Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polysaccharides Extraction
2.3. Experimental Design, Optimization and Validation of Optimized Conditions
2.4. Characterization of GPPP
2.4.1. Chemical Properties Analysis
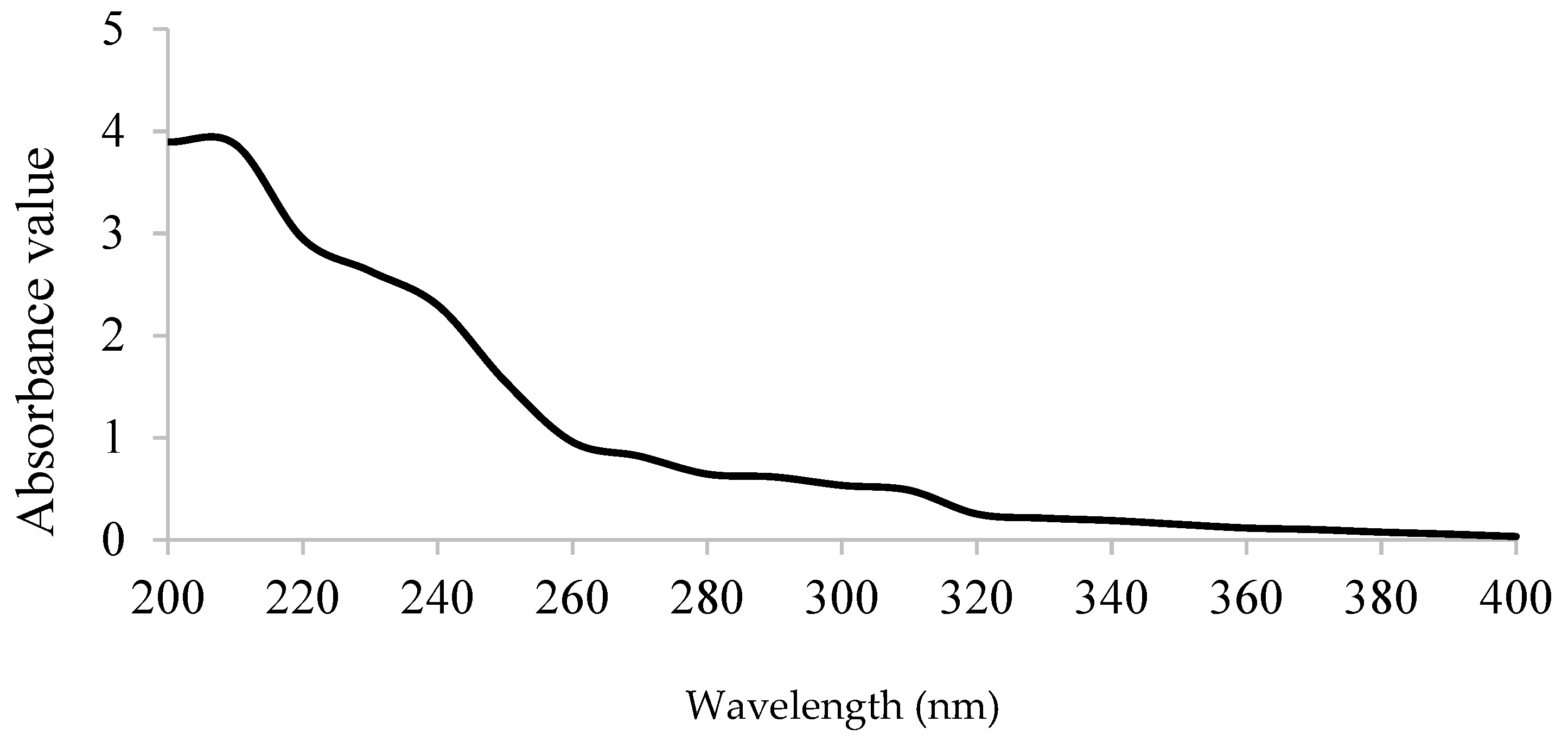
2.4.2. UV-Vis, FT-IR Spectroscopy
2.4.3. Determination of the Degree of Esterification
Titrimetric Method
FT-IR Spectroscopic Method
2.5. Determination of In Vitro Antioxidant Activity
2.5.1. Assay of DPPH Free Radical Scavenging Activity
2.5.2. Determination of Reducing Power
2.5.3. Ferric Reducing Antioxidant Power Assay (FRAP)
