Analysis of Naturally Occurring Steroid Hormones in Infant Formulas by HPLC-MS/MS and Contribution to Dietary Intake
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Standard Solutions
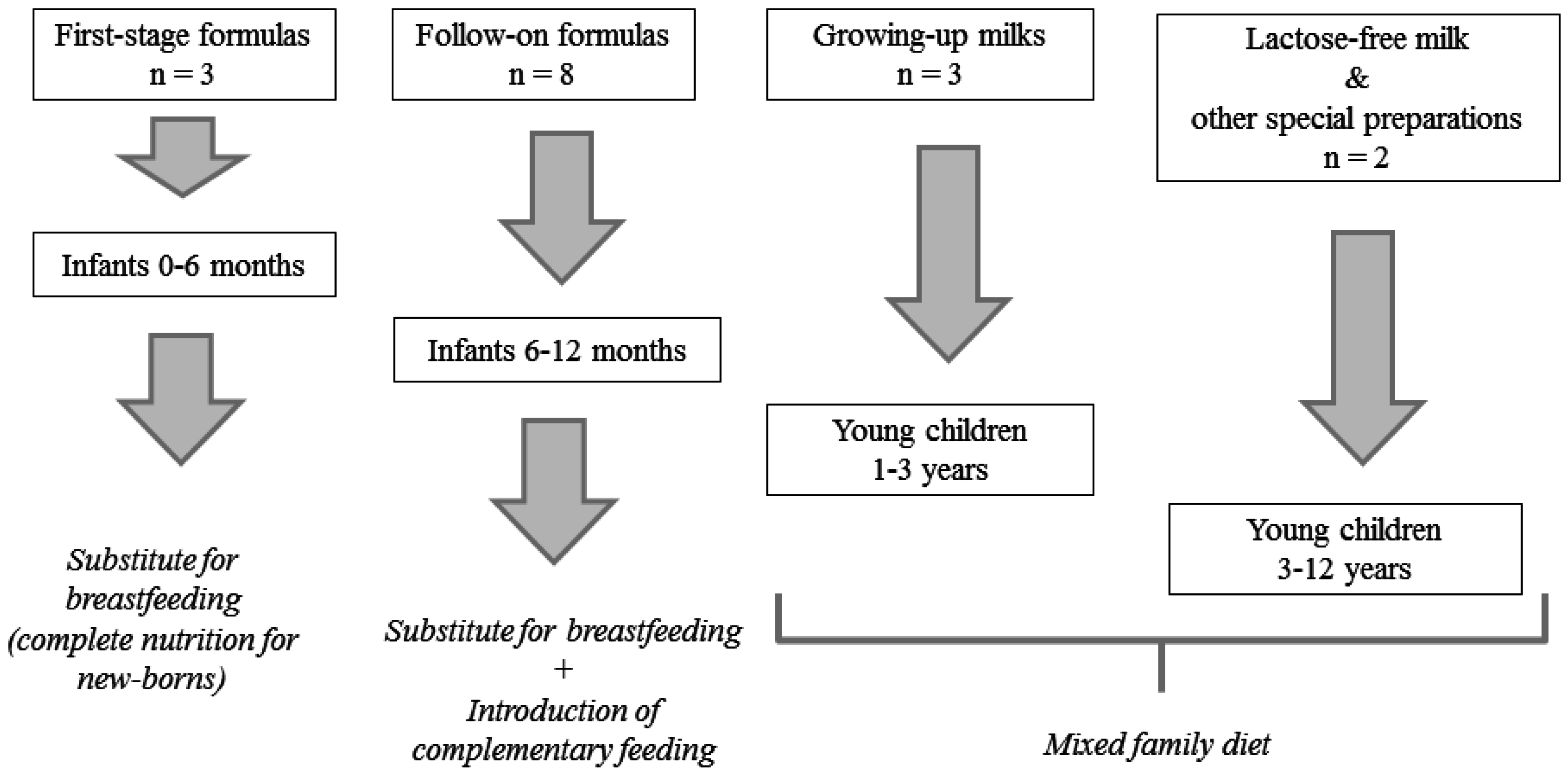
2.2. Samples
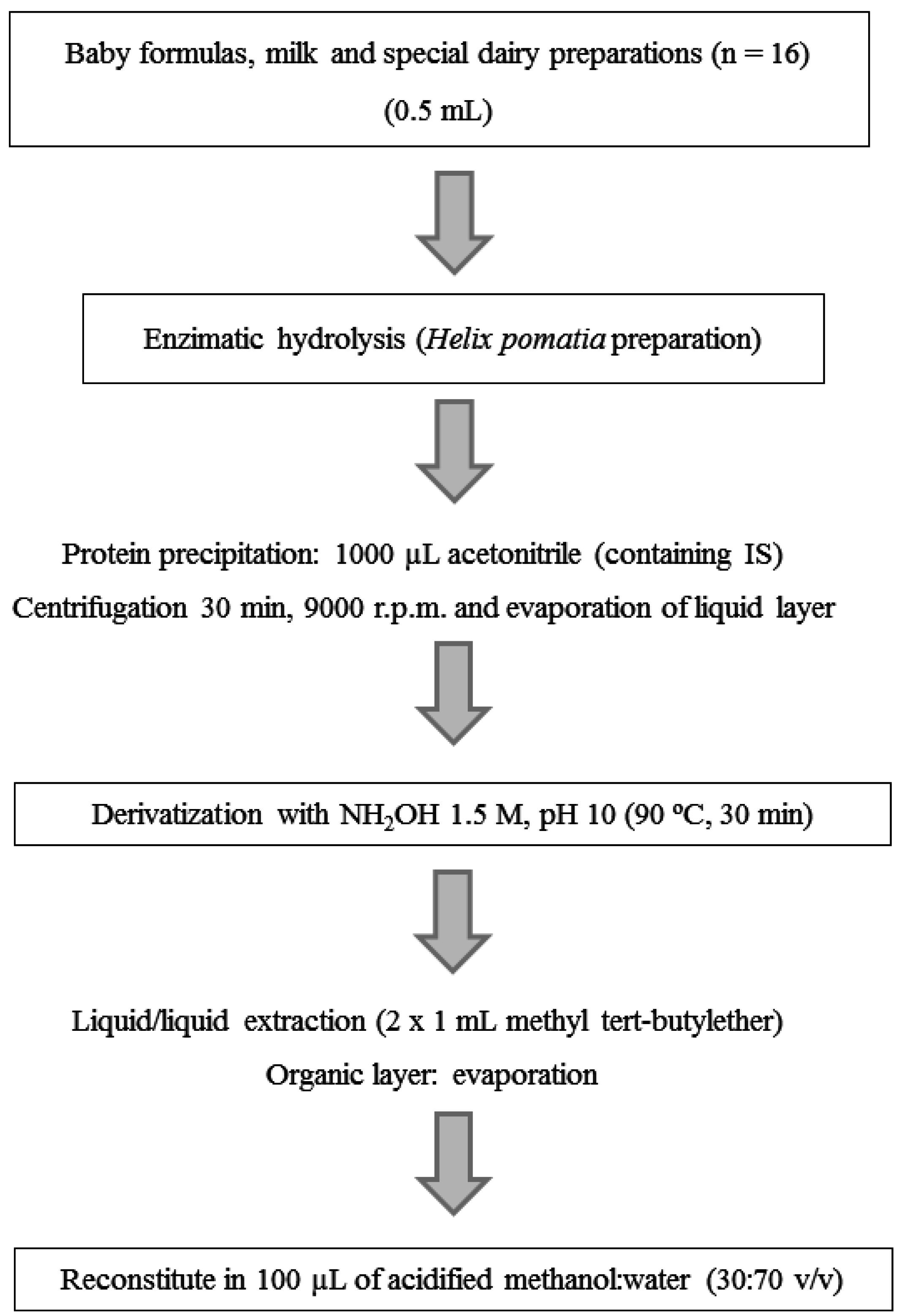
2.3. Sample Preparation
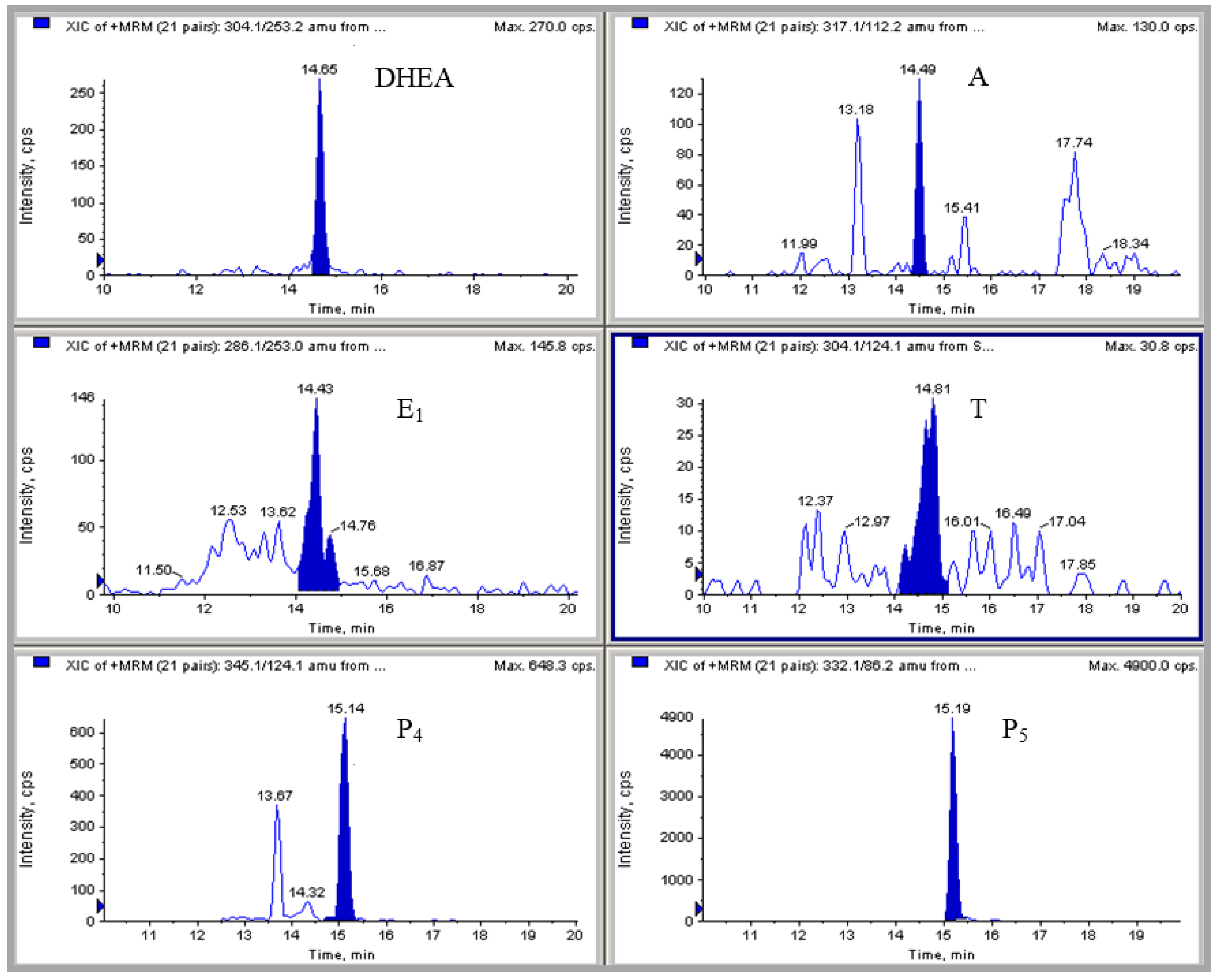
2.4. HPLC-MS/MS Measurement of Hormonal Levels
2.5. Calculation of Daily Intakes
3. Results and Discussion
3.1. Samples
3.2. Analytical Methodology
| Dairy Product (dL per Day) | % Fat | Hormonal Levels (ng·dL−1) * | Estimated Daily Intake (μg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | T | DHEA | E1 | P4 | P5 | A + T + DHEA | E1 | P5 + P4 | |||
| First-stage formula (9 dL) | 1 | 4.0 | 20.9 ± 5.7 | 10.7 ± 2.9 | 28.9 ± 0.8 | 19.3 ± 13.3 | 91.4 ± 31.7 | 614.4 ± 117.1 | 0.5 | 0.2 | 6.4 |
| 2 | 3.2 | 11.2 ± 3.7 | 7.3 ± 0.0 | 10.2 ± 3.3 | 8.8 ± 0.0 | 10.5 ± 3.9 | 157.4 ± 185.6 | 0.3 | 0.1 | 1.5 | |
| 3 | 3.5 | 11.2 ± 3.7 | 7.3 ± 0.0 | 27.9 ± 28.5 | 20.4 ± 20.2 | 12.8 ± 5.6 | 48.4 ± 41.4 | 0.4 | 0.2 | 0.6 | |
| Follow-on formula (7 dL) | 1 | 3.0 | 13.3 ± 3.7 | 9.0 ± 2.9 | 10.2 ± 3.3 | 40.8 ± 50.1 | 12.8 ± 7.5 | 50.5 ± 9.1 | 0.2 | 0.3 | 0.4 |
| 2 | 3.1 | 13.5 ± 3.9 | 7.3 ± 0.0 | 13.1 ± 8.3 | 25.3 ± 14.5 | 31.8 ± 31.1 | 76.0 ± 86.3 | 0.2 | 0.2 | 0.8 | |
| 3 | 2.8 | 11.2 ± 3.7 | 7.3± 0.0 | 16.5 ± 14.2 | 22.7 ± 18.9 | 14.5 ± 7.0 | 45.3 ± 0.8 | 0.2 | 0.2 | 0.4 | |
| 4 | 3.2 | 14.3 ± 4.8 | 11.1 ± 3.3 | 8.3 ± 0.0 | 35.5 ± 41.0 | 20.8 ± 1.7 | 67.2 ± 4.0 | 0.2 | 0.2 | 0.6 | |
| 5 | 2.9 | 15.2 ± 6.0 | 7.3 ± 0.0 | 8.3 ± 0.0 | 8.8 ± 0.0 | 16.2 ± 8.7 | 36.1 ± 26.6 | 0.2 | 0.1 | 0.4 | |
| 6 | 3.2 | 12.2 ± 4.5 | 7.3 ± 0.0 | 11.2 ± 4.1 | 24.9 ± 14.0 | 22.6 ± 0.1 | 74.2 ± 21.8 | 0.2 | 0.2 | 0.7 | |
| 7 | 2.8 | 9.0 ±0.0 | 7.3 ± 0.0 | 8.3 ± 0.0 | 8.8 ± 0.0 | 25.1 ± 0.8 | 30.1 ± 1.5 | 0.2 | 0.1 | 0.4 | |
