The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Cookie Preparation
2.3. Physical Characteristics
2.4. Texture
2.5. Moisture
2.6. Determination of Total Phenolic Content
2.7. Antioxidant Properties
2.8 InVitro Carbohydrate Digestion (Glycaemic Glucose Equivalent-GGE) Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Cookies
3.2. Colour
3.3. Total Phenolic Content (TPC) and Antioxidant Activity of Cookies
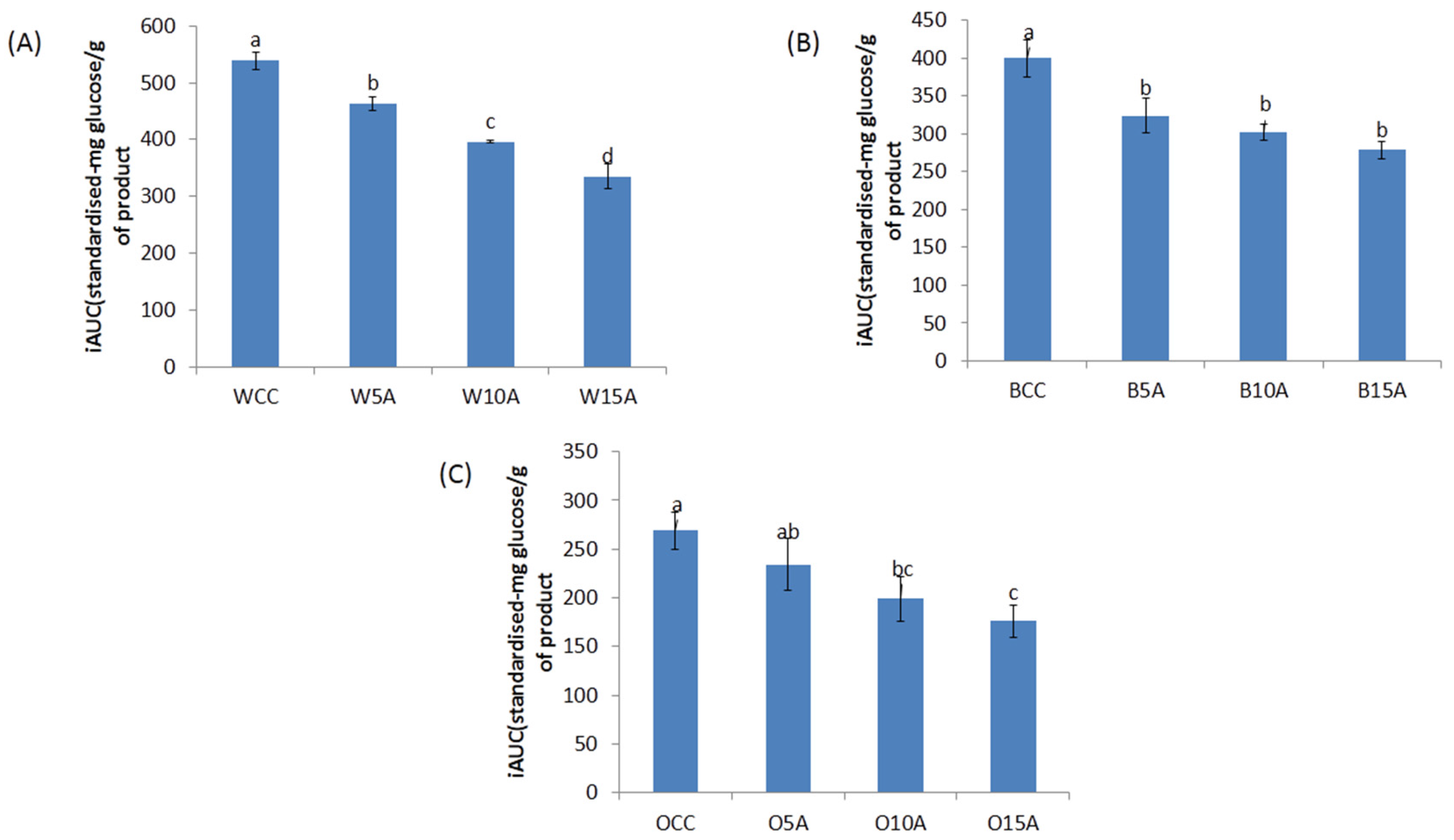
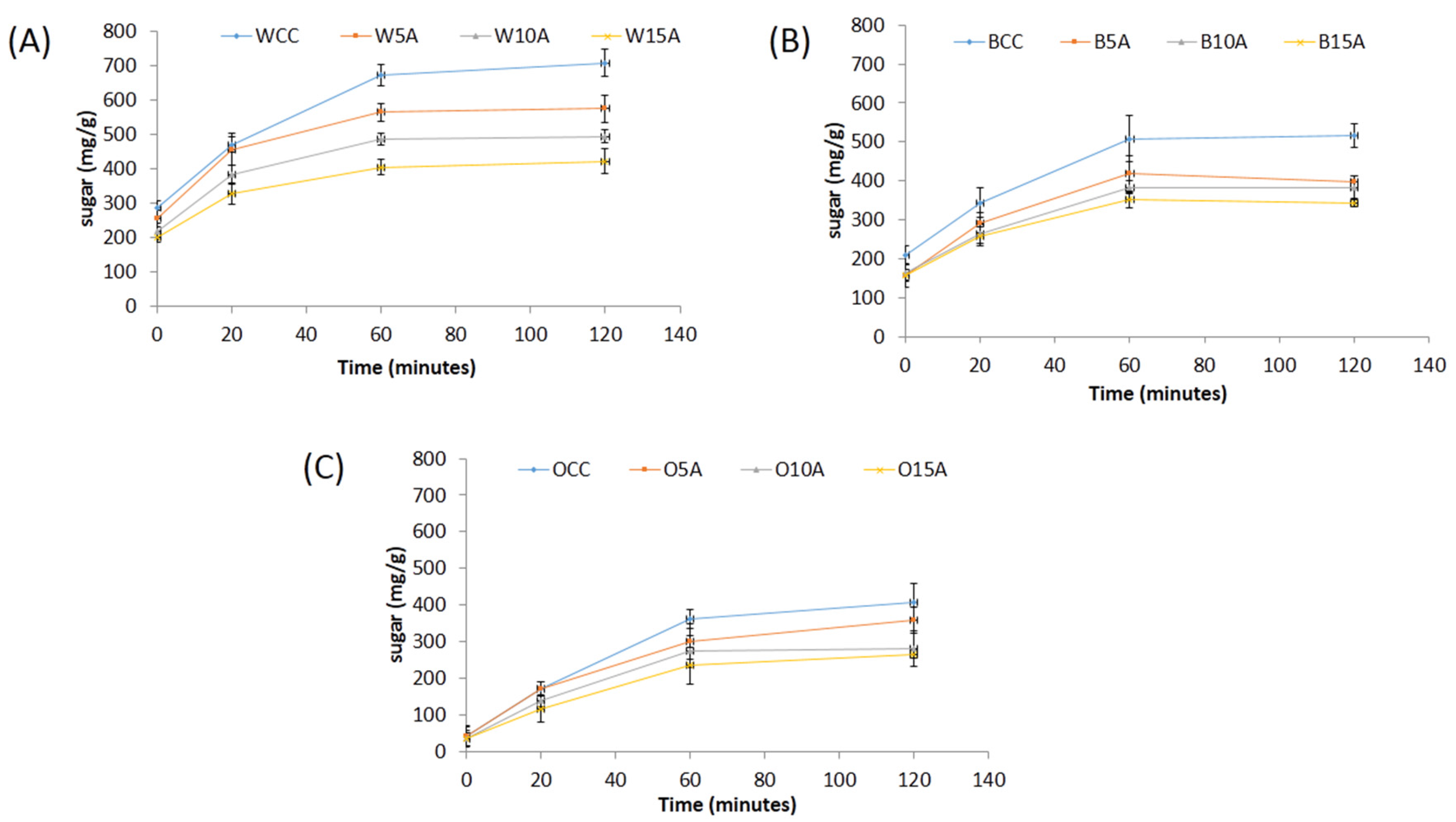
3.4. Glycaemic Glucose Equivalent (GGE) Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersson, A.A.M.; Dimberg, L.; Aman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3,1→4)-β-D-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S.M. Greater Whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, M.D.; Kyro, C.; Olsen, A.; Dragsted, L.O.; Skeie, G.; Lund, E.; Aringman, P.; Nilsson, L.M.; Bueno-de-Mesquita, H.B.; Tjonneland, A.; et al. Self-reported whole-grain intake and plasma alkylresorcinol concentrations in combination in relation to the incidence of colorectal cancer. Am. J. Epidemiol. 2014, 179, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Hergert, N.; Rompato, G.; Lefevre, M. Whole grain oats improve insulin sensitivity and plasma cholesterol profile and modify gut microbiota composition in C57BL/6J mice. J. Nutr. 2015, 145, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Laddomada, B.; Centomani, I.; Paradiso, V.M.; Minervini, D.; Caponio, F.; Summo, C. Bread making aptitude of mixtures of re-milled semolina and selected durum wheat milling by-products. LWT Food Sci. Technol. 2017, 78, 151–159. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Rosell, C.M.; Steel, C.J. Effect of the addition of whole-grain wheat flour and of extrusion process parameters on dietary fibre content, starch transformation and mechanical properties of a ready-to-eat breakfast cereal. Int. J. Food Sci. Technol. 2015, 50, 1504–1514. [Google Scholar] [CrossRef]
- Robin, F.; Theoduloz, C.; Srichuwong, S. Properties of extruded whole grain cereals and pseudocereals flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159. [Google Scholar] [CrossRef]