3. Results and Discussion
3.1. Model Fitting and Statistical Analysis
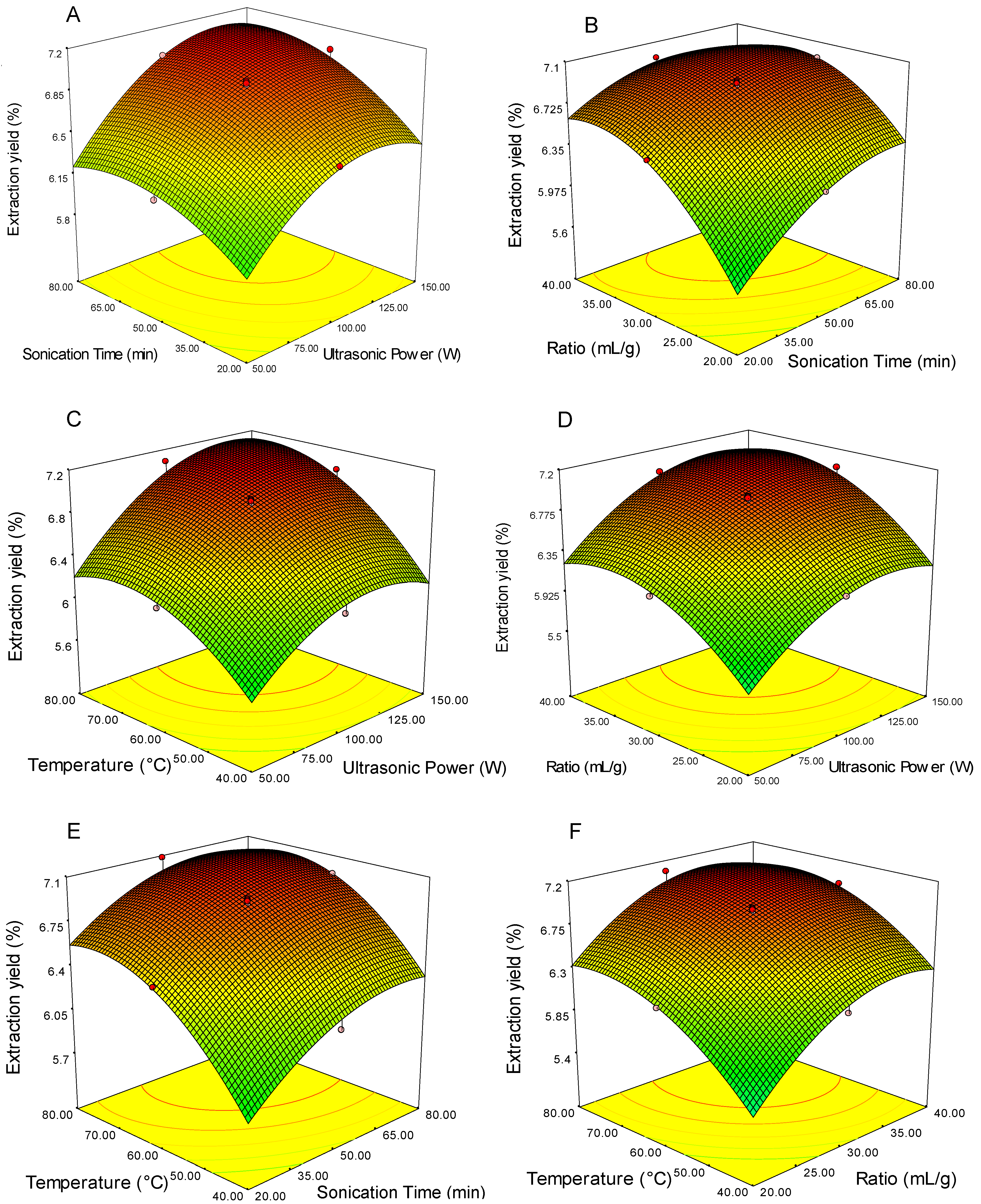
3.2. Analysis of Response Surfaces, Optimization of Extraction Parameters and Validation of the Predictive Model
3.3. Preliminary Characterization of GPPP
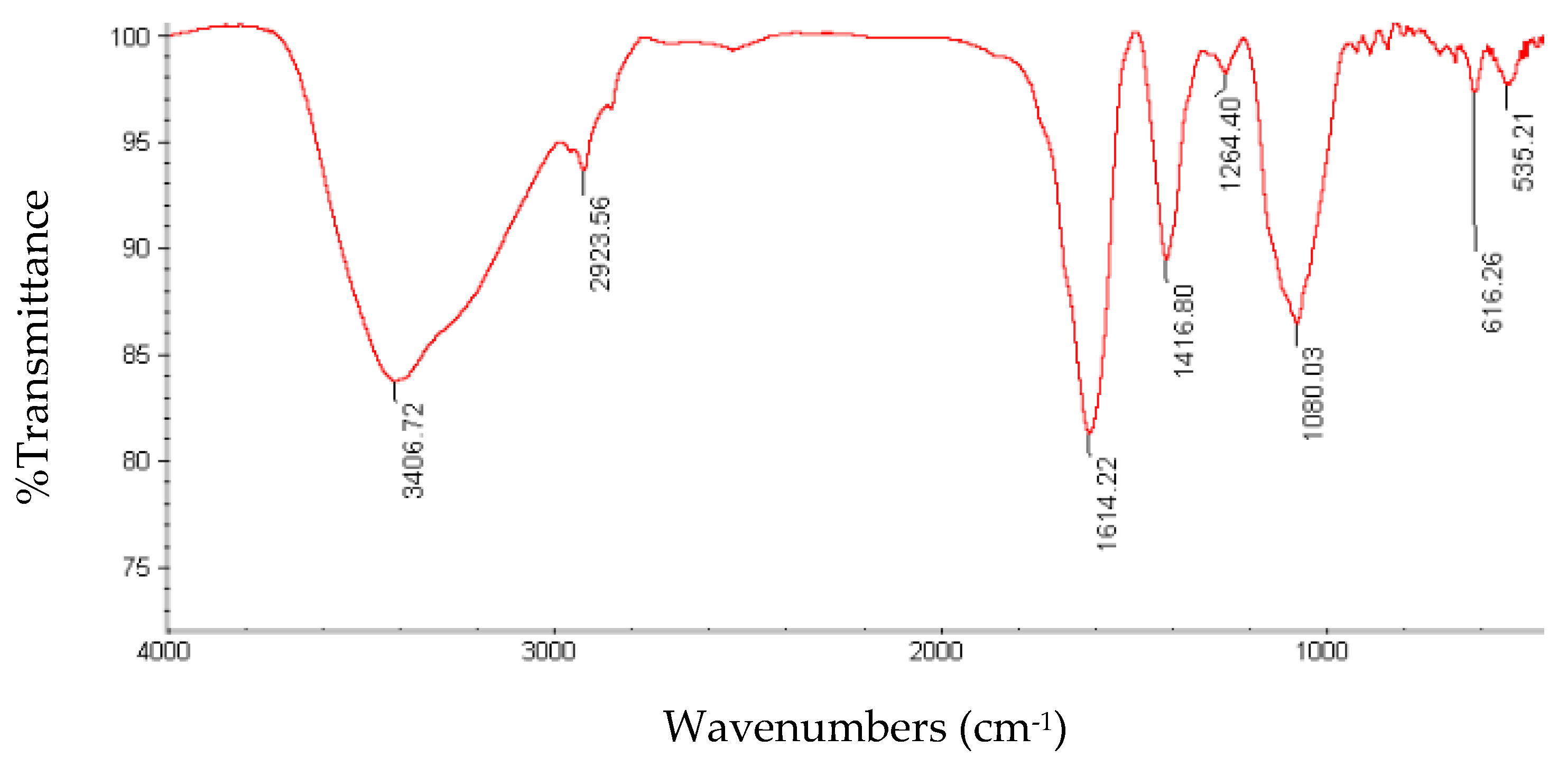
3.4. FT-IR Spectroscopy
3.5. The Degree of Esterification of GPPP
3.6. DPPH Radical Scavenging Activity of GPPP
3.7. Reducing Power
3.8. Ferric Reducing Antioxidant Power (FRAP)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amarakoon, D.; Thavarajah, D.; McPhee, K.; Thavarajah, P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: A potential food-based solution to global micronutrient malnutrition. J. Food Comp. Anal. 2012, 27, 8–13. [Google Scholar] [CrossRef]
- Barzegar, M.; Zare, D.; Stroshine, R.L. An integrated energy and quality approach to optimization of green peas drying in a hot air infrared-assisted vibratory bed dryer. J. Food Eng. 2015, 166, 302–315. [Google Scholar] [CrossRef]
- FAO. Browse Data-By Doman. Available online: http://faostat3.Fao.Org/browse/q/qc/e (accessed on 1 October 2016).