| 8 | 3.0 | 12.2 ± 4.5 | 7.3 ± 0.0 | 8.3 ± 0.0 | 11.9 ± 4.4 | 34.8 ± 5.9 | 319.7 ± 212.4 | 0.2 | 0.1 | 2.5 | |
| Growing-up (5 dL) | 1 | 2.7 | 11.2 ± 3.7 | 10.7 ± 5.8 | 11.4 ± 5.5 | 21.9 ± 17.5 | 54.6 ± 16.4 | 69.0 ± 12.1 | 0.2 | 0.1 | 0.6 |
| 2 | 3.0 | 11.2 ± 3.8 | 7.3 ± 0.0 | 8.3 ± 0.0 | 20.7 ± 20.6 | 21.1 ± 9.0 | 88.3 ± 97.2 | 0.1 | 0.1 | 0.5 | |
| 3 | 3.2 | 17.2 ± 2.0 | 7.3 ± 0.0 | 14.1 ± 7.8 | 8.8 ± 0.0 | 10.9 ± 0.0 | 5.2 ± 0.0 | 0.2 | 0.0 | 0.1 | |
| Milk 3–12 years(5 dL) | 1 | 2.8 | 16.9 ± 2.1 | 7.3 ± 0.0 | 27.9 ± 7.8 | 11.9 ± 4.4 | 102.8 ± 45.1 | 87.9 ± 9.8 | 0.3 | 0.1 | 1.0 |
| Lactose-free milk (5 dL) | 1 | 1.6 | 21.1 ± 1.3 | 7.3 ± 0.0 | 8.3 ± 0.0 | 8.8 ± 0.0 | 13.9 ± 6.6 | 8.8 ± 0.0 | 0.2 | 0.0 | 0.1 |
3.3. Measurement of Natural Steroid Hormones in Dairy Preparations for Infants
| Analysis | Sample | Hormonal Levels (ng·dL−1) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| T | A | P4 | E1 | 17β-E2 | ||||
| Total content (deconjugated) | First-stage formula | Sample 1 | 10.7 | 20.9 | 91.4 | 19.3 | – | Results in this article |
| First-stage formula | Sample 2 | 7.3 | 11.2 | 10.5 | 8.8 | – | ||
| First-stage formula | Sample 3 | 7.3 | 11.2 | 12.8 | 20.4 | – | ||
| Follow-on formula | Sample 1 | 9.0 | 13.3 | 12.8 | 40.8 | – | ||
| Follow-on formula | Sample 2 | 7.3 | 13.5 | 31.8 | 25.3 | – | ||
| Follow-on formula | Sample 3 | 7.3 | 11.2 | 14.5 | 22.7 | – | ||
| Follow-on formula | Sample 4 | 11.1 | 14.3 | 20.8 | 35.5 | – | ||
| Follow-on formula | Sample 5 | 7.3 | 15.2 | 16.2 | 8.8 | – | ||
| Follow-on formula | Sample 6 | 7.3 | 12.2 | 22.6 | 24.9 | – | ||
| Follow-on formula | Sample 7 | 7.3 | 9.0 | 25.1 | 8.8 | – | ||
| Follow-on formula | Sample 8 | 7.3 | 12.2 | 34.8 | 11.9 | – | ||
| Growing-up formula | Sample 1 | 10.7 | 11.2 | 54.6 | 21.9 | – | ||
