Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Sample Treatment
2.4. Instrumental and Chromatographic Conditions
2.5. Statistical Analysis
3. Results
3.1. Analysis of Soda and Energy Drinks
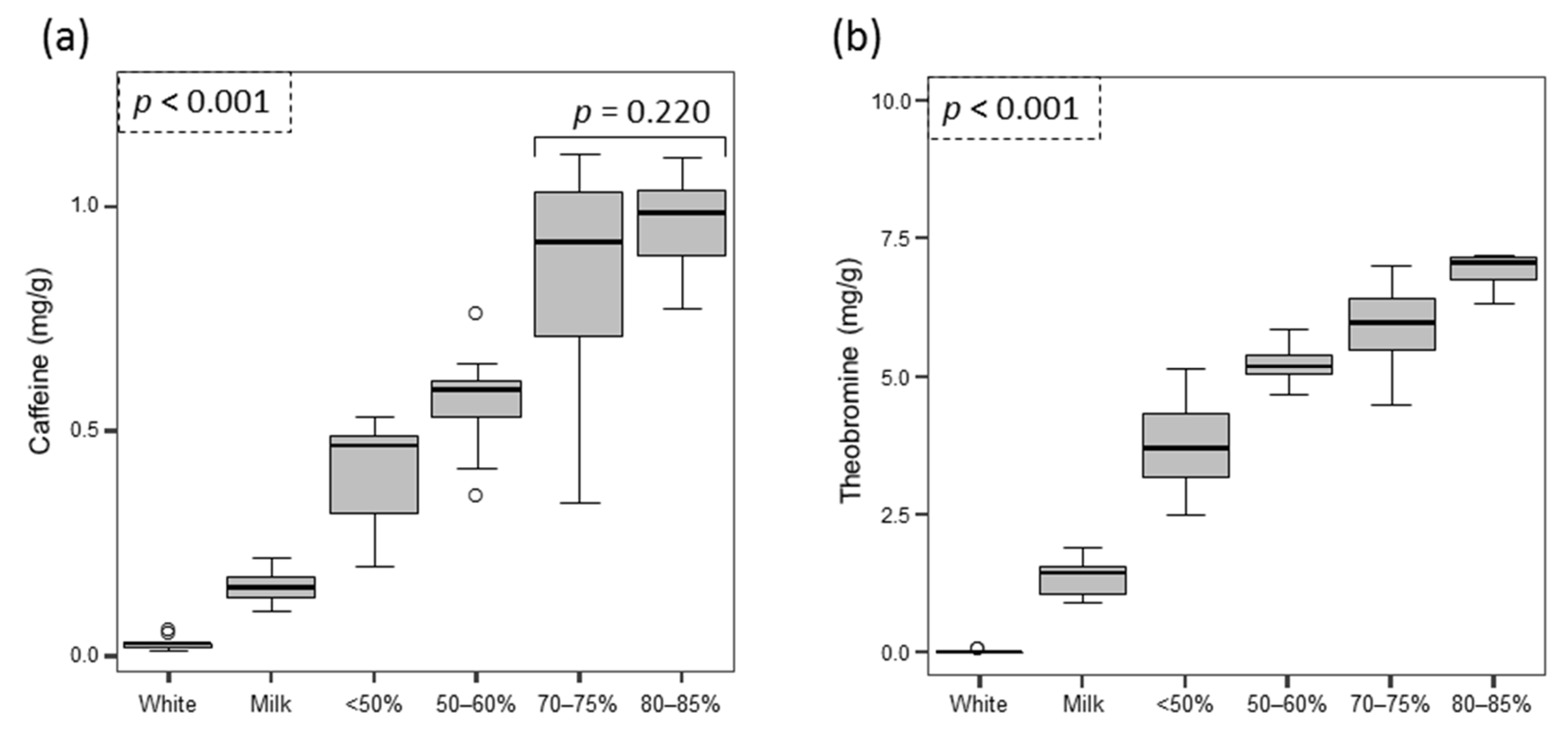
3.2. Chocolates and Chocolate Drinks
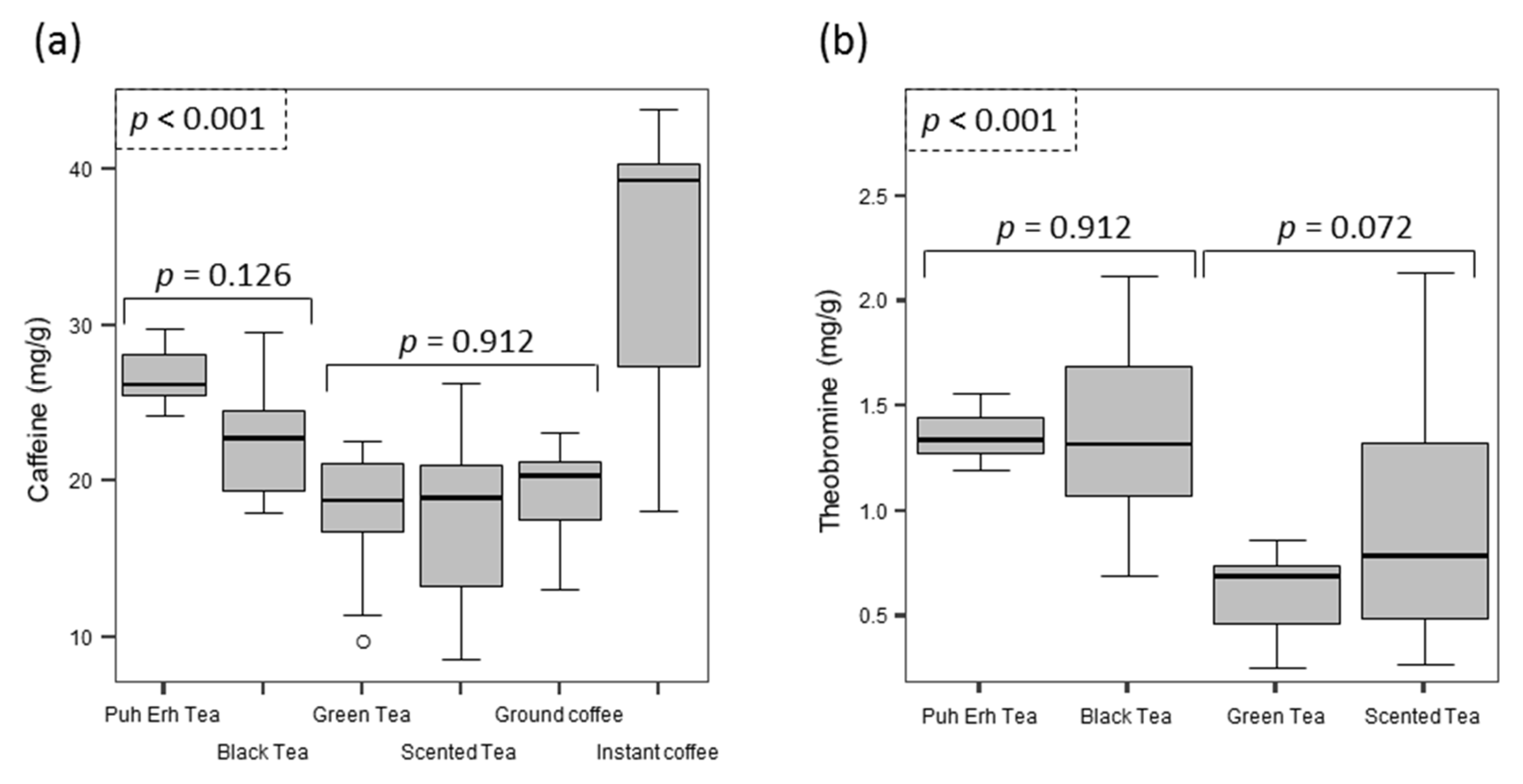
3.3. Tea Leaves
3.4. Coffees
4. Discussion
4.1. Beverages and Chocolate Drinks
4.2. Foods
5. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- James, J.E. Caffeine and Health; Academic Press Ltd.: London, UK, 1991; ISBN 9780123801052. [Google Scholar]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Vernotica, E.M. Is caffeine a flavoring agent in cola soft drinks? Arch. Fam. Med. 2000, 9, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Roper, J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Ridell, L.J. Caffeine as a flavor additive in soft-drinks. Appetite 2007, 49, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated energy drinks—A growing problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Latosinska, M.; Latosinska, J.N. Introductory chapter: Caffeine, a major component of nectar of the gods and favourite beverage of kings, popes, artists and revolutionists, a drug or a poison? In The Question of Caffeine; Latosinska, J.N., Latosinska, M., Eds.; InTech: Rijeka, Croatia, 2017; pp. 1–26. ISBN 978-953-51-3274-5. [Google Scholar]
- Oñatibia-Astibia, A.; Franco, R.; Martínez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010, 20 (Suppl. 1), S85–S94. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Jacobson, K.A. Xanthines as adenosine receptor antagonists. Hand Exp. Pharmacol. 2011, 200, 151–199. [Google Scholar] [CrossRef]
- Chen, J.F.; Chern, Y. Impacts of methylxanthines and adenosine receptors on neurodegeneration: Human and experimental studies. Hand Exp. Pharmacol. 2011, 200, 267–310. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr. Bull. 2008, 33, 15–25. [Google Scholar] [CrossRef]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the US. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Clark, N. Caffeine: A user’s guide. Phys. Sports Med. 1997, 25, 109–110. [Google Scholar] [CrossRef]
