Influence of Traditional Vanilla Curing on Its Physicochemical Properties and Aromatic Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Vanilla Samples
2.2. Physicochemical Analysis
2.3. Ethical Aproval
2.4. Sensory Evaluation Test
2.4.1. Generation of Descriptors
2.4.2. Participants
2.4.3. Liking Level Data and RATA Questions
2.5. Data Analysis
2.5.1. Physicochemical Data
2.5.2. Liking Level Data and RATA Questions
3. Results
3.1. Physicochemical Analysi Results
3.2. Sensory Evaluation Results
3.2.1. Comparison of the Level of Liking Between Samples
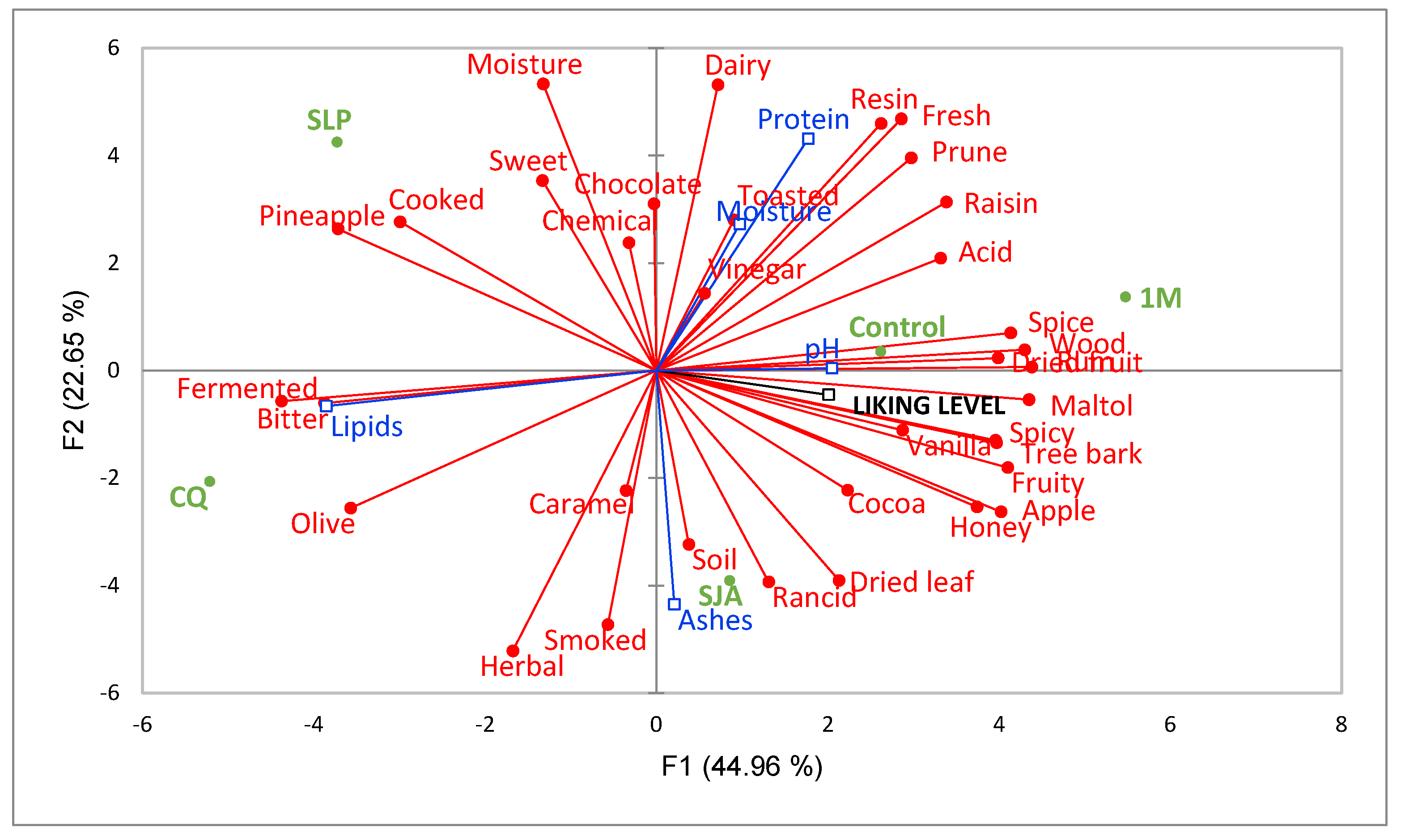
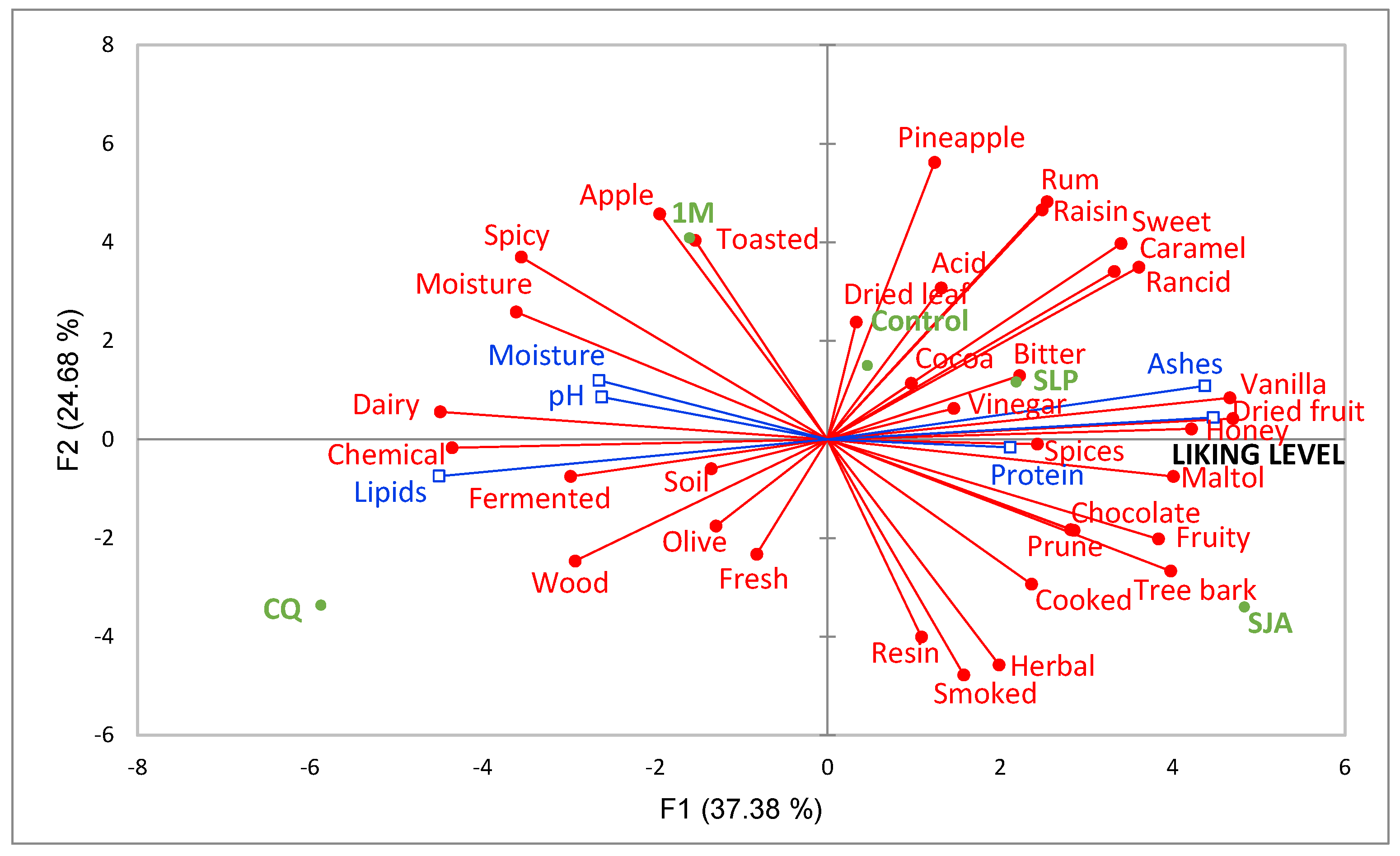
3.2.2. Rate-All-That-Apply (RATA) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Aromatic Descriptor | Frequency (Total) | |
|---|---|---|
| 1 | Wood | 515 |
| 2 | Raisins | 384 |
| 3 | Rum | 239 |
| 4 | Prune | 429 |
| 5 | Apple | 99 |
| 6 | Toasted | 306 |
| 7 | Vinegar | 159 |
| 8 | Dairy | 85 |
| 9 | Herbal | 241 |
| 10 | Rancid | 166 |
| 11 | Bitter | 197 |
| 12 | Sweet | 556 |
| 13 | Spicy | 369 |
| 14 | Vanilla | 747 |
| 15 | Chemical | 177 |
| 16 | Smoked | 254 |
| 17 | Fresh | 134 |
| 18 | Honey | 303 |
| 19 | Cocoa | 287 |
| 20 | Chocolate | 363 |
| 21 | Moisture | 243 |
| 22 | Dried Fruit | 324 |
| 23 | Fermented | 318 |
| 24 | Pineapple | 96 |
| 25 | Soil | 243 |
| 26 | Olive | 168 |
| 27 | Tree Bark | 365 |
| 28 | Acid | 140 |
| 29 | Dried Leaf | 325 |
| 30 | Maltol | 192 |
| 31 | Spicy | 115 |
| 32 | Resin | 210 |
| 33 | Fruity | 252 |
| 34 | Cooked | 87 |
| 35 | Caramel | 421 |
References
- Gallage, N.J.; Møller, B.L. Vanillin–Bioconversion and Bioengineering of the Most Popular Plant Flavor and Its De Novo Biosynthesis in the Vanilla Orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, N.; Masor, N.F.; Zolkeflee, P.; Zakaria, N.S.; Abdul Wahab, N.H.; Asari, A.; Aziz, A.N. Qualitative Phytochemical Analysis, Enzymatic and Non-Enzymatic Antioxidant Activities in Stems and Leaves of Vanilla planifolia (Orchidaceae). Food Res. 2023, 7, 165–172. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.Á. Vanilla (Vanilla planifolia Andr.), Its Residues and Other Industrial by-Products for Recovering High Value Flavor Molecules: A Review. J. Appl. Res. Med. Aromat. Plants 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Diaz-Bautista, M. Quantification of Vanillin in Fruits of Vanilla planifolia by High-Resolution Liquid Chromatography. Lett. Appl. NanoBioSci. 2022, 12, 15. [Google Scholar] [CrossRef]
- Rivero-Angeles, K.D.; Buitimea-Cantúa, G.V.; Dávila-Ortiz, G.; López-Villegas, E.O.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.; Téllez-Medina, D.I. Microstructural Changes in Vanilla planifolia Beans after Using High-Hydrostatic-Pressure Treatment in the Curing Process. Foods 2024, 13, 177. [Google Scholar] [CrossRef]
- Mariezcurrena, M.D.; Zavaleta, H.A.; Waliszewski, K.N.; Sánchez, V. The Effect of Killing Conditions on the Structural Changes in Vanilla (Vanilla planifolia, Andrews) Pods during the Curing Process. Int. J. Food Sci. Tech. 2008, 43, 1452–1457. [Google Scholar] [CrossRef]
- Odoux, E.; Brillouet, J.-M. Anatomy, Histochemistry and Biochemistry of Glucovanillin, Oleoresin and Mucilage Accumulation Sites in Green Mature Vanilla Pod (Vanilla planifolia; Orchidaceae): A Comprehensive and Critical Reexamination. Fruits 2009, 64, 221–241. [Google Scholar] [CrossRef]
- Havkin-Frenkel, D.; French, J.C.; Graft, N.M.; Pak, F.E.; Frenkel, C.; Joel, D.M. Interrelation of Curing and Botany in Vanilla (Vanilla Planifolia) Bean. Acta Hortic. 2004, 629, 93–102. [Google Scholar] [CrossRef]
- Manyatsi, T.S.; Lin, Y.-H.; Jou, Y.-T. The Isolation and Identification of Bacillus Velezensis ZN-S10 from Vanilla (V. planifolia), and the Microbial Distribution after the Curing Process. Sci. Rep. 2024, 14, 16339. [Google Scholar] [CrossRef]
