharide. Appl. Environ. Microbiol. 2008, 74, 4686–4694. [Google Scholar] [CrossRef]
- Chichlowski, M.; de Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Kavanaugh, D.W.; O’Callaghan, J.; Buttó, L.F.; Slattery, H.; Lane, J.; Clyne, M.; Kane, M.; Joshi, L.; Hickey, R.M. Exposure of Bifidobacterium longum subsp. infantis to Milk Oligosaccharides Increases Adhesion to Epithelial Cells and Induces a Substantial Transcriptional Response. PLoS ONE 2013, 8, e67224. [Google Scholar]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide Binding Proteins from Bifidobacterium longum subsp. infantis Reveal a Preference for Host Glycans. PLoS ONE 2011, 6, e17315. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ross, S.A.; Lane, J.A.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Defatted bovine milk fat globule membrane inhibits association of enterohaemorrhagic Escherichia coli O157:H7 with human HT-29 cells. Int. Dairy J. 2016, 59, 36–43. [Google Scholar] [CrossRef]
- Le, T.T.; van Camp, J.; Rombaut, R.; van Leeckwyck, F.; Dewettinck, K. Effect of washing conditions on the recovery of milk fat globule membrane proteins during the isolation of milk fat globule membrane from milk. J. Dairy Sci. 2009, 92, 3592–3603. [Google Scholar] [CrossRef]
- Struijs, K.; Van de Wiele, T.; Le, T.T.; Debyser, G.; Dewettinck, K.; Devreese, B.; Van Camp, J. Milk fat globule membrane glycoproteins prevent adhesion of the colonic microbiota and result in increased bacterial butyrate production. Int. Dairy J. 2013, 32, 99–109. [Google Scholar] [CrossRef]
- Mehra, R.; Barile, D.; Marotta, M.; Lebrilla, C.B.; Chu, C.; German, J.B. Novel High-Molecular Weight Fucosylated Milk Oligosaccharides Identified in Dairy Streams. PLoS ONE 2014, 9, e96040. [Google Scholar] [CrossRef] [Green Version]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar]
- Garrido, D.; Dallas, D.C.; Mills, D.A. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: Mechanisms and implications. Microbiology 2013, 159, 649–664. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Jimenez-Espinoza, R.; Eom, H.-J.; Block, D.E.; Mills, D.A. Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol. 2013, 33, 262–270. [Google Scholar] [CrossRef]
- Perrin, S.; Warchol, M.; Grill, J.P.; Schneider, F. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J. Appl. Microbiol. 2001, 90, 859–865. [Google Scholar] [CrossRef]
- Kim, J.-H.; An, H.J.; Garrido, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Proteomic Analysis of Bifidobacterium longum subsp. infantis Reveals the Metabolic Insight on Consumption of Prebiotics and Host Glycans. PLoS ONE 2013, 8, e57535. [Google Scholar]
- Sela, D.A.; Li, Y.; Lerno, L.; Wu, S.; Marcobal, A.M.; German, J.B.; Chen, X.; Lebrilla, C.B.; Mills, D.A. An Infant-associated Bacterial Commensal Utilizes Breast Milk Sialyloligosaccharides. J. Biol. Chem. 2011, 286, 11909–11918. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.F. Milk: An overview. In Milk Proteins; Academic Press: Cambridge, MA, USA, 2008; pp. 1–54. [Google Scholar]
- Robitaille, G. Growth-promoting effects of caseinomacropeptide from cow and goat milk on probiotics. J. Dairy Res. 2013, 80, 58–63. [Google Scholar] [CrossRef]
- O’Riordan, N.; O’Callaghan, J.; Buttò, L.F.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Bovine glycomacropeptide promotes the growth of Bifidobacterium longum ssp. infantis and modulates its gene expression. J. Dairy Sci. 2018, 101, 6730–6741. [Google Scholar]
- Chaneton, L.; Sáez, J.M.P.; Bussmann, L.E. Antimicrobial activity of bovine β-lactoglobulin against mastitis-causing bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent advances in exploiting goat’s milk: Quality, safety and production aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Barnett, A.; Roy, N.; McNabb, W.; Cookson, A. Effect of a Semi-Purified Oligosaccharide-Enriched Fraction from Caprine Milk on Barrier Integrity and Mucin Production of Co-Culture Models of the Small and Large Intestinal Epithelium. Nutrients 2016, 8, 267. [Google Scholar] [CrossRef]
- Lara-Villoslada, F.; Debras, E.; Nieto, A.; Concha, A.; Gálvez, J.; López-Huertas, E.; Boza, J.; Obled, C.; Xaus, J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr. 2006, 25, 477–488. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Requena, P.; Martinez-Ferez, A.; Guadix, E.; Sanchez de Medina, F.; Zarzuelo, A.; Suarez, M.D.; Boza, J.J.; Martinez-Augustin, O. Goat Milk Oligosaccharides Are Anti-Inflammatory in Rats with Hapten-Induced Colitis. J. Nutr. 2006, 136, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.L.; Costabile, A.; Wilbey, R.A.; Grandison, A.S.; Duarte, L.C.; Roseiro, L.B. In vitro evaluation of the fermentation properties and potential prebiotic activity of caprine cheese whey oligosaccharides in batch culture systems. Biofactors 2012, 38, 440–449. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Guadix, A.; Guadix, E.M. Recovery of caprine milk oligosaccharides with ceramic membranes. J. Membr. Sci. 2006, 276, 23–30. [Google Scholar] [CrossRef]