- Tiwari, B.K.; Singh, N. Pulse Chemistry and Technology; The Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Isolation and characterisation of cell wall polysaccharides from legume by-products: Okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010, 122, 339–345. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.; Chen, G.; Hua, Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Salazar-Montoya, J.A.; Ramos-Ramırez, E.G.; Delgado-Reyes, V.A. Changes of the dynamic properties of tamarind (Tamarindus indica) gel with different saccharose and polysaccharide concentrations. Carbohydr. Polym. 2002, 49, 387–791. [Google Scholar] [CrossRef]
- Jiang, C.h.; Li, X.; Jiao, Y.; Jiang, D.; Zhang, L.; Fan, B.; Zhang, Q. Optimization for ultrasound-assisted extraction of polysaccharides with antioxidant activity in vitro from the aerial root of ficus microcarpa. Carbohydr. Polym. 2014, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, Y.; Hu, X.; Zhang, Z. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014, 155, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Proc. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Wen, L.; Lin, L.; You, L.; Yang, B.; Jiang, G.; Zhao, M. Ultrasound-assited extraction and structural identification of polysaccharides from isodon lophanthoides var. Gerardianus (Bentham) H. Hara. Carbohydr. Polym. 2011, 85, 541–547. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Priya, B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. Fruit peel. Int. J. Biol. Macromol. 2014, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, L.; Hang, F.; Ding, S.; Liu, Y. Composition and antioxidant activity of polysaccharides from jujuba by classical and ultrasound extraction. Int. J. Biol. Macromol. 2014, 63, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Dong, X.F.; Tong, J.M. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity. Int. J. Biol. Macromol. 2013, 62, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Pakrokh Ghavi, P. The extraction process optimization of antioxidant polysaccharides from marshmallow (Althaea officinalis L.) roots. Int. J. Biol.Macromol. 2015, 75, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-H.; Zhang, D.; Yu, B.; Zhao, S.-P.; Wang, J.-W.; Yao, L.; Cao, W.-G. Antioxidant activity and optimization of extraction of polysaccharide from the roots of dipsacus asperoides. Int. J. Biol. Macromol. 2015, 81, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Nivetha, C.V.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Blessing Manoj, J.J. Modeling of polysaccharide extraction from Gossypium arboreum L. Seed using central composite rotatable design. Int. J. Biol. Macromol. 2016, 86, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Moorthy, I.; Prakash Maran, J.; Muneeswari Suryaa, S.; Naganyashreea, S.; Shivamathi, C.S. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Khodaiyan, F.; Hamedi, M. Optimization and characterization of walnut beverage emulsions in relation to their composition and structure. Int. J. Biol. Macromol. 2012, 50, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from mosla chinensis maxim. Cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Liu, Z.G.; Dang, J.; Wang, Q.L.; Yu, M.F.; Jiang, L.; Mei, L.J.; Shao, Y.; Tao, Y.D. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. The United States Pharmacopeia—The National Formulary; United States Pharmacopeial Convention: Rockville, MD, USA, 2003; pp. 1401–1402. [Google Scholar]