- Peña-Barrientos, A.; Perea-Flores, M.D.J.; Martínez-Gutiérrez, H.; Patrón-Soberano, O.A.; González-Jiménez, F.E.; Vega-Cuellar, M.Á.; Dávila-Ortiz, G. Physicochemical, Microbiological, and Structural Relationship of Vanilla Beans (Vanilla planifolia, Andrews) during Traditional Curing Process and Use of Its Waste. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100445. [Google Scholar] [CrossRef]
- Hutchings, S.C.; Deb-Choudhury, S.; Subbaraj, A.K.; Guerrero, L.; Torrico, D.D.; Ham, E.E.; Realini, C.E. Characterizing the Odor of New Zealand Native Plants Using Sensory Analysis and Gas Chromatography–Mass Spectrometry. J. Food Sci. 2025, 90, e70050. [Google Scholar] [CrossRef] [PubMed]
- Ravier, A.; Chalut, P.; Belarbi, S.; Santerre, C.; Vallet, N.; Nhouchi, Z. Impact of the Post-Harvest Period on the Chemical and Sensorial Properties of Planifolia and Pompona Vanillas. Molecules 2024, 29, 839. [Google Scholar] [CrossRef] [PubMed]
- Cuan-Escobar, T.A.; Cuellar-Sánchez, A.; Gómez-Velázquez, H.D.J.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Gutiérrez-Díaz, I.; Luna-Vital, D.A. Effect of Different Killing Methods during Curing on the Phytochemical and Bacterial Composition of Vanilla planifolia Using Multi-Omic Approaches. Food Chem. X 2025, 26, 102269. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, A.; Budiastra, I.W.; Mardjan, S.; Nurfadila, N. Effects of Curing Method on Quality of Vanilla Pod Using a Greenhouse Effectdryer. Food Res. 2024, 8, 380–384. [Google Scholar] [CrossRef]
- Antonio-Gutiérrez, O.; Solano, R.; Lagunez-Rivera, L. Enhancement of Phenolic Compounds in Vanilla Curing with the Application of UVC Light, Microwaves and Ultrasound. J. Food Sci. Technol. 2024, 61, 2020–2026. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Chávez-Leal, V.; Soto-Caballero, M.C.; Tellez-Medina, D.I.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia). Molecules 2023, 28, 7606. [Google Scholar] [CrossRef]
