Characterization and Validation of the Antibacterial Activity of Heyndrickxia coagulans BHE26 Against Helicobacter pylori
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. In Vitro Anti-Bacterial Activity of H. coagulans BHE26
2.3. Characterization of Antimicrobial Compounds from H. coagulans BHE26
2.4. Tolerance to Simulated Gastrointestinal Conditions
2.5. The Agglutination Activity of H. coagulans BHE26
2.5.1. Hydrophobicity
2.5.2. Auto-Aggregation
2.5.3. Co-Aggregation
2.6. Antibiotic Susceptibility and Safety Assessment
2.7. Effect of H. coagulans BHE26 on H. pylori Infected Mice
2.7.1. Animals and Treatments
- •
- Con group—gavaged daily with sterile water (0.2 mL/day);
- •
- Hp_0 group—infected with H. pylori and gavaged daily with sterile water (0.2 mL/day);
- •
- Hp_GMRS group—infected with H. pylori and gavaged daily with 0.2 mL of GMRS medium;
- •
- Hp_E26 group—infected with H. pylori and treated daily with H. coagulans BHE26 suspension (5 × 108 CFU/mL, 0.2 mL/day);
- •
- Hp_E26_FS group—infected with H. pylori and treated daily with 24 h fermented supernatant of H. coagulans BHE26 (0.2 mL/day).
- •
- Con group—gavaged with sterile water throughout the experiment;
- •
- Hp_1 group—gavaged with sterile water for 3 weeks prior to H. pylori infection and continued during infection;
- •
- E26_Hp group—gavaged with H. coagulans BHE26 (5 × 108 CFU/mL, 0.2 mL/day) for 3 weeks before H. pylori challenge and continuously treated during infection;
- •
- E26 group—gavaged with H. coagulans BHE26 (5 × 108 CFU/mL, 0.2 mL/day) for 4 weeks without H. pylori challenge.
2.7.2. Urease Activity
2.7.3. Histopathological Analysis of Gastric Tissue and Measurement of Anti-Bacterial-IgG Antibodies in Mouse Serum
2.7.4. Analysis of Gastric Tissue Microbiota
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anti-Bacterial Activity of H. coagulans BHE26
3.2. Analysis of Antimicrobial Components in the CFC
3.3. Tolerance to Simulated Gastrointestinal Conditions
3.4. The Agglutination Activity of H. coagulans BHE26
3.5. Antibiotic Susceptibility Testing and Safety Evaluation
3.6. Effect of H. coagulans BHE26 on H. pylori-Infected Mice
3.6.1. Urease Activity and W–S Silver Staining Analyses
3.6.2. Measurement of Anti-Bacterial-IgG Antibodies in Mouse Serum
3.6.3. H. coagulans BHE26 Inhibits H. pylori Infection In Vivo
3.7. Bioinformatic Analysis of Gastric Microbiota
