Assessment of Tetracyclines Residues and Tetracycline Resistant Bacteria in Conventional and Organic Baby Foods
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Collection
2.2. Sample Preparation for qPCR
2.3. PCR Conditions
2.4. Quantification Assays
2.5. Quantification of Tetracyclines Residues in Baby Foods
2.6. Statistical Analysis
3. Results and Discussion
| product | tet(A) | tet(B) | ||
|---|---|---|---|---|
| R2 | Slope | R2 | Slope | |
| Poultry meat | 0.9997 | −3.32 | 0.9996 | −3.29 |
| Beef | 0.9887 | −3.17 | 0.9876 | −2.97 |
| Vegetables | 0.9985 | −3.26 | 0.9747 | −3.20 |
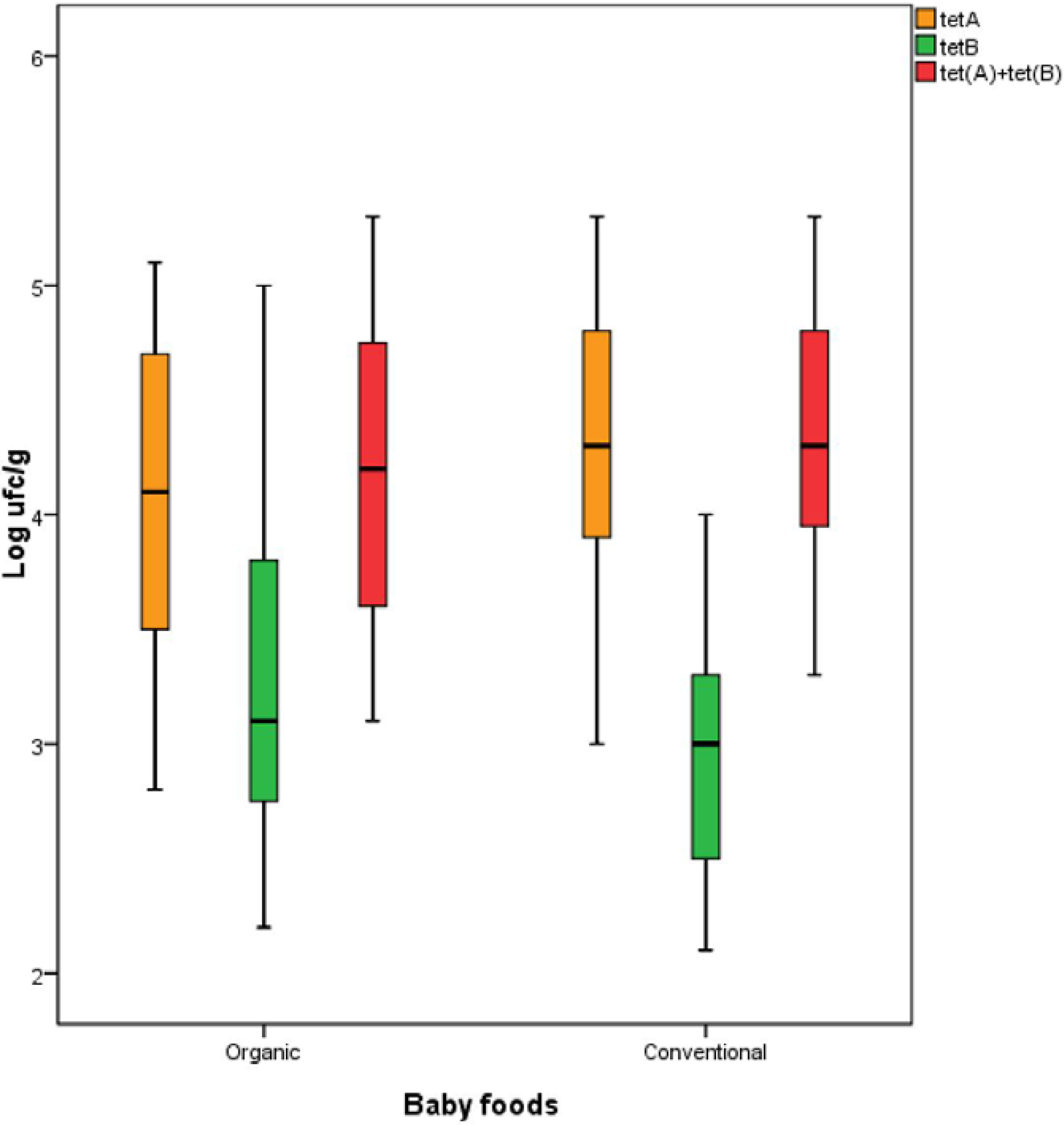
| Farming Method | Type of Sample | Genes [CFU·g−1] | Tetracycline Residues [µg·kg−1] | ||
|---|---|---|---|---|---|
| tet(A) | tet(B) | tet(A) + tet(B) | |||
| Conventional | Poultry meat | 4.5 ± 0.574 * | 3.2 ± 0.526 | 4.6 ± 0.540 | 51.2 ± 5.44 |
| Beef | 4.2 ± 0.426 | 2.7 ± 0.404 | 4.2 ± 0.400 | 51.2 ± 6.74 | |
| Organic | Poultry meat | 4.6 ± 0.471 | 3.9 ± 0.378 | 4.7 ± 0.394 | 53.1 ± 6.39 |
| Beef | 3.7 ± 0.481 | 2.9 ± 0.415 | 3.8 ± 0.449 | 66.2 ± 426 | |
| Vegetables | 3.9 ± 0.499 | 2.5 ± 0.339 | 3.9 ± 0.485 | 53.3 ± 13.9 | |
| Vegetables | Beef | Poultry | |
|---|---|---|---|
| Tet A | 3.9 ± 0.50 | 3.9 ± 0.53 | 4.6 ± 0.53 |
| Tet B | 2.5 ± 0.34 | 2.8 ± 0.42 | 3.5 ± 0.57 |
| Tet (A) + Tet (B) | 3.9 ± 0.49 | 4.0 ± 0.48 | 4.6 ± 0.49 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodríguez, E.; Moreno-Bondi, M.C.; Marazuela, M.D. Multiresidue determination of fluoroquinolone antimicrobials in baby foods by liquid chromatography. Food Chem. 2011, 127, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.; Florini, K.; Barlam, T. When Wonder Drugs Don’T Work, 2nd ed.; Enviromental Defense: Washington, DC, USA, 2001; p. 9. [Google Scholar]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, J.M. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.; Gerald, J.; Friedman, D.; Gill, C. From the farm to the kitchen table: The negative impact of antimicrobial use in animals on humans. Nutr. Rev. 2002, 60, 261–264. [Google Scholar] [PubMed]
- Tollefson, L.; Karp, B.E. Human health impact from antimicrobial use in food animals. Med. Maladies Infect. 2004, 34, 514–521. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin. Pharmacol. 2005, 96, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Cosby, D. Salmonella prevalence in free-range and certified organic chickens. J. Food Protect 2005, 68, 2451–2453. [Google Scholar]
- Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. Off. J. Eur. Communities 2008, 8, 1–115.
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic food: Buying more safety or just peace of mind? A critical review of the literature. Crit. Rev. Food Sci. 2006, 46, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Rajic, A.; Wilhelm, B.J.; Waddell, L.; Parker, S.; McEwen, S.A. Comparison of the prevalence of bacterial enteropathogens, potentially zoonotic bacteria and bacterial resistance to antimicrobials in organic and conventional poultry, swine and beef production: A systematic review and meta-analysis. Epidemiol. Infect. 2009, 137, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Kools, S.A.E.; Moltmann, J.F.; Knacker, T. Estimating the use of veterinary medicines in the European union. Regul. Toxicol. Pharmacol. 2008, 50, 59–65. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 26 EU/EEA Countries in 2012. (EMA/333921/2014). 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/10/WC500175671.pdf (accessed on 3 July 2015).