- Peña-Barrientos, A.; Dávila-Ortiz, G.; Martínez-Gutiérrez, H.; Perea-Flores, M.D.J. Biochemical, Micro and Ultrastructural Changes in Vanilla Pods (Vanilla planifolia, Andrews) during the Curing Process. Plant Physiol. Biochem. 2025, 219, 109377. [Google Scholar] [CrossRef]
- Mahadeo, K.; Taïbi, A.; Meile, J.-C.; Côme, B.; Gauvin-Bialecki, A.; Boubakri, H.; Herrera-Belaroussi, A.; Kodja, H. Exploring Endophytic Bacteria Communities of Vanilla Planifolia. BMC Microbiol. 2024, 24, 218. [Google Scholar] [CrossRef]
- Escobar-Muciño, E.; Luna-Guevara, M.L.; Ramos-Cassellis, M.E.; Amador-Espejo, G.G.; Castañeda-Lucio, M.; Arenas-Hernández, M.M.P. Evaluation of Process Involved in the Production of Aromatic Compounds in Gram-negative Bacteria Isolated from Vanilla (Vanilla planifolia Ex. Andrews) Beans. J. Appl. Microbiol. 2020, 128, 1086–1098. [Google Scholar] [CrossRef]
- Franco, J.L.; Ayres, E.M.M.; De Oliveira, D.; Martins, I.B.A.; Macedo, A.F.; Deliza, R.; Koblitz, M.G.B. Exploring the Potential of the Vanilla Species from the Brazilian Atlantic Forest: Sensory Description and Consumer Acceptance. J. Sens. Stud. 2024, 39, e12896. [Google Scholar] [CrossRef]
- Januszewska, R.; Giret, E.; Clement, F.; Van Leuven, I.; Goncalves, C.; Vladislavleva, E.; Pradal, P.; Nåbo, R.; Landuyt, A.; D’Heer, G.; et al. Impact of Vanilla Origins on Sensory Characteristics of Chocolate. Food Res. Int. 2020, 137, 109313. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.; Domínguez-Soberanes, J.; Licon, C.C.; Estevez-Rioja, A. Sensory Profiles of Cheeses Manufactured in Mexico and the US and the Influence of Judges’ Cultural Context: A Pilot Study. Appl. Sci. 2024, 14, 11980. [Google Scholar] [CrossRef]