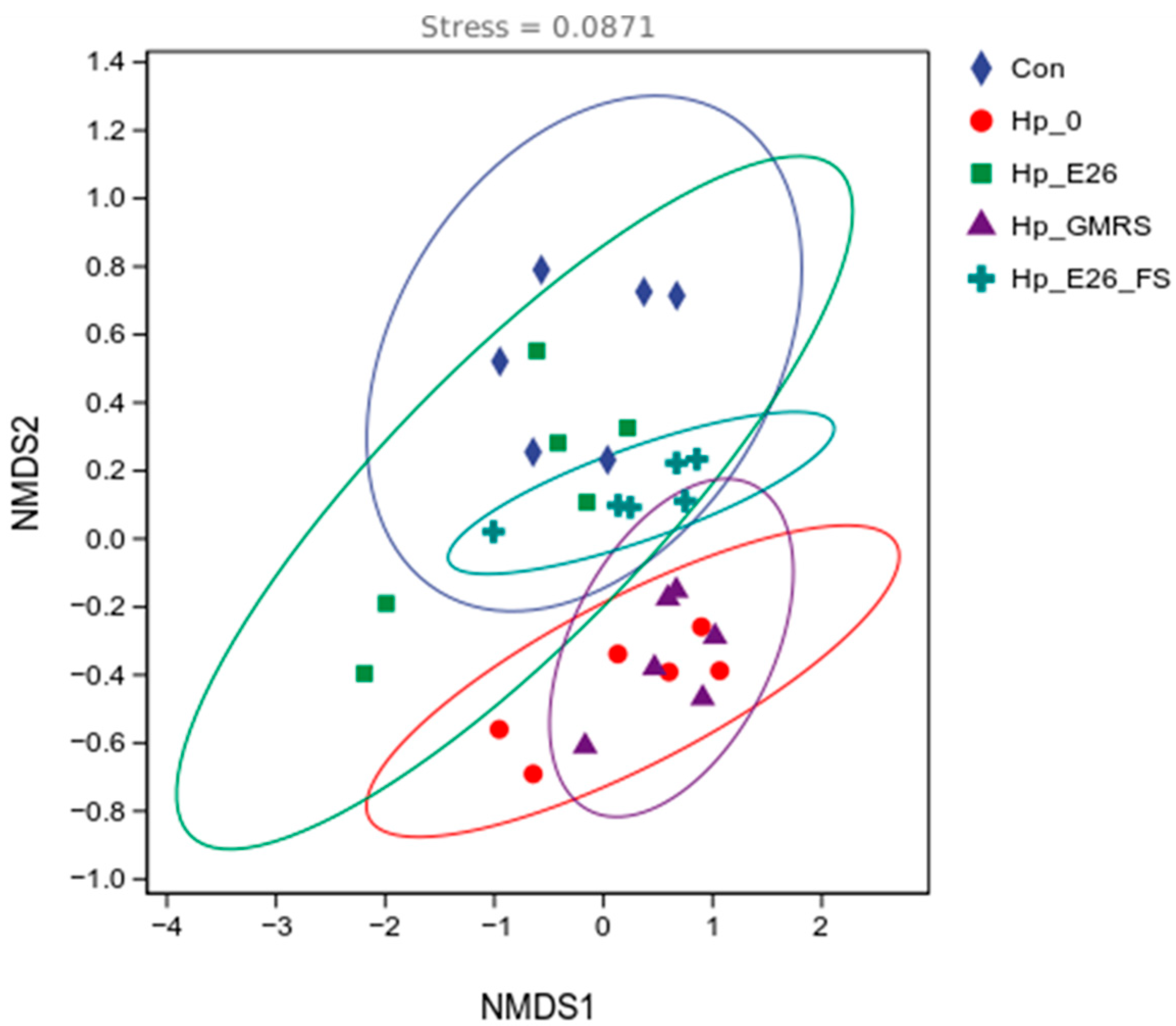
3.7.1. Beta Diversity Analysis
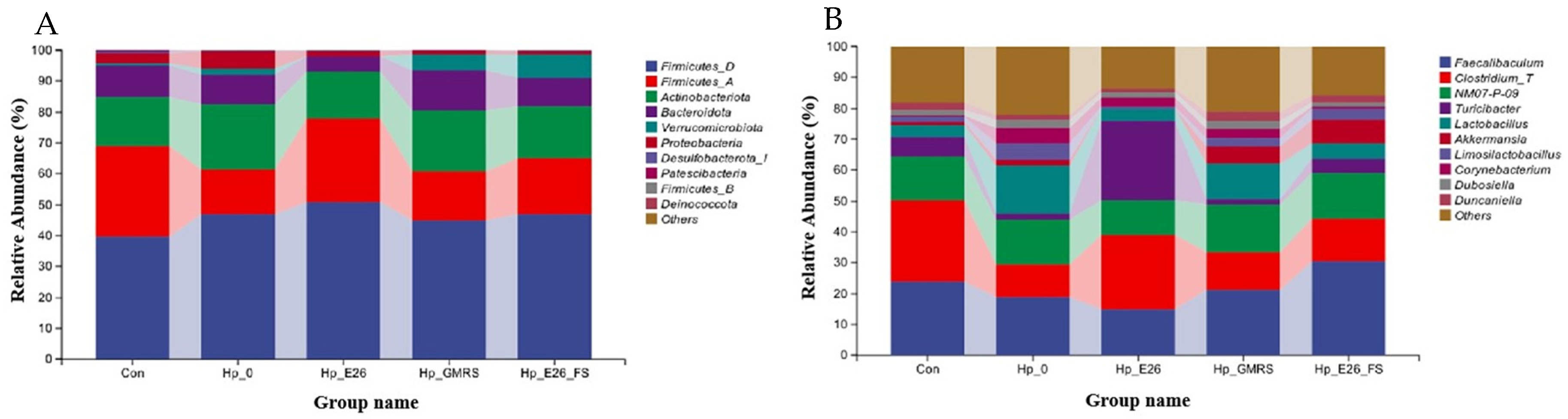
3.7.2. Phylum Level and Genus Level Species Analysis
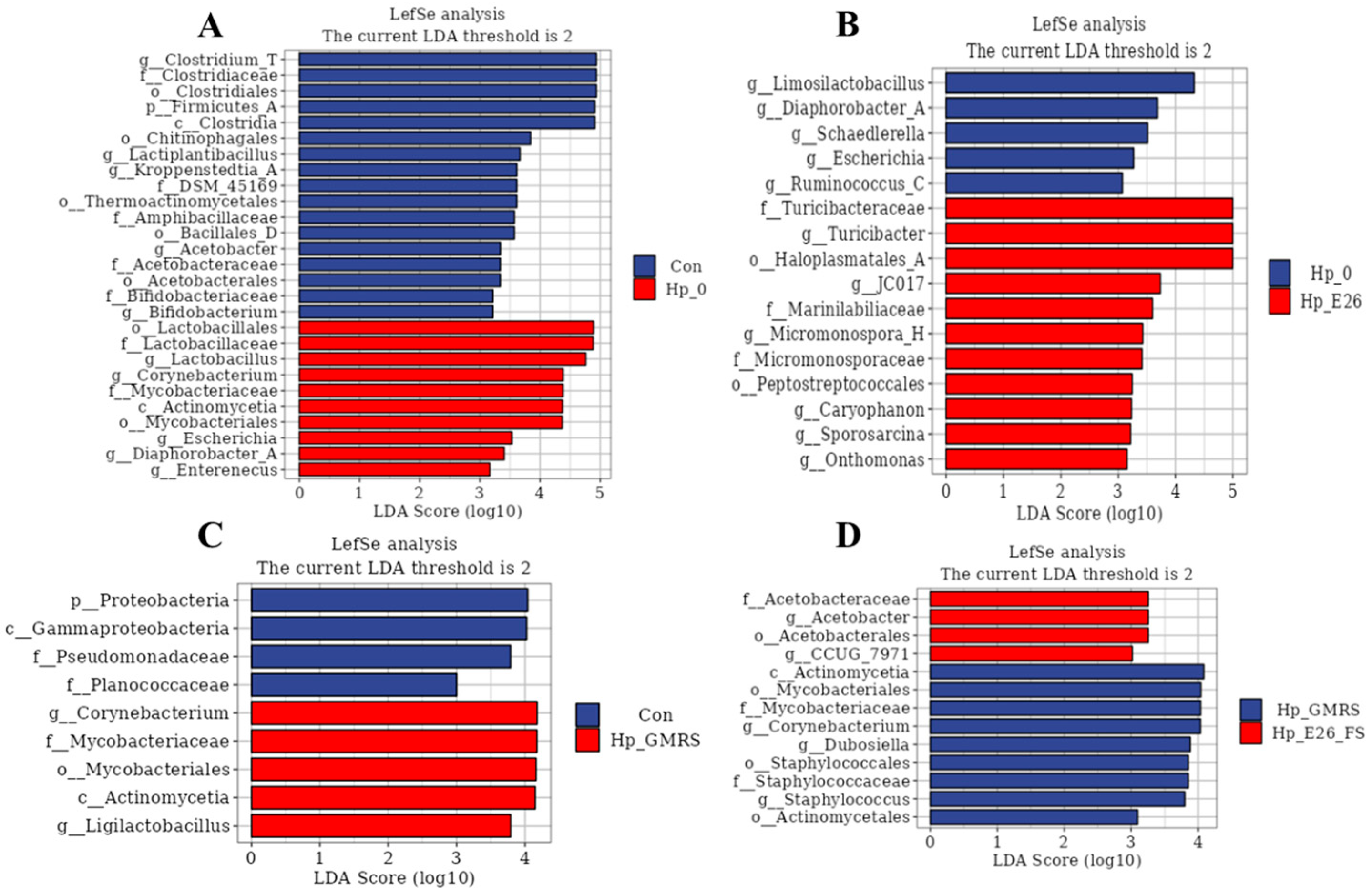
3.7.3. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ruan, X.; Hang, X.; Heng, D.; Cai, C.; Zeng, L.; Zhang, G.; Zhou, L.; Bi, H.; Zhang, L. Antagonist targeting the species-specific fatty acid dehydrogenase/isomerase fabx for anti-H. pylori infection. Adv. Sci. 2025, 12, e2414844. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, Z.; Li, W.; Deng, X.; Yu, L.; Yang, J.; Liu, J.; Cheng, Y.; Huang, W.; Guo, X.; et al. Weizmannia coagulans BCF-01: A novel gastrogenic probiotic for Helicobacter pylori infection control. Gut Microbes 2024, 16, 2313770. [Google Scholar] [CrossRef] [PubMed]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef]
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24, e12653. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, F.; Huang, H.; Xu, X.; Guan, Q.; Xie, M.; Xiong, T. Characterization, mechanism and in vivo validation of Helicobacter pylori antagonism by probiotics screened from infants’ feces and oral cavity. Food Funct. 2024, 15, 1170–1190. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, S.; Wang, C.; Bai, Z.; Tian, P.; Tie, S.; Wang, Y.; Zhang, Y.; Zhao, L.; Gu, S. Weizmannia coagulans BC99 modulate gut microbiota after Helicobacter pylori eradication: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2025, 125, 106681. [Google Scholar] [CrossRef]
- Liou, J.M.; Lee, Y.C.; Wu, M.S. Treatment of Helicobacter pylori infection and its long-term impacts on gut microbiota. J. Gastroenterol. Hepatol. 2020, 35, 1107–1116. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, H.; Ma, X.; Li, Y.; Yang, H.; Li, H.; Su, J.; Zhang, C.; Huang, L. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with Rhizoma Zingiber officinale (Ginger) extract. Food Funct. 2020, 11, 10839–10851. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; Miao, J.; Zhang, Z.; Zheng, L.; Li, F.; Zhou, S.; Zhang, Z.; Li, S.; Liu, H.; et al. Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: A review. Front Microbiol. 2024, 14, 1330029. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhu, M.; He, Y.; Wang, T.; Tian, D.; Shu, J. The impacts of probiotics in eradication therapy of Helicobacter pylori. Arch. Microbiol. 2022, 204, 692. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, C.; Jia, P.; Yan, Y.; Xu, J.; Zhang, J.; Bai, N.; Chen, W.; Gao, W. Effect of Clostridium butyricum and Bacillus coagulans on fecal and serum metabolic profiles in Helicobacter pylori-infected mice. J. Funct. Foods 2025, 130, 106938. [Google Scholar] [CrossRef]