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Norstrom, M. The prevalence of, associations between and conjugal transfer of antibiotic resistance genes in Escherichia coli isolated from Norwegian meat and meat products. J. Antimicrob. Chemoth. 2006, 58, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Dubnau, D. DNA uptake during Bacterial Transformation. Nat. Rev. Microbiol. 2004, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, L.P.L.; Tulinski, P.; Bondt, N.; Mevius, D.; Verwer, C. Prevalence and Molecular Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) in Organic Pig Herds in the Netherlands. Zoonoses Public Health 2014, 61, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Luiz, M.M.; Martínez Vidal, J.L.; Romero González, R.; Garrido Frenich, A. Multiclass method for fast determination of veterinary drug residues in baby food by ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 132, 2171–2180. [Google Scholar] [CrossRef]
- Guarddon, M.; Miranda, J.M.; Rodríguez, J.A.; Vázquez, B.I.; Cepeda, A.; Franco, C.M. Real-Time polymerase chain reaction for the quantitative detection of tetA and tetB bacterial tetracycline resistance genes in food. Int. J. Food Microbiol. 2011, 146, 284–289. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 470/2009 of the european parliament and of the council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Off. J. Eur. Union 2009, L152, 11–22. [Google Scholar]
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. Kinetic PCR analysis—Real-time monitoring of DNA amplification reactions. Biotechnology 1993, 11, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Guarddon, M.; Mondragón, A.; Vázquez, B.I.; Fente, C.A.; Cepeda, A.; Franco, C.M. Antimicrobial resistance in Enterococcus spp. strains isolated from organic chicken, conventional chicken, and turkey meat: A comparative survey. J. Food Prot. 2007, 70, 1021–1024. [Google Scholar]
- Miranda, J.M.; Guarddon, M.; Vázquez, B.I.; Fente, C.A.; Barros-Velázquez, J.; Cepeda, A.; Franco, C.M. Antimicrobial resistance in Enterobacteriaceae strains isolated from organic chicken, conventional chicken and conventional turkey meat: A comparative survey. Food Control 2008, 19, 412–416. [Google Scholar] [CrossRef]
- Wilhelm, B.; Rajic, A.; Waddell, L.; Parker, S.; Harris, J.; Roberts, K.C.; Kydd, R.; Greig, J.; Baynton, A. Prevalence of zoonotic or potentially zoonotic bacteria, antimicrobial resistance, and somatic cell counts in organic dairy production: Current knowledge and research gaps. Foodborne Pathog. Dis. 2009, 6, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Mondragón, A.; Vázquez, B.I.; Fente, C.A.; Cepeda, A.; Franco, C.M. Influence of farming methods on microbiological contamination and prevalence of resistance to antimicrobial drugs in isolates from beef. Meat Sci. 2009, 82, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Bennedsgaard, T.; Barlett, P.; Erskine, R.; Kaneene, J. Comparison of antimicrobial susceptibility of Staphylococcus aureus isolated from bulk tank milk in organic and conventional dairy herds in the midwestern United States and Denmark. J. Food Protect. 2004, 67, 1104–1110. [Google Scholar]
- DANMAP 2012—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: http://www.danmap.org/Downloads/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202012/Danmap_2012.ashx (accessed on 16 July 2015).