- Meyners, M.; Jaeger, S.R.; Ares, G. On the Analysis of Rate-All-That-Apply (RATA) Data. Food Qual. Prefer. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a Rating-Based Variant of Check-All-That-Apply Questions: Rate-All-That-Apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Ares, G. RATA Questions are not Likely to Bias Hedonic Scores. Food Qual. Prefer. 2015, 44, 157–161. [Google Scholar] [CrossRef]
- NMX-F-083-1986l; Alimentos-Determinación de Humedad en Productos Alimenticios. Secretaría de Economía (SE): Mexico City, Mexico, 1986. Available online: https://es.scribd.com/doc/283047993/NMX-F-083-1986 (accessed on 1 April 2025).
- NMX-F-608-NORMEX-2002; Alimentos-Determinación de Proteínas en Alimentos-Método de Prueba. Secretaría de Economía (SE): Mexico City, Mexico, 2012. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5208839&fecha=12/09/2011#gsc.tab=0 (accessed on 1 April 2025).
- NMX-F-615-NORMEX-2004; Alimentos-Determinación de Extracto Etéreo (Método Soxhlet). Secretaría de Economía (SE): Mexico City, Mexico, 2004. Available online: https://www.dof.gob.mx/normasOficiales/7704/seeco12_C/seeco12_C.html#:~:text=615%2DNORMEX%2D2018-,ALIMENTOS%2DDETERMINACI%C3%93N%20DE%20EXTRACTO%20ETEREO%20(M%C3%89TODO%20SOXHLET)%20EN%20ALIMENTOS,615%2DNORMEX%2D2004).&text=La%20presente%20Norma%20Mexicana%20establece,de%20origen%20vegetal%20y%20animal (accessed on 1 April 2025).
- NMX-F-607-NORMEX-2002; Alimentos-Determinación de Cenizas En Alimentos-Métodos de Prueba. Secretaría de Economía (SE): Mexico City, Mexico, 2002. Available online: https://www.dof.gob.mx/nota_detalle.php%3Fcodigo%3D5311757%26fecha%3D27/08/2013 (accessed on 1 April 2025).
- NMX-F-317-S-1978; Alimentos-Determinación de pH En Alimentos-Método de Prueba. Secretaría de Economía (SE): Mexico City, Mexico, 1978. Available online: http://www.economia-nmx.gob.mx/normas/nmx/1978/nmx-f-317-s-1978.pdf (accessed on 1 April 2025).