| Growing-up formula | Sample 2 | 7.3 | 11.2 | 21.1 | 20.7 | – | ||
| Growing-up formula | Sample 3 | 7.3 | 17.2 | 10.9 | 8.8 | – | ||
| Milk 3–12 years | Sample 1 | 7.3 | 16.9 | 102.8 | 11.9 | – | ||
| Lactose-free | Sample 1 | 7.3 | 21.1 | 13.9 | 8.8 | – | ||
| Free hormones only | Infant formula milk powder | Sample 1 | – | – | – | 3.6 | 1.9 | [15] |
| Sample 2 | – | – | – | 5.0 | 2.5 | |||
| Sample 3 | – | – | – | 14.6 | 3.3 | |||
| Sample 4 | – | – | – | 14.4 | 3.5 | |||
| Sample 5 | – | – | – | 2.7 | 3.7 | |||
| Sample 6 | – | – | – | 2.7 | 3.8 | |||
| Free and sulfate conjugates | Skim fraction | from raw milk | – | – | – | 0.1 | – | [26] |
| Raw milk whole | 50 cows | – | – | – | 0.2 | – | ||
| Fat fraction | from raw milk | – | – | – | 2.8 | – | ||
| Whole milk | pasteurized | – | – | – | 1.0 | – | ||
| Raw bovine milk | 173 cows | – | – | – | 0.7 | – | ||
| Total content (deconjugated) | Bovine raw milk | pregnant | 10.3 | 36.7 | 82.4 | 14 | – | [9] |
| Bovine raw milk | non-pregnant | 8 | 10.9 | 8.2 | 8.8 | – | ||
| Total content (deconjugated) | Bovine colostrum | 1 day | 10 | 18 | 646 | 130.0 | 30 | [20] |
| Bovine milk | 26–30 days | 10 | 10 | 213 | – | – | ||
| Human colostrum | 1 day | 25 | 131 | 239 | 27.0 | – | ||
| Human colostrum | 3 days | 19 | 98 | 101 | 17.0 | – | ||
| Free hormones only | Raw bovine milk | Sample 1 | – | – | – | 7.5 | 310 | [19] |
| Sample 2 | – | – | – | 8.0 | 320 | |||
| Sample 3 | – | – | – | 2.5 | 9 | |||
| Whole | Sample 1 | – | – | – | 3.5 | 110 | ||
| Sample 2 | – | – | – | 13.0 | 120 | |||
| Human milk | Sample 1 | – | – | – | 5.5 | 54 | ||
| Sample 2 | – | – | – | 17.0 | 49 | |||
| Powdered infant milk | Sample 1 | – | – | – | <0.01 | <0.12 | ||
| Total content (deconjugated) | Human milk | concentration range (n = 4) | – | – | – | – | 790 to 1850 | [18] |
