Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Essential Oils
2.3. Chromatographic Analyses
2.4. Sensory Evaluation with a Trained Panel
2.5. Statistical Analysis
3. Results and Discussion
3.1. Volatile Composition of Essential Oil of Dill
3.2. Volatile Composition of Essential Oil of Parsley
3.3. Volatile Composition of Essential Oil of Coriander
3.4. Volatile Composition of Essential Oil of Mint
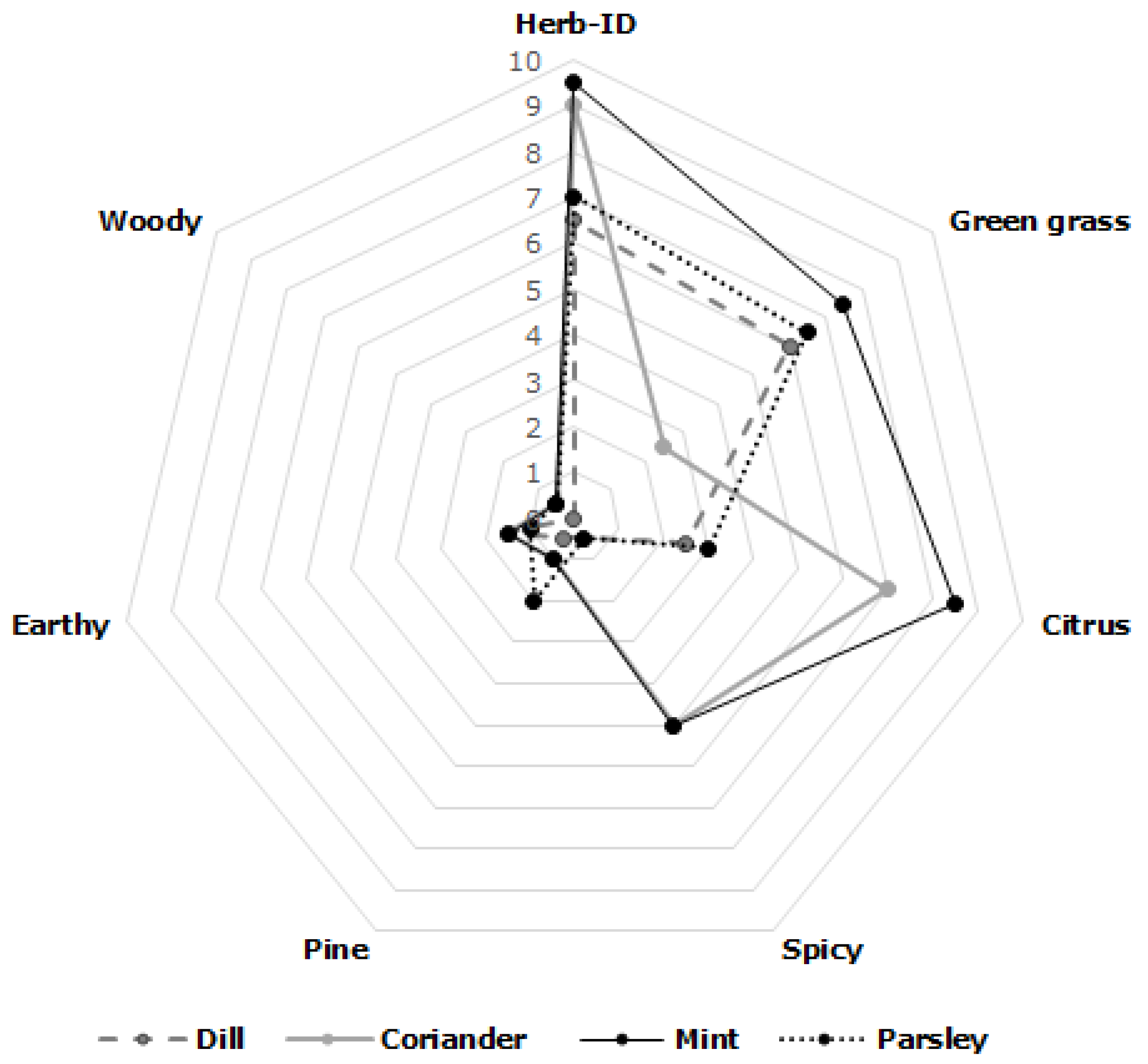
3.5. Descriptive Sensory Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, A.; Figiel, A.; Lech, K.; Szumny, A.; Martínez-Tomé, J.; Carbonell-Barrachina, A.A. Drying methods affect the aroma of (Origanum majorana L.), analyzed by GC–MS and descriptive sensory analysis. Ind. Crops Prod. 2015, 74, 218–227. [Google Scholar]
- Huopalahti, R.; Linko, R. Composition and content of aroma compounds in dill, Anethum graveolens L., at three different growth stages. J. Agric. Food Chem. 1983, 31, 331–333. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Tsai, M.L.; Wu, C.T.; Lin, T.F.; Lin, W.C.; Huang, Y.C.; Yang, C.H. Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharmac. Res. 2013, 12, 577–582. [Google Scholar] [CrossRef]
- Costa, S.S.; Gariepy, Y.; Sandra, C.S.; Rocha, C.S.S.; Raghavan, V. Microwave extraction of mint essential oil—Temperature calibration for the oven. J. Food Eng. 2014, 126, 1–6. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Rúiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2011, 51, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, A.; Lech, K.; Szumny, A.; Figiel, A.; Carbonell-Barrachina, A.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012, 48, 217–225. [Google Scholar]
- Calín-Sánchez, A.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, A.A. Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Drying Technol. 2013, 31, 224–235. [Google Scholar]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Sánchez-Palomo, E.; González-Viñas, M.A. Impact of drying and storage time on sensory characteristics of rosemary (Rosmarinus officinalis L.). J. Sens. Stud. 2007, 22, 34–48. [Google Scholar]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coisson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, H.R.; Nadjafib, F.; Bannayana, M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind. Crops Prod. 2008, 27, 315–321. [Google Scholar] [CrossRef]
- Callan, N.W.; Johnson, D.L.; Westcott, M.P.; Welty, L.E. Herb and oil composition of dill (Anethum graveolens L.): Effects of crop maturity and plant density. Ind. Crops Prod. 2007, 25, 282–287. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Akoumianakis, C.A.; Passam, H.C.; Polissiou, M.G. The effect of sowing date and growth stage on the essential oil composition of three types of parsley (Petroselinum crispum). J. Sci. Food Agric. 2004, 84, 1606–1610. [Google Scholar] [CrossRef]