- Waehrens, S.S.; Zhang, S.; Hedelund, P.I.; Petersen, M.A.; Byrne, D.V. Application of the fast sensory method ‘Rate-All-That-Apply’ in chocolate Quality Control compared with DHS-GC-MS. Int. J. Food Sci. Technol. 2016, 52, 1877–1887. [Google Scholar] [CrossRef]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Desings to Balance the Effect of Order of Presentation and First-Order Carry-over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Van Dyk, S.; McGlasson, W.B.; Williams, M.; Gair, C. Influence of Curing Procedures on Sensory Quality of Vanilla Beans. Fruits 2010, 65, 387–399. [Google Scholar] [CrossRef]
- Liu, Y.; Toro-Gipson, R.S.D.; Drake, M. Sensory Properties and Consumer Acceptance of Ready-to-drink Vanilla Protein Beverages. J. Sens. Stud. 2021, 36, e12704. [Google Scholar] [CrossRef]
- Oppermann, A.K.L.; De Graaf, C.; Scholten, E.; Stieger, M.; Piqueras-Fiszman, B. Comparison of Rate-All-That-Apply (RATA) and Descriptive Sensory Analysis (DA) of Model Double Emulsions with Subtle Perceptual Differences. Food Qual. Prefer. 2017, 56, 55–68. [Google Scholar] [CrossRef]
- Gutiérrez-Méndez, N.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Nevárez-Moorillón, G.V.; Rivera-Chavira, B. Evaluation of Aroma Generation of Lactococcus Lactis with an Electronic Nose and Sensory Analysis. J. Dairy Sci. 2008, 91, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, C.; Collard, F.X.; Bianchini, J.-P.; Raharivelomanana, P. Evaluation of Chemical Variability of Cured Vanilla Beans (Vanilla tahitensis and Vanilla planifolia). Nat. Prod. Commun. 2009, 4, 1934578X0900401016. [Google Scholar] [CrossRef]
- NOM-182-SCFI-2011; Vainilla de Papantla, Extractos y Derivados-Especificaciones, Información Comercial y Métodos de Ensayo (Prueba). Secretaría de Economía (SE): Mexico City, Mexico, 2000. Available online: https://www.dof.gob.mx/normasOficiales/4477/seeco/seeco.htm (accessed on 1 April 2025).
- Sreedhar, R.V.; Venkatachalam, L.; Neelwarne, B. Hyperhydricity-Related Morphologic and Biochemical Changes in Vanilla (Vanilla planifolia). J. Plant Growth Regul. 2009, 28, 46–57. [Google Scholar] [CrossRef]
- Hernández-Hernández, J. Mexican Vanilla Production. In Handbook of Vanilla Science and Technology; Wiley Blackwell: Hoboken, NJ, USA, 2011; pp. 3–24. [Google Scholar]
- Brunschwig, C.; Senger-Emonnot, P.; Aubanel, M.-L.; Pierrat, A.; George, G.; Rochard, S.; Raharivelomanana, P. Odor-active compounds of Tahitian vanilla flavor. Food Res. Int. 2012, 46, 148–157. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Peña-Mojica, E.; Ortega-Galeana, A.; López-Cruz, J.I.; Ledesma-Escobar, C.A.; Rivera-Rivera, M.; Paz-Gamboa, E. Maya Vanilla (Vanilla cribbiana Soto Arenas): A New Species in Commerce. Plants 2025, 14, 300. [Google Scholar] [CrossRef]
- Zhang, S.; Mueller, C. Comparative Analysis of Volatiles in Traditionally Cured Bourbon and Ugandan Vanilla Bean (Vanilla planifolia) Extracts. J. Agric. Food Chem. 2012, 60, 10433–10444. [Google Scholar] [CrossRef]