3.4. Hormonal Intake Calculation
| CRITERIA | PARAMETER | P4 | T | βE2 |
|---|---|---|---|---|
| JECFA, 1999 [32] | ADI a | 0–30 μg·kg−1 bw | 0–2 μg·kg−1 bw | 0–50 ng·kg−1 bw |
| ADI adult 60 kg | 1.8 mg·day−1 | 120 μg·day−1 | 3 μg·day−1 | |
| ADI child 0.5 year old (8 kg) | 0.24 mg·day−1 | 16 μg·day−1 | 0.4 μg·day−1 | |
| ADI child 1 year old (10 kg) | 0.3 mg·day−1 | 20 μg·day−1 | 0.5 μg·day−1 | |
| ADI child 3 years old (15 kg) | 0.45 mg·day−1 | 30 μg·day−1 | 0.75 μg·day−1 | |
| ADI child 12 years old (40 kg) | 1.2 mg·day−1 | 80 μg·day−1 | 2 μg day−1 | |
| NOEL b (LOEL c for P4) | 3.3 mg·kg−1 bw·day−1 | 1.7 mg·kg−1 bw·day−1 | 5 μg·kg−1 bw·day−1 | |
| FDA, 2006 [33] | DAILY PRODUCTION | 150 μg | 32 μg | 6 μg |
| permitted increase exposure | 1.5 μg | 0.32 μg | 0.06 μg |
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Infant Formula and Infant Nutrition: Bioactive Proteins of Human Milk and Implications for Composition of Infant Formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Milk—The Promoter of Chronic Western Diseases. Med. Hypotheses 2009, 72, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities. Council Directive 96/22/EC of 29 April 1996 Concerning the Prohibition on the use in Stockfarming of Certain Substances having a Hormonal or Thyrostatic Action and of Beta-Agonists, and Repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC. Off. J. Eur. Commun. 1996, L125, 3–9. [Google Scholar]
- Poelmans, S.; de Wasch, K.; Noppe, H.; van Hoof, N.; van Cruchten, S.; le Bizec, B.; Deceuninck, Y.; Sterk, S.; van Rossum, H.J.; Hoffman, M.K.; et al. Endogenous Occurrence of some Anabolic Steroids in Swine Matrices. Food Addit. Contam. 2005, 22, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Scherpenisse, P.; Bergwerff, A.A. Naturally Occurring Estrogens in Processed Milk and in Raw Milk (from Gestated Cows). J. Agric. Food Chem. 2006, 54, 9785–9791. [Google Scholar] [CrossRef] [PubMed]