- Woof, J.M. Immunoglobulins and their receptors, and subversion of their protective roles by bacterial pathogens. Biochem. Soc. Trans. 2016, 44, 1651–1658. [Google Scholar] [CrossRef]
- Mehra, R.; Marnila, P. Milk immunoglobulins for health promotion. Int. Dairy J. 2006, 16, 1262–1271. [Google Scholar] [CrossRef]
- Korhonen, H.; Marnila, P.; Gill, H.S. Bovine milk antibodies for health. Br. J. Nutr. 2000, 84 (Suppl. 1), S135–S146. [Google Scholar] [CrossRef]
- Kvistgaard, A.S.; Pallesen, L.T.; Arias, C.F.; Lopez, S.; Petersen, T.E.; Heegaard, C.W.; Rasmussen, J.T. Inhibitory Effects of Human and Bovine Milk Constituents on Rotavirus Infections. J. Dairy Sci. 2004, 87, 4088–4096. [Google Scholar] [CrossRef]
- Garrido, D.; Nwosu, C.; Ruiz-Moyano, S.; Aldredge, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteom. 2012, 11, 775–785. [Google Scholar] [CrossRef]
- Chen, P.-W.; Liu, Z.-S.; Kuo, T.-C.; Hsieh, M.-C.; Li, Z.-W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. BioMetals 2017, 30, 237–248. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar]
- Arnold, R.R.; Cole, M.F.; McGhee, J.R. A Bactericidal Effect for Human Lactoferrin. Science 1977, 197, 263–265. [Google Scholar] [CrossRef]
- Longhi, C.; Conte, M.P.; Seganti, L.; Polidoro, M.; Alfsen, A.; Valenti, P. Influence of lactoferrin on the entry process of Escherichia coli HB101 (pRI203) in HeLa cells. Med. Microbiol. Immunol. 1993, 182, 25–35. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Inagaki, M.; Nagai, S.; Yabe, T.; Nagaoka, S.; Minamoto, N.; Takahashi, T.; Matsuda, T.; Nakagomi, O.; Nakagomi, T.; Ebina, T.; et al. The Bovine Lactophorin C-Terminal Fragment and PAS6/7 Were Both Potent in the Inhibition of Human Rotavirus Replication in Cultured Epithelial Cells and the Prevention of Experimental Gastroenteritis. Biosci. Biotechnol. Biochem. 2010, 74, 1386–1390. [Google Scholar] [CrossRef] [Green Version]
- Douëllou, T.; Montel, M.C.; Sergentet, D.T. Invited review: Anti-adhesive properties of bovine oligosaccharides and bovine milk fat globule membrane-associated glycoconjugates against bacterial food enteropathogens. J. Dairy Sci. 2017, 100, 3348–3359. [Google Scholar] [CrossRef]
- Ubeda, C.; Djukovic, A.; Isaac, S. Roles of the intestinal microbiota in pathogen protection. Clin. Transl. Immunol. 2017, 6, e128. [Google Scholar] [CrossRef] [Green Version]
| Powder | Abbreviation | Source |
|---|---|---|
| Goat milk oligosaccharides | GMO | Isolated in-house |
| Bovine milk oligosaccharides | BMO | Isolated in-house |
| Human milk oligosaccharides | HMO | Isolated in-house |
| 3′-sialyllactose and 6′-sialyllactose | 3′ and 6′ SL | Merck |
| Glycomacropeptide | GMP | Agropur foods international |
| Lactoferrin | LF | Upfront Chromatography A/S |
| β-casein | β-C | Merck |
| Lacprodan® MFGM-10 | MFGM-10 | Arla food ingredients |
| β-lactoglobulin | β-L | Merck |
| Lacprodan® PL-20 | PL-20 | Arla food ingredients |
| Butter milk fraction | BF | Isolated in-house |
| P95 | P95 | Beneo Orafti |
| Immunoglobulin G Enriched Powder | IGEP | Upfront Chromatography A/S |
| Powder | 3′ Sialyllactose (μg/mL) | 6′ Sialyllactose (μg/mL) |
|---|---|---|
| GMO | 37.14 | 35.28 |
| HMO | 46.90 | 59.00 |
| BMO | 48.22 | 26.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinn, E.M.; Slattery, H.; Thompson, A.P.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods 2018, 7, 196. https://doi.org/10.3390/foods7120196
Quinn EM, Slattery H, Thompson AP, Kilcoyne M, Joshi L, Hickey RM. Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods. 2018; 7(12):196. https://doi.org/10.3390/foods7120196
Chicago/Turabian StyleQuinn, Erinn M., Helen Slattery, Aoife P. Thompson, Michelle Kilcoyne, Lokesh Joshi, and Rita M. Hickey. 2018. "Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells" Foods 7, no. 12: 196. https://doi.org/10.3390/foods7120196
APA StyleQuinn, E. M., Slattery, H., Thompson, A. P., Kilcoyne, M., Joshi, L., & Hickey, R. M. (2018). Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods, 7(12), 196. https://doi.org/10.3390/foods7120196