- Radulescu, V.; Popescu, M.L.; Ilies, D.C. Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia 2010, 58, 594–600. [Google Scholar]
- Orhan, I.; Senol, F.S.; Ozturk, N.; Celik, S.A.; Pulur, A.; Kan, Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013, 59, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Amin, W.M.A.; Sleem, A.A. Chemical and biological study of aerial parts of dill (Anethum graveolens L.). Egyptian J. Biomed. Sci. 2007, 23, 1–18. [Google Scholar] [CrossRef]
- Vokk, R.; Lõugas, T.; Mets, K.; Kravets, M. Dill (Anethum graveolens L.) and Parsley (Petroselinum crispum (Mill.) Fuss) from Estonia: Seasonal Differences in Essential Oil Composition. Agron Res. 2011, 9, 515–520. [Google Scholar]
- Zlatev, S.K. Dynamics of accumulation of the essential oil in the dill (Anethum graveolens Linnaeus) during its ontogenical development. Riv. Ital. Essenze Profumi Piante Off. Aromi Saponi Cosmet. Aerosol 1975, 57, 203–209. [Google Scholar]
- El-Gengaihi, S.E.; Hornok, L. The effect of plant age on content and composition of dill essential oil Anethum graveolens L. Acta Hortic. 1978, 73, 213. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Essential oil composition of the coriander (Coriandrum sativum L.) Herb depending on the development stage. Acta Agrobotanica. 2013, 66, 53–60. [Google Scholar]
- Cadwallader, K.R.; Benitez, D.; Pajjanapimol, S.; Suriyaphan, O.; Singh, T. Characteristic aroma components of the cilantro mimics. Nat. Flavors Fragr. 2005, 117, 128. [Google Scholar]
- Donega, M.A.; Mello, S.C.; Moraes, R.M.; Cantrell, C.L. Nutrient uptake, biomass yield and quantitative analysis of aliphatic aldehydes in cilantro plants. Ind. Crops Prod. 2013, 44, 127–131. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Sintim, H.Y.; Poudyal, S.; Bufalo, J.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E.; Ciampa, L.; Zheljazkov, V.D. Diurnal effects of Mentha canadensis oil concentration and composition at two different havests. HortScience 2015, 50, 85–89. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E. Mentha Canadensis L, a subtropical plant, can withstand first few fall frosts when grown in northern climate. Ind. Crops Prod. 2013, 49, 521–525. [Google Scholar] [CrossRef]
- Brar, S.K.; Gill, B.S.; Brar, A.S.; Kaur, T. Planting date and Straw mulch affect biomass yield, oil yield and oil quality of Japanese mint (Mentha arvensis L.) harvested at successive intervals. J. Essent. Oil Bear. Pl. 2014, 17, 676–695. [Google Scholar] [CrossRef]