- Do, A.D.; Su, C.H.; Hsu, Y.M. Antagonistic activities of Lactobacillus rhamnosus JB3 against Helicobacter pylori infection through lipid raft formation. Front. Immunol. 2022, 12, 796177. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Nair, A.S.; Dubhashi, A.V. In-vitro transit tolerance of probiotic Bacillus species in human gastrointestinal tract. Int. J. Sci. Res. 2016, 5, 1899–1902. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential use of Bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Maresca, E.; Aulitto, M.; Contursi, P. Harnessing the dual nature of Bacillus (Weizmannia) coagulans for Sustainable production of biomaterials and development of functional food. Microb. Biotechnol. 2024, 17, e14449. [Google Scholar] [CrossRef]
- Jang, Y.J.; Moon, J.S.; Kim, J.E.; Kim, D.; Choi, H.S.; Oh, I. Blending three probiotics alleviates loperamide-induced constipation in sprague-dawley (sd)-rats. Food Sci. Anim. Resour. 2024, 44, 119–131. [Google Scholar] [CrossRef]
- Fijan, S.; Fijan, T.; Connil, N. Overview of probiotic strains of Weizmannia coagulans, previously known as bacillus coagulans, as food supplements and their use in human health. Appl. Microbiol. 2023, 3, 935–947. [Google Scholar] [CrossRef]
- Fan, Q.; Gao, Y.; Zhou, Y.; Wu, J.; Wang, H.; Dong, Y.; Gai, Z.; Wu, Y.; Fang, S.; Gu, S. Weizmannia coagulans BC99 relieves constipation symptoms by regulating inflammatory, neurotransmitter, and lipid metabolic pathways: A randomized, double-blind, placebo-controlled trial. Foods 2025, 14, 654. [Google Scholar] [CrossRef]
- Li, C.; Zhai, S.; Duan, M.; Cao, L.; Zhang, J.; Wang, Y.; Wu, Y.; Gu, S. Weizmannia coagulans BC99 enhances intestinal barrier function by modulating butyrate formation to alleviate acute alcohol intoxication in rats. Nutrients 2024, 16, 4142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Z.; Zhang, Z.; Liu, T.; Fan, Y.; Liu, T.; Peng, N. Bacillus coagulans in combination with chitooligosaccharides regulates gut microbiota and ameliorates the DSS-induced colitis in mice. Microbiol. Spectrum. 2022, 10, e0064122. [Google Scholar] [CrossRef]