- Courant, F.; Antignac, J.P.; Maume, D.; Monteau, F.; Andre, F.; le Bizec, B. Determination of Naturally Occurring Oestrogens and Androgens in Retail Samples of Milk and Eggs. Food Addit. Contam. 2007, 24, 1358–1366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daxenberger, A.; Ibarreta, D.; Meyer, H.H.D. Possible Health Impact of Animal Oestrogens in Food. Hum. Reprod. Update 2001, 7, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Regal, P.; Cepeda, A.; Fente, C. Development of an LC-MS/MS Method to Quantify Sex Hormones in Bovine Milk and Influence of Pregnancy in their Levels. Food Addit. Contam. A 2012, 29, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Pape-Zambito, D.A.; Magliaro, A.L.; Kesinger, R.S. 17β-Estradiol and Estrone Concentrations in Plasma and Milk during Bovine Pregnancy. J. Dairy Sci. 2007, 91, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Oshima, T.; Ohyama, K. Exposure to Exogenous Estrogen through Intake of Commercial Milk Produced from Pregnant Cows. Pediatr. Int. 2010, 52, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities. Commission Directive 2006/141/EC of 22 December 2006 on Infant Formulae and Follow-on Formulae and Amending Directive 1999/21/EC. Off. J. Eur. Commun. 2006, L401, 1–33. [Google Scholar]
- Commission of the European Communities. Commission Directive 2013/46/EU of 28 August 2013 amending Directive 2006/141/EC with regard to Protein Requirements for Infant Formulae and Follow-on Formulae. Off. J. Eur. Commun. 2013, L230, 16–19. [Google Scholar]
- Wang, H.; Zhou, Y.; Jiang, Q. Simultaneous Analysis of Nine Estrogens in Milk Powder with Ultra-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Chin. J. Anal. Chem. 2011, 39, 1323–1328. [Google Scholar] [CrossRef]