- Lu, X.K.; Brennan, M.A.; Serventi, L.; Mason, S.; Brennan, C.S. How the inclusion of mushroom powder can affect the physicochemical characteristics of pasta. Int. J. Food Sci. Technol. 2016, 51, 2433–2439. [Google Scholar] [CrossRef]
- Sobota, A.; Rzedzicki, Z.; Zarzycki, P.; Kuzawinska, E. Application of common wheat bran for the industrial production of high-fibre pasta. Int. J. Food Sci. Technol. 2015, 50, 111–119. [Google Scholar] [CrossRef]
- Grigor, J.M.; Brennan, C.S.; Hutchings, S.C.; Rowlands, D.S. The sensory acceptance of fibre-enriched cereal foods: A meta-analysis. Int. J. Food Sci. Technol. 2016, 51, 3–13. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Simoes, T.; Horta, A.; Silva, J.; Tecelao, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O'Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Aguilera-Morales, M.; Casas-Valdez, M.; Carrillo-Dominguez, B.; Gonzalez-Acosta, B.; Perez-Gil, F. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J. Food Compost. Anal. 2005, 18, 79–88. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Wu, H.Y.; Hong, H.L.; Zhu, N.; Han, L.M.; Suo, Q.L. Two ethoxyquinoline metabolites from the alga Heamatococcus pluvialis. Chem. Nat. Compd. 2014, 50, 578–580. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.D.; de Morais, A.; de Morais, R. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; D'Orazio, N.; Franceschelli, S.; Speranza, L. Marine carotenoids and cardiovascular risk markers. Mar. Drugs 2011, 9, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists. Approved Methods of the American Association of Cereal Chemists, 9th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1995. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. Synergistic effect of different dietary fibres in pasta on in vitro starch digestion? Food Chem. 2015, 172, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.R.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. Effect of sugar replacement with stevianna and inulin on the texture and predictive glycaemic response of muffins. Int. J. Food Sci. Technol. 2016, 51, 1979–1987. [Google Scholar] [CrossRef]