- Chen, X.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.P.; Zhang, H.; Chen, W. In vitro screening of lactobacilli with antagonistic activity against Helicobacter pylori from traditionally fermented foods. J. Dairy Sci. 2010, 93, 5627–5634. [Google Scholar] [CrossRef]
- Ding, C.H.; Fan, X.; Gao, J.; Zheng, D.G.; Du, L.G.; Hao, Z.H.; Ma, Y.X.; Wang, S.S. Screening of Haiendrix coagulans with antagonistic effect on Helicobacter pylori and its application in fermented milk. J. Henan Univ. Technol. Nat. Sci. Ed. 2025, 46, 81–89. [Google Scholar] [CrossRef]
- Paucar-Carrión, C.; Espinoza-Monje, M.; Gutiérrez-Zamorano, C.; Sánchez-Alonzo, K.; Carvajal, R.I.; Rogel-Castillo, C.; Sáez-Carrillo, K.; García-Cancino, A. Incorporation of Limosilactobacillus fermentum UCO-979C with anti-Helicobacter pylori and immunomodulatory activities in various ice cream bases. Foods 2022, 11, 333. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sun, L.; Man, C.; Jiang, Y. Evaluation of gastrointestinal tolerance and regulation of immune-related genes of Lactobacillus reuteri J1 in vitro. Sci. Technol. Food Ind. 2019, 40, 101–106,113. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Noriega, L.; Gueimonde, M.; Sánchez, B.; Margolles, A.; de los Reyes-Gavilán, C.G. Effect of the adaptation to high bile salts concentrations on glycosidic activity, survival at low pH and cross-resistance to bile salts in Bifidobacterium. Int. J. Food Microbiol. 2004, 94, 79–86. [Google Scholar] [CrossRef]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of Lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 Cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef]
- Huang, H.; Peng, F.; Li, J.Y.; Liu, Z.G.; Xie, M.Y.; Xiong, T. Isolation and characteristics of lactic acid bacteria with antibacterial activity against Helicobacter pylori. Food Biosci. 2021, 44, 101446. [Google Scholar] [CrossRef]
- Sutton, P.; Wilson, J.; Lee, A. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 2000, 18, 2677–2685. [Google Scholar] [CrossRef]
- Li, M.; Shao, D.; Zhou, J.; Gu, J.; Qin, J.; Chen, W.; Wei, W. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2020, 32, 755–767. [Google Scholar] [CrossRef]
- Soliman, N.S.; Soliman, M.S.; Elhossary, W.; El-Kholy, A.A. Analysis of gastric mucosa-associated microbiota in functional dyspepsia using 16S rRNA gene next-generation sequencing. BMC Microbiol. 2025, 25, 386. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Faas, M.M.; de Vos, P. Disease managing capacities and mechanisms of host effects of lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2021, 61, 1365–1393. [Google Scholar] [CrossRef]