- Knight, C.A.; Knight, I.; Mitchell, D.C.; Zepp, J.E. Beverage caffeine intake in US consumers and subpopulations of interest: Estimates from the Share of Intake Panel survey. Food Chem. Toxicol. 2004, 42, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, H.; González de Mejia, E. Caffeine (1,3,7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Are we dependent upon coffee and caffeine? A review on human an animal data. Neurosci. Biobehav. Rev. 1999, 23, 563–576. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet. Gynecol. 2010, 116, 467–468. [Google Scholar] [CrossRef]
- Bernstein, G.; Carroll, M.; Crosby, R.; Perwein, A.; Go, F.; Benowitz, N. Caffeine effects on learning, performance and anxiety in normal school-age children. J. Am. Acad. Child. Adolesc. Psychiatry 1994, 33, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Caffeine in Food. 2012. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/caffeine-foods/foods.html (accessed on 2 November 2017).
- Pendleton, M.; Brown, S.; Thomas, C.; Odle, B. Potential toxicity of caffeine when used as a dietary supplement for weight loss. J. Diet. Suppl. 2013, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sinchai, T.; Plasen, S.; Sanvarinda, Y.; Jaisin, Y.; Govitrapong, P.; Morales, N.P.; Ratanachamnong, P.; Plasen, D. Caffeine potentiates methamphetamine-induced toxicity both in vitro and in vivo. Neurosci. Lett. 2011, 502, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, J.A.; Gómez, G.; González, B.; Rivero-Echeto, M.C.; Cadet, J.L.; García-Rill, E.; Urbano, F.J.; Bisagno, V. Combined effects of simultaneous exposure to caffeine and cocaine in the mouse striatum. Neurotox. Res. 2016, 29, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Arria, A.M.; O’Brien, M.C. The high risk of energy drinks. JAMA 2011, 305, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Code of Federal Regulations, Title 21: Food and Drugs, Section 182.1180 (21 CFR 182.1180); Food and Drug Administration: Silver Spring, MD, USA, 2016; p. 480.