- Okpala, L.; Okoli, E.; Udensi, E. Physico-chemical and sensory properties of cookies made from blends of germinated pigeon pea, fermented sorghum, and cocoyam flours. Food Sci. Nutr. 2013, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Cho, A.; Lim, S.T. Utilization of germinated and heat-moisture treated brown rices in sugar-snap cookies. LWT Food Sci. Technol. 2014, 57, 260–266. [Google Scholar] [CrossRef]
- Brennan, C.S.; Samyue, E. Evaluation of starch degradation and textural characteristics of dietary fiber enriched biscuits. Int. J. Food Prop. 2004, 7, 647–657. [Google Scholar] [CrossRef]
- Giami, S.Y.; Achinewhu, S.C.; Ibaakee, C. The quality and sensory attributes of cookies supplemented with fluted pumpkin (Telfairia occidentalis Hook) seed flour. Int. J. Food Sci. Technol. 2005, 40, 613–620. [Google Scholar] [CrossRef]
- Mais, A.; Brennan, C.S. Characterisation of flour, starch and fibre obtained from sweet potato (kumara) tubers, and their utilisation in biscuit production. Int. J. Food Sci. Technol. 2008, 43, 373–379. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.M. Pasting properties of starch and protein in selected cereals and quality of their food products. Food Chem. 2006, 95, 9–18. [Google Scholar] [CrossRef]
- Yamsaengsung, R.; Berghofer, E.; Schoenlechner, R. Physical properties and sensory acceptability of cookies made from chickpea addition to white wheat or whole wheat flour compared to gluten-free amaranth or buckwheat flour. Int. J. Food Sci. Technol. 2012, 47, 2221–2227. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.J.; Liu, S.X. Physical properties of gluten-free sugar cookies made from amaranth-oat composites. LWT Food Sci. Technol. 2015, 63, 214–220. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Solvent retention capacity (SRC) testing of wheat flour: Principles and value in predicting flour functionality in different wheat-based food processes and in wheat breeding—A review. Cereal Chem. 2011, 88, 537–552. [Google Scholar] [CrossRef]