- Chornchoem, P.; Tandhavanant, S.; Saiprom, N.; Preechanukul, A.; Thongchompoo, N.; Sensorn, I.; Chantratita, W.; Chantratita, N. Metagenomic evaluation, antimicrobial activities, and immune stimulation of probiotics from dietary supplements and dairy products. Sci. Rep. 2025, 15, 11537. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Y.; Ma, Y.; Li, J.; Li, J.; Huo, Z.; Yang, P.; Zhang, C. Screening probiotics for Anti-Helicobacter pylori and investigating the effect of probiotics on patients with Helicobacter pylori Infection. Foods 2024, 13, 1851. [Google Scholar] [CrossRef]
- Sornsenee, P.; Surachat, K.; Wong, T.; Kaewdech, A.; Saki, M.; Romyasamit, C. Lyophilized cell-free supernatants of Limosilactobacillus fermentum T0701 exhibited antibacterial activity against Helicobacter pylori. Sci. Rep. 2024, 14, 13632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.X.; Fang, H.Y.; Yang, H.B.; Tien, N.Y.; Wang, M.C.; Wu, J.J. Lactobacillus pentosus strain LPS16 produces lactic acid, inhibiting multidrug-resistant Helicobacter pylori. J. Microbiol. Immunol. Infect. 2016, 49, 168–174. [Google Scholar] [CrossRef]
- Gao, F.; Sui, L.; Mu, G.; Zhu, X.; Qian, F. Screening of potential probiotics with anti-Helicobacter pylori activity from infant feces through principal component analysis. Food Biosci. 2021, 42, 101045. [Google Scholar] [CrossRef]
- Hyronimus, B.; Le Marrec, C.; Sassi, A.H.; Deschamps, A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef]
- Nithya, V.; Halami, P.M. Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann. Microbiol. 2013, 63, 129–137. [Google Scholar] [CrossRef]
- Chantanawilas, P.; Pahumunto, N.; Teanpaisan, R. Aggregation and adhesion ability of various probiotic strains and Candida species: An in vitro study. J. Dent. Sci. 2024, 19, 2163–2171. [Google Scholar] [CrossRef]
- Tuo, Y.F.; Yu, H.L.; Ai, L.Z.; Wu, Z.J.; Guo, B.H.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef]