- Chen, C.; Mi, X.; Yuan, Y.; Chen, G.; Ren, L.; Wang, K.; Zhu, D.; Qian, Y. A Preliminary Risk Assessment of Potential Exposure to Naturally Occurring Estrogens from Beijing (China) Market Milk Products. Food Chem. Toxicol. 2014, 71, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, H.; Zhang, J.; Sun, N.; Sun, H. Analysis of Infant Formula for Steroid Hormones by Gas Chromatography-Tandem Mass Spectrometry using Microwave-Assisted Extraction and Gel Permeation Chromatography Clean Up. Food Anal. Methods 2014, 7, 798–805. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of Infants to Phyto-Oestrogens from Soy-Based Infant Formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, K.; Hong, J.K.; Park, S.J.; Chung, B.C. Determination of Non-Steroidal Estrogens in Breast Milk, Plasma, Urine and Hair by Gas chromatography/mass Spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Jurado-Sanchez, B.; Souhail, B.; Ballesteros, E. Simultaneous Determination of 20 Pharmacologically Active Substances in Cow’s Milk, Goat’s Milk, and Human Breast Milk by Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 5125–5132. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, L.; Zhang, Y.; Sheng, Q.; Zhao, A. Qualitative and Quantitative Comparison of Hormone Contents between Bovine and Human Colostrums. Int. Dairy J. 2011, 21, 54–57. [Google Scholar] [CrossRef]
- Commission of the European Communities. Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Commun. 2002, L221, 8–36. [Google Scholar]