- Chevallier, S.; Colonna, P.; Lourdin, D. Contribution of major ingredients during baking of biscuit dough systems. J. Cereal Sci. 2000, 31, 241–252. [Google Scholar] [CrossRef]
- Spiller, G.A.; Dewell, A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: A randomized clinical trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gujral, H.S. Cookie making behavior of wheat-barley flour blends and effects on antioxidant properties. LWT Food Sci. Technol. 2014, 55, 301–307. [Google Scholar] [CrossRef]
- Matsiu, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef]
- Paliwal, C.; Ghosh, T.; Bhayani, K.; Maurya, R.; Mishra, S. Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar. Drugs 2015, 13, 5384–5401. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Tan, S.P.; Leong, L.P.; Henry, J.K. Total antioxidant capacity and starch digestibility of muffins baked with rice, wheat, oat, corn and barley flour. Food Chem. 2014, 164, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef] [PubMed]
| Sample | Wholemeal Flour (g) | Astaxanthin Powder (g) | Other Ingredients |
|---|---|---|---|
| Control | 225.00 | - | Vegetable shortening (64.0 g), sugar (130 g), salt (2.1 g), sodium bicarbonate (2.5 g), dextrose solution (33 g), water (16 g) |
| 5% Astaxanthin powder | 213.75 | 11.25 | Vegetable shortening (64.0 g), sugar (130 g), salt (2.1 g), sodium bicarbonate (2.5 g), dextrose solution (33 g), water (16 g) |
| 10% Astaxanthin powder | 202.50 | 22.50 | |
| 15% Astaxanthin powder | 191.25 | 33.75 |
| Sample | Increase in Height (%) | Increase in Diameter (%) | Weight Loss (%) | Moisture Content (%) | Hardness (kg) |
|---|---|---|---|---|---|
| WCC | 94.39 ± 3.06 a | 3.93 ± 0.226 | 9.71 ± 0.04 a | 7.50 ± 0.11 c | 9.26 ± 0.13 a |
| W5A | 71.44 ± 8.39 b | 2.96 ± 1.139 b | 9.63 ± 0.02 a,b | 7.83 ± 0.01 b | 7.79 ± 0.16 b |
| W10A | 59.39 ± 3.06 b,c | 2.27 ± 0.216 b | 9.48 ± 0.12 a,b | 7.91 ± 0.07 b | 7.35 ± 0.58 b |
| W15A | 52.94 ± 0.75 c | 1.15 ± 0.925 | 9.44 ± 0.12 b | 8.21 ± 0.03 a | 7.06 ± 0.48 b |