- Lanz, R.; Kuhnert, P.; Boerlin, P. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 2003, 91, 73–84. [Google Scholar] [CrossRef]
- Schwaiger, K.; Hölzel, C.; Bauer, J. Resistance gene patterns of tetracycline resistant Escherichia coli of human and porcine origin. Vet. Microbiol. 2010, 142, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jouini, A.; Ben Slama, K.; Saenz, Y.; Klibi, N.; Costa, D.; Vinue, L.; Zarazaga, M.; Boudabous, A.; Torres, C. Detection of multiple-antimicrobial resistance and characterization of the implicated genes in Escherichia coli isolates from foods of animal origin in Tunis. J. Food Protect 2009, 72, 1082–1088. [Google Scholar]
- Sengelov, G.; Halling-Sorensen, B.; Aarestrup, F. Susceptibility of Escherichia coli and Enterococcus faecium isolated from pigs and broiler chickens to tetracycline degradation products and distribution of tetracycline resistance determinants in E. coli from food animals. Vet. Microbiol. 2003, 95, 91–101. [Google Scholar] [CrossRef]
- Fan, W.; Hamilton, T.; Webster-Sesay, S.; Nikolich, M.P.; Lindler, L.E. Multiplex real-time SYBR Green IPCR assay for detection of tetracycline efflux genes of Gram-negative bacteria. Mol. Cell. Probes 2007, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Guarddon, M.; Miranda, J.M.; Vázquez, B.I.; Cepeda, A.; Franco, C.M. Direct quantification and distribution of tetracycline-resistant genes in meat samples by real-time polymerase chain reaction. J. Food Sci. 2012, 77, M372–M376. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Nielsen, K. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Agerso, Y.; Wulff, G.; Vaclavik, E.; Halling Sorensen, B.; Jensen, L. Effect of tetracycline residues in pig manure slurry on tetracycline-resistant bacteria and resistance gene tet(M) in soil microcosms. Environ. Int. 2006, 32, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Vidaver, A. Uses of antimicrobials in plant agriculture. Clin. Infect. Dis. 2002, 34, S107–S110. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Casewell, M.; Cox, T.; de Groot, B.; Friis, C.; Jones, R.; Nigtingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Khachatourians, G. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can. Med. Assoc. J. 1998, 159, 1129–1136. [Google Scholar]
- McGowan, E. Comment on “Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado”. Environ. Sci. Technol. 2007, 41, 2651–2652. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–72.
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev Sci. Tech. 2012, 31, 199–210. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarddon, M.; Miranda, J.M.; Vázquez, B.I.; Cepeda, A.; Franco, C.M. Assessment of Tetracyclines Residues and Tetracycline Resistant Bacteria in Conventional and Organic Baby Foods. Foods 2015, 4, 306-317. https://doi.org/10.3390/foods4030306
Guarddon M, Miranda JM, Vázquez BI, Cepeda A, Franco CM. Assessment of Tetracyclines Residues and Tetracycline Resistant Bacteria in Conventional and Organic Baby Foods. Foods. 2015; 4(3):306-317. https://doi.org/10.3390/foods4030306
Chicago/Turabian StyleGuarddon, Mónica, José M. Miranda, Beatriz I. Vázquez, Alberto Cepeda, and Carlos M. Franco. 2015. "Assessment of Tetracyclines Residues and Tetracycline Resistant Bacteria in Conventional and Organic Baby Foods" Foods 4, no. 3: 306-317. https://doi.org/10.3390/foods4030306
APA StyleGuarddon, M., Miranda, J. M., Vázquez, B. I., Cepeda, A., & Franco, C. M. (2015). Assessment of Tetracyclines Residues and Tetracycline Resistant Bacteria in Conventional and Organic Baby Foods. Foods, 4(3), 306-317. https://doi.org/10.3390/foods4030306