- Assembly of Life Sciences (U.S.); Food and Nutrition Board; Committee on Codex Specifications. Food Chemical Codex, 3rd ed.; National Academy of Sciences: Washington, DC, USA, 1981; pp. 283–286. [Google Scholar]
- Xie, J.-H.; Shen, M.-Y.; Xie, M.-Y.; Nie, S.-P.; Chen, Y.; Li, C.; Huang, D.-F.; Wang, Y.-X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (batal.) iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, T.J.; Ding, L.L. Optimization of polysaccharide extraction from angelica sinensis using response surface methodology. Food Sci. 2012, 33, 146–149. [Google Scholar]
- Luque-Garcı́a, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, H. Ultrasonic degradation of polysaccharide from a red algae (Porphyra yezoensis). J. Agric. Food Chem. 2006, 54, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, W.; Liu, S. Response surface methodology for optimization of the ultrasonic extraction of polysaccharides from Codonopsis pilosula Nannf. var. Modesta L.T.Shen. Carbohydr. Polym. 2011, 84, 503–508. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.M.; Chen, P.L.; Cao, Y.Y.; Tan, X.L. Optimization of ultrasonic-assisted enzymatic hydrolysis for the extraction of luteolin and apigenin from celery. J. Food Sci. 2011, 76, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.E.M.; Moreschi, S.R.M.; Meireles, M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr. Polym. 2006, 63, 340–346. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Thangam, R.; Suresh, V.; Kannan, S. Optimized extraction of polysaccharides from cymbopogon citratus and its biological activities. Int. J. Biol. Macromol. 2014, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Wen, Q.; Li, L.; Wu, H.; Li, X. Antioxidant activities of water-soluble polysaccha-ride extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydr. Polym. 2010, 81, 323–329. [Google Scholar] [CrossRef]
- Hu, J.; Jia, X.; Fang, X.; Li, P.; He, C.; Chen, M. Ultrasonic extraction, antioxidant and anticancer activities of novel polysaccharides from Chuanxiong rhizome. Int. J. Biol. Macromol. 2016, 85, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, J.; Deng, K.; Ai, L. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Gordon, M.H. The mechanism of antioxidant action in vitro. In Food Antioxidants; Elsevier Applied Science: London, UK, 1990; pp. 1–18. [Google Scholar]
- Zheng, Y.; Li, Y.; Wang, W.-D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.J.; Ding, C.B.; Yuan, S.; Zhang, Z.W.; Du, L.; Yuan, M. Extraction, purification and characterization of polysaccharides from hawk tea. Carbohydr. Polym. 2014, 99, 319–324. [Google Scholar] [CrossRef] [PubMed]

| Run Order | Uncoded Variable Levels | Extraction Yield (%) | ||||
|---|---|---|---|---|---|---|
| X1: Ultrasonic Power (W) | X2: Sonication Time (min) | X3: Ratio of Water to Raw Material (mL/g) | X4: Extraction Temperature (°C) | Experimental | Predicted | |
| 1 | 50.00 | 20.00 | 20.00 | 40.00 | 4.45 | 4.41 |
| 2 | 150.00 | 20.00 | 20.00 | 40.00 | 4.70 | 4.66 |