| BCC | 94.33 ± 6.78 a | 5.23 ± 1.168 | 10.31 ± 0.11 a | 7.74 ± 0.02 d | 4.12 ± 0.12 c |
| B5A | 83.50 ± 1.04 a,b | 4.76 ± 0.444 | 10.41 ± 0.11 a | 7.79 ± 0.02 c | 4.98 ± 0.20 b |
| B10A | 74.61 ± 2.91 b,c | 3.67 ± 0.731 b | 10.46 ± 0.20 a | 7.90 ± 0.01 b | 5.26 ± 0.22 b |
| B15A | 65.50 ± 4.84 c | 2.72 ± 0.314 | 10.67 ± 0.28 a | 8.17 ± 0.02 a | 6.21 ± 0.10 a |
| OCC | 70.94 ± 0.91 a | 23.20 ± 0.25 a | 11.54 ± 0.17 a | 5.55 ± 0.05 d | 7.57 ± 0.05 a |
| O5A | 67.94 ± 2.46 a,b | 12.81 ± 0.49 a | 11.14 ± 0.23 a,b | 6.09 ± 0.04 c | 7.23 ± 0.14 a,b |
| O10A | 64.55 ± 0.25 b | 7.80 ± 0.05 c | 10.63 ± 0.22 b,c | 6.66 ± 0.04 b | 7.02 ± 0.12 b |
| O15A | 55.55 ± 0.91 c | 3.62 ± 0.14 d | 10.23 ± 0.23 c | 7.12 ± 0.09 a | 6.16 ± 0.23 c |
| Sample | L* | a* | b* | △E |
|---|---|---|---|---|
| Surface Cookie colour | ||||
| WCC | 90.40 ± 0.42 a | −5.79 ± 0.35 a | 33.05 ± 0.09 a | 96.43 ± 0.44 a |
| W5A | 84.14 ± 0.26 b | −7.37 ± 0.08 b | 29.16 ± 0.23 b | 89.36 ± 0.32 b |
| W10A | 82.20 ± 0.10 c | −8.72 ± 0.20 c | 27.42 ± 0.06 c | 87.09 ± 0.13 c |
| W15A | 81.42 ± 0.32 c | −8.27 ± 0.08 c | 26.45 ± 0.34 d | 86.01 ± 0.40 d |
| BCC | 94.43 ± 0.45 a | −8.16 ± 0.76 a | 34.55 ± 0.27 a | 100.89 ± 0.58 a |
| B5A | 86.97 ± 0.19 b | −9.25 ± 0.04 b | 32.16 ± 0.07 b | 93.19 ± 0.20 b |
| B10A | 84.77 ± 0.23 c | −7.88 ± 0.25 a | 30.06 ± 0.15 c | 90.29 ± 0.27 c |
| B15A | 83.10 ± 0.14 d | −7.83 ± 0.01 a | 27.95 ± 0.14 d | 88.02 ± 0.18 d |
| OCC | 91.31 ± 0.69 a | −5.64 ± 0.34 a | 35.24 ± 0.15 a | 98.04 ± 0.71 a |
| O5A | 84.19 ± 0.14 b | −7.63 ± 0.34 b | 30.64 ± 0.15 b | 89.91 ± 0.20 b |
| O10A | 82.21 ± 0.13 c | −7.87 ± 0.13 b | 28.26 ± 0.11 c | 87.31 ± 0.17 c |
| O15A | 80.77 ± 0.15 d | −8.17 ± 0.13 b | 26.56 ± 0.19 d | 85.39 ± 0.22 d |
| Ground Cookie colour | ||||
| WCC | 87.20 ± 0.20 a | −0.32 ± 0.03 a | 45.33 ± 0.10 a | 97.55 ± 0.30 a |
| W5A | 77.82 ± 0.06 b | −6.32 ± 0.04 c | 43.72 ± 0.26 b | 90.29 ± 0.01 b |
| W10A | 75.42 ± 0.22 c | −6.98 ± 0.03 d | 38.31 ± 0.15 c | 84.88 ± 0.13 c |
| W15A | 69.10 ± 0.30 d | −6.20 ± 0.02 b | 34.13 ± 0.12 d | 77.32 ± 0.22 d |
| BCC | 95.13 ± 0.07 a | −13.41 ± 0.21 c | 43.14 ± 0.41 a | 105.32 ± 0.13 a |