- Hernández-Fernández, M.Á.; Rojas-Avila, A.; Vazquez-Landaverde, P.A.; Cornejo-Mazón, M.; Dávila-Ortiz, G. Volatile Compounds and Fatty Acids in Oleoresins from Vanilla planifolia Andrews Obtained by Extraction with Supercritical Carbon Dioxide. CyTA J. Food 2019, 17, 419–430. [Google Scholar] [CrossRef]
- Azees, S. Vanilla. In Chemistry of Spices; CABI: Wallingford, CT, USA, 2008; pp. 287–311. ISBN 978-1-84593-405-7. [Google Scholar]
- Ramachandra Rao, S.; Ravishankar, G. Vanilla Flavour: Production by Conventional and Biotechnological Routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional Composition, Mineral Content, Antioxidant Activity and Quantitative Estimation of Water Soluble Vitamins and Phenolics by RP-HPLC in Some Lesser Used Wild Edible Plants. Heliyon 2019, 5, e01431. [Google Scholar] [CrossRef]
- Devi, C.B.; Bains, K.; Kaur, H. Effect of Drying Procedures on Nutritional Composition, Bioactive Compounds and Antioxidant Activity of Wheatgrass (Triticum aestivum L). J. Food Sci. Technol. 2019, 56, 491–496. [Google Scholar] [CrossRef]
- Kouam, I.D.; Moungang, S.; Koulagna, H.I.; Ntsoli, G.P.; Titti, R.W.; Yaouba, A. Influence of Organic and Mineral Fertilizers and a Foliar Biostimulant on the Yield and Nutritional Quality of Strawberries (Fragaria ×ananassa Duch.) under Field Conditions. Biochem. Syst. Ecol. 2024, 117, 104917. [Google Scholar] [CrossRef]
- Peña-Barrientos, A.; Perea-Flores, M.D.J.; Vega-Cuellar, M.Á.; Flores-Vela, A.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Davila-Ortiz, G. Chemical and Microstructural Characterization of Vanilla Waste Compounds (Vanilla Planifolia, Jackson) Using Eco-Friendly Technology. Waste Biomass Valor 2022, 13, 271–286. [Google Scholar] [CrossRef]
- Yang, H.; Barros-Rios, J.; Kourteva, G.; Rao, X.; Chen, F.; Shen, H.; Liu, C.; Podstolski, A.; Belanger, F.; Havkin-Frenkel, D.; et al. A Re-Evaluation of the Final Step of Vanillin Biosynthesis in the Orchid Vanilla planifolia. Phytochemistry 2017, 139, 33–46. [Google Scholar] [CrossRef]
- Lopes, E.M.; Linhares, R.G.; De Oliveira Pires, L.; Castro, R.N.; Souza, G.H.M.F.; Koblitz, M.G.B.; Cameron, L.C.; Macedo, A.F. Vanilla bahiana, a Contribution from the Atlantic Forest Biodiversity for the Production of Vanilla: A Proteomic Approach through High-Definition nanoLC/MS. Food Res. Int. 2019, 120, 148–156. [Google Scholar] [CrossRef]
- Hariom; Shyamala, B.N.; Prakash, M.; Bhat, K.K. Vanilla Flavor Evaluation by Sensory and Electronic Nose Techniques. J. Sens. Stud. 2006, 21, 228–239. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Márquez, O.; Pardio, V.T. Quantification and Characterisation of Polyphenol Oxidase from Vanilla Bean. Food Chem. 2009, 117, 196–203. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Luna-Guevara, M.L.; Amador-Espejo, G.G.; Herrera-Cabrera, B.H.; Arévalo-Galarza, M.L.; Ruiz-Espinosa, H. Caracterización fisicoquímica y sensorial de Vanilla planifolia jacks. Ex Andrews con diferentes esquemas de beneficiado. Agroproductividad 2016, 9, 34–40. [Google Scholar]