| 3 | 50.00 | 80.00 | 20.00 | 40.00 | 5.17 | 5.12 |
| 4 | 150.00 | 80.00 | 20.00 | 40.00 | 5.60 | 5.67 |
| 5 | 50.00 | 20.00 | 40.00 | 40.00 | 5.40 | 5.43 |
| 6 | 150.00 | 20.00 | 40.00 | 40.00 | 5.80 | 5.81 |
| 7 | 50.00 | 80.00 | 40.00 | 40.00 | 5.62 | 5.60 |
| 8 | 150.00 | 80.00 | 40.00 | 40.00 | 6.34 | 6.29 |
| 9 | 50.00 | 20.00 | 20.00 | 80.00 | 5.10 | 5.14 |
| 10 | 150.00 | 20.00 | 20.00 | 80.00 | 5.80 | 5.83 |
| 11 | 50.00 | 80.00 | 20.00 | 80.00 | 5.65 | 5.66 |
| 12 | 150.00 | 80.00 | 20.00 | 80.00 | 6.70 | 6.66 |
| 13 | 50.00 | 20.00 | 40.00 | 80.00 | 6.00 | 5.94 |
| 14 | 150.00 | 20.00 | 40.00 | 80.00 | 6.73 | 6.77 |
| 15 | 50.00 | 80.00 | 40.00 | 80.00 | 5.90 | 5.93 |
| 16 | 150.00 | 80.00 | 40.00 | 80.00 | 7.00 | 7.05 |
| 17 | 50.00 | 50.00 | 30.00 | 60.00 | 6.20 | 6.25 |
| 18 | 150.00 | 50.00 | 30.00 | 60.00 | 7.00 | 6.94 |
| 19 | 100.00 | 20.00 | 30.00 | 60.00 | 6.47 | 6.45 |
| 20 | 100.00 | 80.00 | 30.00 | 60.00 | 6.95 | 6.95 |
| 21 | 100.00 | 50.00 | 20.00 | 60.00 | 6.20 | 6.21 |
| 22 | 100.00 | 50.00 | 40.00 | 60.00 | 6.95 | 6.92 |
| 23 | 100.00 | 50.00 | 30.00 | 40.00 | 6.15 | 6.23 |
| 24 | 100.00 | 50.00 | 30.00 | 80.00 | 7.08 | 6.98 |
| 25 | 100.00 | 50.00 | 30.00 | 60.00 | 6.91 | 6.91 |
| 26 | 100.00 | 50.00 | 30.00 | 60.00 | 6.93 | 6.91 |
| 27 | 100.00 | 50.00 | 30.00 | 60.00 | 6.88 | 6.91 |
| 28 | 100.00 | 50.00 | 30.00 | 60.00 | 6.88 | 6.91 |
| 29 | 100.00 | 50.00 | 30.00 | 60.00 | 6.92 | 6.91 |
| 30 | 100.00 | 50.00 | 30.00 | 60.00 | 6.89 | 6.91 |
| Source | Sum of Squares | Degree of Freedom | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 16.37 | 14 | 1.17 | 321.03 | <0.0001 |
| X1-Ultrasonic power | 2.12 | 1 | 2.12 | 582.38 | <0.0001 |
| X2-Sonication time | 1.12 | 1 | 1.12 | 306.04 | <0.0001 |
| X3-ratio of water to raw material | 2.25 | 1 | 2.25 | 618.74 | <0.0001 |
| X4-Extraction temperature | 2.52 | 1 | 2.52 | 690.65 | <0.0001 |
| X1 X2 | 0.09 | 1 | 0.09 | 25.53 | <0.0001 |
| X1 X3 | 0.01 | 1 | 0.01 | 4.64 | 0.0479 |
| X1 X4 | 0.20 | 1 | 0.20 | 54.35 | <0.0001 |
| X2 X3 | 0.29 | 1 | 0.29 | 78.56 | <0.0001 |
| X2 X4 | 0.03 | 1 | 0.03 | 9.91 | 0.0066 |
| X3 X4 | 0.04 | 1 | 0.04 | 12.69 | 0.0028 |
| Residual | 0.05 | 15 | 3.64 × 10−3 | 11.74 | |
| Std. dev. | 0.06 | R2 | 0.99 | ||
| Mean | 6.21 | Adj-R2 | 0.99 | ||
| CV% | 0.97 | Pred-R2 | 0.97 | ||
| PRESS Signal-to-noise ratio | 0.33 >61 | Adequate precision | 61.85 |
| Method | Degree of Esterification (%) |
|---|---|
| Titrimetric method | 53.28 ± 0.08 |
| FT-IR spectroscopy | 51.36 ± 0.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalili Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods 2016, 5, 78. https://doi.org/10.3390/foods5040078
Jalili Safaryan M, Ganjloo A, Bimakr M, Zarringhalami S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods. 2016; 5(4):78. https://doi.org/10.3390/foods5040078
Chicago/Turabian StyleJalili Safaryan, Maryam, Ali Ganjloo, Mandana Bimakr, and Soheila Zarringhalami. 2016. "Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods" Foods 5, no. 4: 78. https://doi.org/10.3390/foods5040078
APA StyleJalili Safaryan, M., Ganjloo, A., Bimakr, M., & Zarringhalami, S. (2016). Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods, 5(4), 78. https://doi.org/10.3390/foods5040078