- De sousa barros, A.; De morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; Dos santos fontenelle, R.O.; De menezes, J.E.S.A.; Da Silva, F.W.F.; De Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Rohloff, J. Monoterpene composition of essential oil from peppermint (Mentha piperita L.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J. Agric. Food Chem. 1999, 47, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Vázquez-Araújo, L.; García-Martínez, S.; Ruiz, J.J.; Carbonell-Barrachina, A.A. Volatile compounds of traditional and virus-resistant breeding lines of Muchamiel tomatoes. Eur. Food Res. Technol. 2009, 230, 315–323. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, A.; Hernández, F.; Szumny, A.; Martínez, J.J.; Legua, P.; Martínez, R.; Carbonell-Barrachina, A.A. Chemical, functional and quality properties of Japanese plum (Prunus salicina Lindl.) as affected by mulching. Sci. Hort. 2012, 134, 114–120. [Google Scholar] [CrossRef]
- Vendramini, A.L.; Trugo, L.C. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar]
- Gironés-Vilaplana, A.; Calín-Sánchez, Á.; Moreno, D.A.; Carbonell-Barrachina, Á.A.; García-Viguera, C. Novel maqui liquor using traditional pacharán processing. Food Chem. 2015, 173, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
| Compound | Herb | RT (min) | Retention Indexes (RI) | Descriptor ¶ | |
|---|---|---|---|---|---|
| Exp. † | Lit. † | ||||
| trans-2-Hexenal ‡ | dill | 11.09 | 806 | 800 | Green, banana, aldehydic ‡ |
| Octane | coriander | 12.12 | 808 | 800 | |
| α-Thujene | dill | 13.19 | 873 | 905 | Woody, green, herb |
| Santene | mint | 13.29 | 879 | 880 | |
| α-Pinene | dill, parsley, mint | 13.58 | 896 | 909 | Fresh, camphor, sweet, pine, earthy, woody |
| Camphene | mint | 14.38 | 944 | 945 | Fresh, woody, fir, terpene |
| Sabinene | dill, parsley | 14.89 | 975 | 975 | Woody, terpene, citrus, pine, spice |
| Myrcene | dill, parsley, mint | 15.15 | 991 | 991 | Peppery, terpene, spicy |
| β-Pinene | dill, parsley | 15.25 | 997 | 990 | Dry, woody, pine, hay, green |
| cis-3-Hexenyl acetate | parsley, coriander, mint | 15.70 | 1008 | 1009 | Fresh, green, sweet, fruity, banana, apple |
| α-Phellandrene | dill, parsley | 16.20 | 1020 | 1013 | Citrus, herbal, terpene, green, woody, peppery |
| α-Terpinene | mint | 16.68 | 1031 | 1018 | Woody, terpene, lemon, herbal, citrus |
| p-Cymene | dill, parsley, mint | 16.88 | 1036 | 1034 | Fresh, citrus, terpene, woody, spice |
| Limonene | dill, parsley, coriander, mint | 17.08 | 1040 | 1039 | Terpene, pine, herbal, peppery |
| β-Phellandrene | dill, parsley, coriander | 17.25 | 1044 | 1036 | Mint, terpentine |
| trans-β-Ocimene | dill, parsley, mint | 17.38 | 1047 | 1047 | Citrus, tropical, green, terpene, woody |
| γ-Terpinene | parsley, mint | 18.20 | 1066 | 1066 | Woody, terpene, lemon, lime, tropical, herbal |
| trans-Sabinene hydrate | mint | 19.00 | 1084 | 1087 | Warm, balsamic, woody |
| Terpinolene | dill, parsley | 19.47 | 1095 | 1097 | Fresh, woody, sweet, pine, citrus. |