- Courant, F.; Aksglaede, L.; Antignac, J.P.; Monteau, F.; Sorensen, K.; Andersson, A.; Skakkebaek, N.; Juul, A.; le Bizec, B. Assessment of Circulating Sex Steroid Levels in Prepubertal and Pubertal Boys and Girls by a Novel Ultrasensitive Gas Chromatography-Tandem Mass Spectrometry Method. J. Clin. Endocrinol. Metab. 2010, 95, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Raecker, T.; Thiele, B.; Boehme, R.M.; Guenther, K. Endocrine Disrupting Nonyl- and Octylphenol in Infant Food in Germany: Considerable Daily Intake of Nonylphenol for Babies. Chemosphere 2011, 82, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Vasco, E.R.; Alvito, P.C. Occurrence and Infant Exposure Assessment of Nitrates in Baby Foods Marketed in the Region of Lisbon, Portugal. Food Addit. Contam. B 2011, 4, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Chromatographic Analysis of Natural and Synthetic Estrogens in Milk and Dairy Products. TrAC Trends Anal. Chem. 2013, 44, 58–77. [Google Scholar] [CrossRef]
- Macrina, A.L.; Ott, T.L.; Roberts, R.F.; Kensinger, R.S. Estrone and Estrone Sulfate Concentrations in Milk and Milk Fractions. J. Acad. Nutr. Diet. 2012, 112, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; LaganÃ, A. Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry for the Analysis of Free and Conjugated Natural Estrogens in Cow Milk without Deconjugation. Anal. Bioanal. Chem. 2015, 407, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Wolford, S.T.; Argoudelis, C.J. Measurement of Estrogens in Cow’s Milk, Human Milk, and Dairy Products. J. Dairy Sci. 1979, 62, 1458–1463. [Google Scholar] [CrossRef]
- Colazo, M.G.; Ambrose, D.J.; Kastelic, J.P.; Small, J.A. Comparison of 2 Enzyme Immunoassays and a Radioimmunoassay for Measurement of Progesterone Concentrations in Bovine Plasma, Skim Milk, and Whole Milk. Can. J. Vet. Res. 2008, 72, 32–36. [Google Scholar] [PubMed]
- Courant, F.; Antignac, J.P.; Laille, J.; Monteau, F.; Andre, F.; le Bizec, B. Exposure Assessment of Prepubertal Children to Steroid Endocrine Disruptors. 2. Determination of Steroid Hormones in Milk, Egg, and Meat Samples. J. Agric. Food Chem. 2008, 56, 3176–3184. [Google Scholar] [CrossRef] [PubMed]