- Kole, J.; Barnhill, A. Caffeine content labelling: A missed opportunity for promoting personal and public health. J. Caffeine Res. 2013, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Official Journal of the European Union. 22 November 2011, pp. 18–63. Available online: https://www.fsai.ie/uploadedFiles/Reg1169_2011.pdf (accessed on 30 November 2017).
- Brunetto, M.R.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, A.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by high-performance liquid chromatographic method with on-line cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Trognitz, B.; Cros, E.; Assemat, S.; Davrieux, F.; Forestier-Chiron, N.; Ayestas, E.; Kuant, A.; Scheldeman, X.; Hermann, M. Diversity of cacao trees in Waslala, Nicaragua: Associations between genotype spectra, product quality and yield potential. PLoS ONE 2013, 8, e54079. [Google Scholar] [CrossRef] [PubMed]
- Baggott, M.J.; Childs, E.; Hart, A.B.; de Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 2013, 228, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.H.; Korson, R.; Cater, M.R.; Ackerly, C.C. Effects of short-term and long-term theobromine administration to male dogs. Toxicol. Appl. Pharmacol. 1980, 53, 481–496. [Google Scholar] [CrossRef]
- Frary, C.D.; Johnson, R.K.; Wang, M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet. Assoc. 2005, 105, 110–113. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Method 980.14. Theobromine and Caffeine in Cacao Products; AOAC International: Rockville, MD, USA, 1981. [Google Scholar]
- Khokhar, S.; Magnusdottir, S.G.M. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- ISO 20481:2008. Coffee and Coffee Products—Determination of Caffeine Content Using High Performance Liquid Chromatography (HPLC)—Reference Method; Technical Committee ISO/TC 34, Food Products, Subcommittee SC 15, Coffee: Saint Denis, France, 2011. [Google Scholar]
- Carlin-Sinclair, A.; Marc, I.; Menguy, L.; Prim, D. The determination of methylxanthines in chocolate and cocoa by different separation techniques: HPLC, instrumental TLC, and MECC. J. Chem. Educ. 2009, 86, 1307–1310. [Google Scholar] [CrossRef]
- EU Directive 2000/36/EC of the European Parliament and of the Council of 23 June 2000 relating to cocoa and chocolate products intended for human consumption. Off. J. 2000, L197, 19–25.