| B5A | 82.96 ± 0.62 b | −5.86 ± 0.23 a | 43.95 ± 0.65 a | 94.06 ± 0.30 b |
| B10A | 74.46 ± 0.63 c | −6.56 ± 0.12 b | 44.02 ± 0.09 a | 86.74 ± 0.56 c |
| B15A | 71.13 ± 0.77 d | −6.04 ± 0.38 a,b | 39.85 ± 1.38 b | 81.76 ± 0.24 d |
| OCC | 93.15 ± 0.59 a | −9.32 ± 0.1.34 b | 49.41 ± 1.35 a | 105.86 ± 0.02 a |
| O5A | 85.92 ± 0.27 b | −8.36 ± 0.09 a,b | 39.58 ± 0.21 b,c | 94.97 ± 0.16 b |
| O10A | 73.24 ± 0.36 c | −7.33 ± 0.03 a | 36.04 ± 0.07 c | 81.96 ± 0.29 c |
| O15A | 71.19 ± 0.47 d | −6.99 ± 0.57 a | 41.34 ± 2.68 b | 82.64 ± 0.97 c |
| Sample | TPC (mg GAE/g Sample) | DPPH (μmol TE/g Sample) | ORAC (mmol TE/g Sample) |
|---|---|---|---|
| WCC | 0.59 ± 0.01 d | 0.54 ± 0.01 d | 0.09 ± 0.001 b |
| W5A | 0.80 ± 0.01 c | 0.95 ± 0.03 c | 0.11 ± 0.001 a |
| W10A | 0.95 ± 0.01 b | 1.10 ± 0.01 b | 0.12 ± 0.001 a |
| W15A | 1.14 ± 0.01 a | 1.26 ± 0.03 a | 0.12 ± 0.004 a |
| BCC | 0.63 ± 0.01 c | 1.36 ± 0.01 d | 0.08 ± 0.003 b |
| B5A | 0.95 ± 0.02 b | 1.69 ± 0.02 c | 0.09 ± 0.002 a |
| B10A | 1.15 ± 0.09 a | 1.74 ± 0.01 b | 0.09 ± 0.002 a |
| B15A | 1.27 ± 0.01 a | 1.79 ± 0.01 a | 0.10 ± 0.002 a |
| OCC | 0.87 ± 0.01 d | 1.13 ± 0.01 d | 0.08 ± 0.001 c |
| O5A | 1.03 ± 0.01 c | 1.22 ± 0.01 c | 0.10 ± 0.002 b |
| O10A | 1.28 ± 0.01 b | 1.34 ± 0.01 b | 0.10 ± 0.001 a |
| O15A | 1.44 ± 0.01 a | 1.46 ± 0.01 a | 0.11 ± 0.001 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. https://doi.org/10.3390/foods6080057
Hossain AKMM, Brennan MA, Mason SL, Guo X, Zeng XA, Brennan CS. The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods. 2017; 6(8):57. https://doi.org/10.3390/foods6080057
Chicago/Turabian StyleHossain, A. K. M. Mofasser, Margaret A. Brennan, Susan L. Mason, Xinbo Guo, Xin An Zeng, and Charles S. Brennan. 2017. "The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies" Foods 6, no. 8: 57. https://doi.org/10.3390/foods6080057
APA StyleHossain, A. K. M. M., Brennan, M. A., Mason, S. L., Guo, X., Zeng, X. A., & Brennan, C. S. (2017). The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods, 6(8), 57. https://doi.org/10.3390/foods6080057