- Farke, C.; Rattenberger, E.; Roiger, S.U.; Meyer, H.H.D. Bovine Calostrum: Determination of Naturally Occurring Steroid Hormones by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Agric. Food Chem. 2011, 59, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Summary and Conclusions of the Fifty-second Meeting on Joint Expert Committee on Food Additives; JECFA: Rome, Italy, 1999; pp. 1–24. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: General Principles for Evaluating the Safety of Compounds Used in Food-Producing Animals; U.S. Food and Drug Administration: Washington, DC, USA, 2006; pp. 1–42.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro, R.; Regal, P.; Díaz-Bao, M.; Fente, C.A.; Cepeda, A. Analysis of Naturally Occurring Steroid Hormones in Infant Formulas by HPLC-MS/MS and Contribution to Dietary Intake. Foods 2015, 4, 605-621. https://doi.org/10.3390/foods4040605
Barreiro R, Regal P, Díaz-Bao M, Fente CA, Cepeda A. Analysis of Naturally Occurring Steroid Hormones in Infant Formulas by HPLC-MS/MS and Contribution to Dietary Intake. Foods. 2015; 4(4):605-621. https://doi.org/10.3390/foods4040605
Chicago/Turabian StyleBarreiro, Rocío, Patricia Regal, Mónica Díaz-Bao, Cristina A. Fente, and Alberto Cepeda. 2015. "Analysis of Naturally Occurring Steroid Hormones in Infant Formulas by HPLC-MS/MS and Contribution to Dietary Intake" Foods 4, no. 4: 605-621. https://doi.org/10.3390/foods4040605
APA StyleBarreiro, R., Regal, P., Díaz-Bao, M., Fente, C. A., & Cepeda, A. (2015). Analysis of Naturally Occurring Steroid Hormones in Infant Formulas by HPLC-MS/MS and Contribution to Dietary Intake. Foods, 4(4), 605-621. https://doi.org/10.3390/foods4040605