- Zhang, L.; Kujawinski, D.M.; Federherr, E.; Schmidt, T.C.; Jochmann, M.A. Caffeine in your drink: Natural or synthetic? Anal. Chem. 2012, 84, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.J. Theobromine and the pharmacology of cocoa. In Methylxanthines, Vol 200 of the Series Handbook of Experimental Pharmacology; Fredholm, B.B., Ed.; Springer: Heidelberg, Germany, 2011; pp. 201–234. ISBN 9783642134425. [Google Scholar]
- Wanyika, H.N.; Gatebe, E.G.; Gitu, L.M.; Ngumba, E.K.; Maritim, C.W. Determination of caffeine content of tea and instant coffee brands in the Kenyan market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar]
- Nogueria, T.; do Lago, C.L. Determination of caffeine in coffee products by dynamic complexation with 3,4-dimethoxycinnamate and separation by CZE. Electrophoresis 2007, 28, 3570–3574. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crouzier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking. Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Heidelberg, Germany, 2009; pp. 938–951. ISBN 9783540699330. [Google Scholar]
- EU. Directive 1999/4/EC of the European Parliament and of the Council of 22 February 1999 relating to coffee extracts and chicory extracts. Off. J. 1999, L66, 26–29. [Google Scholar]
- International Coffee Organization main webpage. Available online: http://www.ico.org (accessed on 30 November 2017).
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2003, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.R.; Goldberger, B.A.; Cone, E.J. Caffeine content of specialty coffees. J. Anal. Toxicol. 2003, 27, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Gloess, A.N.; Schömbäch, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yoretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan coffee brew chemical analysis in comparison to expresso, moka and American brews. Food Res. Int. 2014, 61, 153–160. [Google Scholar] [CrossRef]
- How to Brew a Coffee. Available online: http:www.ncausa.org/About-Coffee/How-to-Brew-Coffee (accessed on 30 November 2017).
- De Camargo, M.C.R.; Toledo, M.C.F. HPLC determination of caffeine in tea, chocolate products and carbonated beverages. J. Sci. Food Agric. 1999, 79, 1861–1864. [Google Scholar] [CrossRef]
- Srdjenovic, B.; Djordjevic-Milic, V.; Grujic, N.; Injac, R.; Lepojevic, Z. Simultaneous HPLC Determination of Caffeine, Theobromine, and Theophylline in Food, Drinks, and Herbal Products. J. Chromatogr. Sci. 2008, 46, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Castle, S.M.; Siswanto, P.J.; Cifuentes-Gómez, T.; Spencer, J.P.E. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O. Chocolate Science and Technology, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2016; ISBN 9781118913789. [Google Scholar]
- Kwok, C.S.; Boekholdt, S.M.; Lentjes, M.A.H.; Loke, Y.K.; Luben, R.N.; Yeong, J.K.; Wareham, N.J.; Myint, P.K.; Khaw, K.T. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015, 101, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, B.M.; Aeby, V.G.; Overton, R.F.; Carpenter-Aeby, T.; Barcer-Heidal, K. A survey of energy drink consumption patterns among college students. Nutr. J. 2007, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Marczinski, C.A. Alcohol mixed with energy drinks: Consumption patterns and motivations for use in U.S. college students. Int. J. Environ. Res. Public Health 2011, 8, 3232–3245. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, J.M. Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption. Foods 2017, 6, 109. https://doi.org/10.3390/foods6120109
Sanchez JM. Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption. Foods. 2017; 6(12):109. https://doi.org/10.3390/foods6120109
Chicago/Turabian StyleSanchez, Juan M. 2017. "Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption" Foods 6, no. 12: 109. https://doi.org/10.3390/foods6120109
APA StyleSanchez, J. M. (2017). Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption. Foods, 6(12), 109. https://doi.org/10.3390/foods6120109