- Juntarachot, N.; Sunpaweravong, S.; Kaewdech, A.; Wongsuwanlert, M.; Ruangsri, P.; Pahumunto, N.; Teanpaisan, R. Characterization of adhesion, anti-adhesion, co-aggregation, and hydrophobicity of Helicobacter pylori and probiotic strains. J. Taibah Univ. Med. Sci. 2023, 18, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Holz, C.; Busjahn, A.; Mehling, H.; Arya, S.; Boettner, M.; Habibi, H.; Lang, C. Significant reduction in Helicobacter pylori load in humans with non-viable Lactobacillus reuteri DSM17648: A pilot study. Probiotics Antimicrob. Proteins 2014, 7, 91–100. [Google Scholar] [CrossRef]
- Ismail, N.I.; Nawawi, K.N.M.; Hsin, D.C.C.; Hao, K.W.; Mahmood, N.R.K.N.; Chearn, G.L.C.; Wong, Z.; Tamil, A.M.; Joseph, H.; Raja Ali, R.A. Probiotic containing Limosilactobacillus reuteri DSM 17648 as an adjunct treatment for Helicobacter pylori infection: A randomized, double-blind, placebo-controlled trial. Helicobacter 2023, 28, e13017. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Teng, D.; Mao, R.Y.; Hao, Y.; Wang, X.M.; Wang, J.H. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Statement on how to interpret the QPS qualification on ‘acquired antimicrobial resistance genes’. EFSA J. 2023, 21, e08323. [Google Scholar] [CrossRef]
- Heo, G.; Kong, H.; Kim, N.; Lee, S.; Sul, S.; Jeong, D.-W.; Lee, J.-H. Antibiotic susceptibility of Bacillus velezensis. FEMS Microbiol. Lett. 2022, 369, fnac017. [Google Scholar] [CrossRef] [PubMed]
- Auclair-Ouellet, N.; Tremblay, A.; Kassem, O.; Caballero-Calero, S.E.; Bronner, S.; Binda, S. Probiotics as adjuvants to standard Helicobacter pylori treatment: Evidence for the use of Lacidofil®, an established blend of thoroughly characterized strains. Microorganisms 2025, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- Shadvar, N.; Akrami, S.; Mousavi Sagharchi, S.-M.-A.; Askandar, R.H.; Merati, A.; Aghayari, M.; Kaviani, N.; Afkhami, H.; Kashfi, M. A review for non-antibiotic treatment of Helicobacter pylori: New insight. Front. Microbiol. 2024, 15, 1379209. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Wu, L.Y.; Sun, X.; Gu, Q.; Zhou, Q.Q. Effect of Lactobacillus plantarum ZFM4 in Helicobacter pylori-infected C57BL/6 mice: Prevention is better than cure. Front. Cell. Infect. Microbiol. 2024, 13, 1320819. [Google Scholar] [CrossRef]
- Yu, Z.H.; Cao, M.; Peng, J.S.; Wu, D.Y.; Li, S.; Wu, C.M.; Qing, L.T.; Zhang, A.D.; Wang, W.J.; Huang, M.; et al. Lacticaseibacillus casei T1 attenuates Helicobacter pylori-induced inflammation and gut microbiota disorders in mice. BMC Microbiol. 2023, 23, 39. [Google Scholar] [CrossRef]