| Undecane | dill, coriander | 19.63 | 1098 | 1099 | Fusel-like |
| Linalool | coriander, mint | 19.82 | 1103 | 1103 | Citrus, orange, floral, terpy, rose |
| Nonanal | coriander, mint | 20.03 | 1107 | 1107 | Aldehydic, rose, fresh, orris, orange, peel |
| 1,3,8-p-Menthatriene | parsley | 20.74 | 1125 | 1115 | Turpentine, camphor, herbal, woody |
| cis-Limonene oxide | mint | 21.87 | 1149 | 1140 | Fresh, citrus |
| trans-Limonene oxide | mint | 22.04 | 1153 | 1147 | Fresh, citrus, mild, green |
| cis-p-Mentha-2.8-dien-1-ol | mint | 23.74 | 1192 | 1193 | |
| trans-p-Mentha-2.8-dien-1-ol | mint | 24.07 | 1199 | 1196 | Fresh, minty |
| Dill ether | dill | 24.40 | 1206 | 1187 | Herbal, dill, spicy |
| α-Terpineol | parsley | 24.74 | 1213 | 1200 | Pine, terpene, lilac, citrus, woody, floral |
| Decanal | coriander | 24.79 | 1214 | 1207 | Sweet, aldehydic, orange, waxy, citrus rind |
| cis-Carveol | mint | 25.05 | 1220 | 1221 | Caraway, spicy, citrus, fruity |
| trans-Carveol | mint | 25.43 | 1228 | 1217 | Caraway, green, oily |
| Carvone | dill, coriander, mint | 27.13 | 1264 | 1262 | Herbaceous, grapefruit, pepper, spicy, woody |
| E-2-Decenal | coriander | 27.54 | 1273 | 1278 | Earthy, coriander green, mushroom, aldehydic |
| 1-Decanol | coriander | 28.45 | 1292 | 1287 | Floral, orange, sweet, clean watery |
| Tridecane | dill | 29.06 | 1297 | 1299 | Citrus, fruity, Fusel-like |
| Bornyl acetate | mint | 29.10 | 1305 | 1291 | Woody, camphor, mentholic, spicy |
| Undecanal | coriander | 29.63 | 1317 | 1310 | Fresh, citrus, waxy, aldehydic |
| Carvomenthyl acetate | mint | 30.70 | 1339 | 1344 | |
| E-2-Undecenal | coriander | 32.40 | 1375 | 1371 | Aldehydic, citrus |
| 1-Undecanol | coriander | 33.88 | 1407 | 1386 | Earthy, soapy, waxy, fatty, honey, coconut |
| β-Bourbonene | mint | 34.08 | 1412 | 1407 | Herbal, Woody |
| Decyl acetate | coriander | 34.13 | 1412 | 1410 | Waxy, sweet, fatty, creamy |
| β-Caryophyllene | mint | 34.26 | 1416 | 1418 | Sweet, woody, spice clove dry |
| Dodecanal | coriander | 34.39 | 1419 | 1420 | Orange, fatty, herbaceous |
| trans-β-Caryophyllene | parsley, mint | 35.68 | 1448 | 1455 | Woody, spicy |
| Z-2-Dodecenal | coriander | 36.37 | 1463 | 1467 | Green, citrus, fruity, mandarin orange, herbal |
| E-2-Dodecenal | coriander | 37.12 | 1480 | 1468 | Citrus, mandarin orange, aldehydic |
| α-Humulene | mint | 37.48 | 1489 | 1489 | |
| E-2-Dodecen-1-ol | coriander | 37.78 | 1495 | 1483 | Oily, fatty |
| 1-Dodecanol | coriander | 38.08 | 1502 | 1485 | Earthy, soapy, waxy, fatty, honey, coconut |
| Germacrene-D | dill, parsley, mint | 38.42 | 1475 | 1477 | Woody, spice |
| Tridecanal | coriander | 38.96 | 1522 | 1518 | Fresh, aldehydic, citrus, grapefruit peel |