- Dong, Y.; Han, M.; Qi, Y.M.; Wu, Y.; Zhou, Z.P.; Jiang, D.C.; Gai, Z.H. Enhancement of host defense against Helicobacter pylori infection through modulation of the gastrointestinal microenvironment by Lactiplantibacillus plantarum Lp05. Front. Immunol. 2025, 15, 1469885. [Google Scholar] [CrossRef]
- Hong, S.S.; Lee, H.A.; Kim, J.Y.; Jeong, J.W.; Shim, J.J.; Lee, J.L.; Sim, J.H.; Chung, Y.; Kim, O. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus paracasei HP7. Lab. Anim. Res. 2018, 34, 216–222. [Google Scholar] [CrossRef]
- Sgouras, D.; Maragkoudakis, P.; Petraki, K.; Martinez-Gonzalez, B.; Eriotou, E.; Michopoulos, S.; Kalantzopoulos, G.; Tsakalidou, E.; Mentis, A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 2004, 70, 518–526. [Google Scholar] [CrossRef]
- Shen, S.Q.; Ren, F.F.; Qin, H.M.; Bukhari, I.; Yang, J.; Gao, D.F.; Ouwehand, A.C.; Lehtinen, M.J.; Zheng, P.Y.; Mi, Y. Lactobacillus acidophilus NCFM and Lactiplantibacillus plantarum Lp-115 inhibit Helicobacter pylori colonization and gastric inflammation in a murine model. Front. Cell. Infect. Microbiol. 2023, 13, 1196084. [Google Scholar] [CrossRef]
- He, C.; Peng, C.; Xu, X.B.; Li, N.S.; Ouyang, Y.; Zhu, Y.; Lu, N.H. Probiotics mitigate Helicobacter pylori-induced gastric inflammation and premalignant lesions in INS-GAS mice with the modulation of gastrointestinal microbiota. Helicobacter 2022, 27, e12898. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterol. 2021, 21, 388. [Google Scholar] [CrossRef]
- Verma, J.; Anwar, M.T.; Linz, B.; Backert, S.; Pachathundikandi, S.K. The Influence of Gastric Microbiota and Probiotics in Helicobacter pylori Infection and Associated Diseases. Biomedicines 2025, 13, 61. [Google Scholar] [CrossRef]
- Bai, X.-F.; Tian, D.; Wang, T.-Y.; Shu, J.-C.; He, Y.-J.; Zhu, M.-J. The impact of probiotics on gut microbiota in the eradication of Helicobacter pylori infection: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6736–6743. [Google Scholar] [PubMed]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Chen, Z.Q.; Zhou, Q.Q.; Li, P.; Wu, S.Y.; Zhou, T.; Gu, Q. Exopolysaccharide from Lacticaseibacillus paracasei alleviates gastritis in Helicobacter pylori-infected mice by regulating gastric microbiota. Front. Nutr. 2024, 11, 1426358. [Google Scholar] [CrossRef] [PubMed]




| Strains | Fermentation Broth (mm) | Fermentation Supernatant (mm) |
|---|---|---|
| Negative control (GMRS) | 6.00 ± 0.00 c | 6.00 ± 0.00 c |
| Lactobacillus plantarum CN2018 | 14.29 ± 0.23 a | 12.44 ± 0.59 b |
| H. coagulans BHE26 | 13.19 ± 0.46 b | 13.76 ± 0.22 a |
| Types of Antibiotic | Disc Content (µg) | Diameter of Inhibition Zone (mm) | Susceptibility | ||
|---|---|---|---|---|---|
| Resistant (R) | Intermediate (I) | Susceptible (S) | H. coagulans BHE26 | ||
| Penicillin | 10 | ≤14 | - | ≥18 | S |
| Ampicillin | 10 | ≤13 | 14~16 | ≥17 | S |
| Erythromycin | 15 | ≤13 | 14~22 | ≥23 | S |
| Gentamicin | 10 | ≤12 | 13~14 | ≥15 | S |
| Ciprofloxacin | 5 | ≤12 | 13~18 | ≥18 | S |
| Ceftriaxone | 30 | ≤11 | 12~16 | ≥17 | R |
| Lincomycin | 2 | ≤10 | 11~15 | ≥16 | S |
| Tetracycline | 30 | ≤10 | 11~15 | ≥16 | S |
| Chloramphenicol | 30 | ≤10 | 11~15 | ≥16 | S |
| Trimethoprim-Sulfamethoxazole | 25 | ≤10 | 11~15 | ≥16 | S |
| Strains | Hemolytic Activity |
|---|---|
| H. coagulans BHE26 | γ |
| S. aureus | β |
| Group | Urease Activity (Positive/Total) | W-S Silver Staining (Positive/Total) | Group | Urease Activity (Positive/Total) | W-S Silver Staining (Positive/Total) |
|---|---|---|---|---|---|
| Con | 0/5 | 0/5 | Con | 0/6 | 0/6 |
| Hp_0 | 8/10 | 9/10 | Hp_1 | 6/6 | 6/6 |
| Hp_E26 | 4/10 | 4/10 | E26_Hp | 2/12 | 3/12 |
| Hp_GMRS | 8/10 | 8/10 | E26 | 0/12 | - |
| Hp_E26_FS | 3/10 | 5/10 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, N.; Ding, C.; Gao, J.; Du, L.; Zheng, D.; Hao, Z.; Ren, Z.; Lou, H. Characterization and Validation of the Antibacterial Activity of Heyndrickxia coagulans BHE26 Against Helicobacter pylori. Foods 2026, 15, 131. https://doi.org/10.3390/foods15010131
Wang N, Ding C, Gao J, Du L, Zheng D, Hao Z, Ren Z, Lou H. Characterization and Validation of the Antibacterial Activity of Heyndrickxia coagulans BHE26 Against Helicobacter pylori. Foods. 2026; 15(1):131. https://doi.org/10.3390/foods15010131
Chicago/Turabian StyleWang, Nannan, Changhe Ding, Jun Gao, Lingguang Du, Dongge Zheng, Zhihui Hao, Zhuoran Ren, and Haiwei Lou. 2026. "Characterization and Validation of the Antibacterial Activity of Heyndrickxia coagulans BHE26 Against Helicobacter pylori" Foods 15, no. 1: 131. https://doi.org/10.3390/foods15010131
APA StyleWang, N., Ding, C., Gao, J., Du, L., Zheng, D., Hao, Z., Ren, Z., & Lou, H. (2026). Characterization and Validation of the Antibacterial Activity of Heyndrickxia coagulans BHE26 Against Helicobacter pylori. Foods, 15(1), 131. https://doi.org/10.3390/foods15010131