| Nerolidol | parsley | 38.97 | 1525 | 1528 | Floral, green, citrus, woody |
| Myristicin | dill, parsley | 39.97 | 1543 | 1532 | Spice, warm, balsam, woody |
| E-2-Tridecenal | coriander | 41.58 | 1582 | 1571 | Citrus, peel tangerine |
| 1-Tetradecanol | coriander | 42.81 | 1615 | 1618 | Fruity, coconut |
| Tetradecanal | coriander | 43.31 | 1632 | 1623 | Dairy, creamy, fishy with a fruity, pear nuance. |
| Compound | ANOVA † | D1 | D2 |
|---|---|---|---|
| Concentration, (mg·kg−1·fw) | |||
| trans-2-Hexenal | *** | 0.18 b ¥ | 1.81 a |
| α-Thujene | *** | 1.35 b | 1.81 a |
| α-Pinene | *** | 7.84 b | 8.70 a |
| Sabinene | *** | 0.35 b | 0.51 a |
| Myrcene | *** | 2.41 b | 3.20 a |
| β-Pinene | *** | 0.66 a | 0.31 b |
| α-Phellandrene | *** | 342 b | 474 a |
| p-Cymene | *** | 12.5 a | 3.92 b |
| Limonene | *** | 17.6 b | 21.4 a |
| β-Phellandrene | *** | 46.0 b | 60.0 a |
| trans-β-Ocimene | *** | 5.13 b | 7.50 a |
| Terpinolene | *** | 2.53 a | 0.33 b |
| Undecane | *** | 4.38 a | 1.11 b |
| Dill ether | *** | 46.2 b | 62.9 a |
| Carvone | NS | 0.02 a | 0.02 a |
| Tridecane | *** | 0.56 a | 0.20 b |
| Germacrene-D | *** | 4.19 a | 1.38 b |
| Myristicin | *** | 13.8 a | 0.02 b |
| TOTAL | *** | 508 b | 649 a |
| Compound | ANOVA † | P1 | P2 | P3 |
|---|---|---|---|---|
| Concentration, (mg·kg−1·fw) | ||||
| α-Pinene | *** | 7.07 c ¥ | 8.53 b | 9.65 a |
| Sabinene | *** | 0.30 b | 0.38 b | 0.55 a |
| Myrcene | *** | 27.0 a | 27.1 a | 24.3 b |
| β-Pinene | *** | 2.47 c | 4.14 a | 3.64 b |
| cis-3-Hexenyl acetate | *** | 1.73 a | 0.35 b | 0.33 b |
| α-Phellandrene | *** | 6.46 c | 11.0 a | 8.63 b |
| p-Cymene | *** | 1.40 b | 1.41 b | 1.83 a |
| Limonene | *** | 12.5 b | 11.3 b | 13.7 a |
| β-Phellandrene | *** | 101 c | 122 a | 110 b |
| Trans-β-Ocimene | *** | 2.89 b | 2.33 c | 3.69 a |
| γ-Terpinene | *** | 0.45 a | 0.29 b | 0.31 b |
| Terpinolene | *** | 22.8 a | 17.2 b | 18.5 b |
| 1,3,8-p-Menthatriene | *** | 222 a | 159 c | 192 b |
| α-Terpineol | *** | 0.26 c | 0.40 b | 0.82 a |
| trans-β-Caryophyllene | *** | 0.61 c | 1.64 b | 2.15 a |
| Germacrene-D | *** | 0.96 c | 1.63 a | 1.39 b |
| Nerolidol | *** | 0.15 b | 0.07 c | 0.23 a |
| Myristcin | *** | 45.1 a | 45.9 a | 25.9 b |
| TOTAL | *** | 455 a | 414 b | 418 b |
| Compound | ANOVA † | C1 | C2 | C3 |
|---|---|---|---|---|
| Concentration, (mg·kg−1·fw) | ||||
| Octane | *** | 16.9 c ¥ | 20.7 a | 18.2 b |
| cis-3-Hexenyl acetate | *** | 0.64 b | 1.17 a | 1.11 a |
| Limonene | *** | 1.06 a | 0.18 b | 0.00 c |
| β-Phellandrene | NS | 0.04 c | 0.14 b | 1.39 a |
| Undecane | *** | 0.45 b | 1.09 a | 0.36 b |
| Linalool | *** | 0.06 a | 0.14 a | 0.05 a |
| Nonanal | NS | 0.01 c | 0.92 a | 0.22 b |
| Decanal | *** | 30.3 b | 25.5 c | 36.4 a |
| Carvone | *** | 3.06 a | 0.01 c | 0.37 b |
| E-2-Decenal | *** | 0.27 b | 0.39 b | 1.47 a |
| 1-Decanol | *** | 3.70 c | 5.05 b | 6.64 a |
| Undecanal | *** | 2.23 c | 3.93 b | 6.70 a |
| E-2-Undecenal | NS | 0.01 c | 0.44 b | 0.75 a |
| 1-Undecanol | NS | 0.05 b | 0.11 a | 0.05 b |
| Decyl acetate | NS | 0.01 a | 0.03 a | 0.00 a |
| Dodecanal | *** | 24.1 a | 24.4 a | 17.6 b |
| Z-2-Dodecenal | *** | 0.12 b | 0.26 a | 0.10 b |
| E-2-Dodecenal | *** | 15.0 c | 39.9 a | 25.7 b |
| E-2-Dodecen-1-ol | *** | 1.90 a | 1.00 b | 0.04 c |
| 1-Dodecanol | *** | 1.80 a | 0.21 b | 0.01 c |
| Tridecanal | *** | 1.90 a | 1.18 b | 1.22 b |
| E-2-Tridecenal | *** | 1.33 b | 4.69 a | 4.59 a |
| 1-Tetradecanol | *** | 0.12 b | 0.27 a | 0.08 b |
| Tetradecanal | *** | 1.61 b | 1.98 a | 0.88 c |
| TOTAL | *** | 107 c | 134 a | 124 b |
| Compound | ANOVA † | M1 | M2 |
|---|---|---|---|
| Concentration, (mg·kg−1·fw) | |||
| Santene | *** | 22.5 a ¥ | 24.05 a |
| Camphene | NS | 2.17 b | 3.50 a |
| β-Pinene | *** | 12.3 a | 13.1 a |
| Myrcene | *** | 23.6 b | 28.3 a |
| cis-3-Hexenyl acetate | NS | 0.56 a | 0.62 a |
| p-Cymene | NS | 1.29 b | 2.55 a |
| α-Terpinene | NS | 0.54 b | 2.90 a |
| Limonene | *** | 590 b | 735 a |
| trans-β-Ocimene | *** | 19.1 a | 18.2 a |
| γ-Terpinene | NS | 1.31 b | 5.17 a |
| trans-Sabinene hydrate | *** | 34.3 b | 73.6 a |
| Nonanal | *** | 10.6 a | 12.0 a |
| Linalool | NS | 1.29 b | 2.23 a |
| cis-Limonene oxide | NS | 1.28 a | 1.23 a |
| trans-Limonene oxide | NS | 2.84 a | 1.79 b |
| cis-p-Mentha-2,8-dien-1-ol | NS | 6.43 a | 6.39 a |
| trans-p-Mentha-2,8-dien-1-ol | NS | 4.45 b | 11.4 a |
| cis-Carveol | *** | 65.3 b | 85.8 a |
| trans-Carveol | NS | 8.12 a | 9.73 a |
| Carvone | *** | 2462 a | 1854 b |
| Bornyl acetate | NS | 0.51 a | 0.43 a |
| Carvomenthyl acetate | *** | 12.0 b | 14.7 a |
| β-Bourbonene | *** | 14.6 a | 14.0 a |
| β-Caryophyllene | NS | 2.30 b | 3.59 a |
| trans-Caryophyllene | *** | 22.1 b | 35.5 a |
| Alloaromadendrene | NS | 1.74 b | 2.57 a |
| α-Humulene | NS | 3.58 b | 5.11 a |
| TOTAL | *** | 3326 a | 2968 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods 2016, 5, 41. https://doi.org/10.3390/foods5020041
El-Zaeddi H, Martínez-Tomé J, Calín-Sánchez Á, Burló F, Carbonell-Barrachina ÁA. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods. 2016; 5(2):41. https://doi.org/10.3390/foods5020041
Chicago/Turabian StyleEl-Zaeddi, Hussein, Juan Martínez-Tomé, Ángel Calín-Sánchez, Francisco Burló, and Ángel A. Carbonell-Barrachina. 2016. "Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain" Foods 5, no. 2: 41. https://doi.org/10.3390/foods5020041
APA StyleEl-Zaeddi, H., Martínez-Tomé, J., Calín-Sánchez, Á., Burló, F., & Carbonell-Barrachina, Á. A. (2016). Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods, 5(2), 41. https://doi.org/10.3390/foods5020041