- Podstolski, A. Enzymes Characterizad from Vanilla. In Handbook of Vanilla Science and Technology; Blackwell Publishing Ltd.: Chichester, UK, 2011; pp. 281–288. [Google Scholar]
- Torres, R.; Montes, E.J.; Pérez, O.A.; Andrade, R.D. Relación del Color y del Estado de Madurez con las Propiedades Fisicoquímicas de Frutas Tropicales. Inf. Tecnol. 2013, 24, 51–56. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Kerler, J.; Braster, M.; Apriyantono, A.; Stam, H.; Van Verseveld, H.W. Microorganisms with a Taste for Vanilla: Microbial Ecology of Traditional Indonesian Vanilla Curing. Appl. Environ. Microbiol. 2001, 67, 1995–2003. [Google Scholar] [CrossRef]
- Brunschwig, C.; Collard, F.-X.; Lepers-Andrzejewski, S.; Raharivelomanana, P. Tahitian Vanilla (Vanilla ×tahitensis): A Vanilla Species with Unique Features. In Active Ingredients from Aromatic and Medicinal Plants; El-Shemy, H.A., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-2975-2. [Google Scholar]
- Espejel, A.; Barrera Rodríguez, A.I.; Pérez, M.G.; Ramírez-García, A.G. Atributos tangibles e intangibles y diferenciación sensorial de la vainilla mexicana. Polibotanica 2022, 54, 241–255. [Google Scholar] [CrossRef]
- Shigeto, A.; Nurasti, E.N.; Sugiarto, A.; Togawa, M.; Kumazawa, K. The Influence of Harvest Maturity on the Aroma Quality of Vanilla. J. Jpn. Soc. Food Sci. Technol. 2017, 64, 502–506. [Google Scholar] [CrossRef]
- Brunschwig, C.; Rochard, S.; Pierrat, A.; Rouger, A.; Senger-Emonnot, P.; George, G.; Raharivelomanana, P. Volatile Composition and Sensory Properties of Vanilla ×tahitensis Bring New Insights for Vanilla Quality Control. J. Sci. Food Agric. 2016, 96, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Inai, Y.; Miyazawa, N.; Kurobayashi, Y.; Fujita, A. Key Odorants in Cured Madagascar Vanilla Beans (Vanilla planiforia) of Differing Bean Quality. Biosci. Biotechnol. Biochem. 2013, 77, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.A.; Günata, Z. GC–MS and GC–Olfactometry Analysis of Aroma Compounds in a Representative Organic Aroma Extract from Cured Vanilla (Vanilla planifolia G. Jackson) Beans. Food Chem. 2006, 99, 728–735. [Google Scholar] [CrossRef]

| Samples | Variety | Area of Production | Date Harvested | Periods of Curing |
|---|---|---|---|---|
| SJA | Vanilla planifolia | San José Acateno, Puebla | 2020 | 8 and 30 cycles |
| SLP | Vanilla planifolia | Cerro Quemado, Oaxaca | 2020 | 8 and 30 cycles |
| CQ | Vanilla planifolia | Jalpilla, San Luis Potosí | 2020 | 8 and 30 cycles |
| EPM | Vanilla planifolia | Primero de Mayo, Veracruz | 2020 | 8 and 30 cycles |
| Control | Vanilla planifolia | Puebla | 2020 | N.A. * |
| Sample | Period | Moisture | Ether Extract | Ashes | Protein | pH |
|---|---|---|---|---|---|---|
| Control | Period 1 | 41.02 ± 0.40 a | 12.98 ± 1.22 ab | 7.43 ± 0.15 a | 7.90 ± 0.82 a | 4.69 ± 0.01 c |
| SLP | 31.44 ± 0.70 c | 13.13 ± 0.61 ab | 5.45 ± 0.20 c | 6.78 ± 0.67 ab | 4.74 ± 0.02 b | |
| SJA | 21.06 ± 0.96 c | 11.63 ± 0.79 bc | 6.99 ± 0.79 ab | 4.73 ± 0.34 cd | 4.93 ± 0.01 a | |
| CQ | 36.61 ± 0.99 ab | 15.00 ± 0.86 a | 6.66 ± 0.91 abc | 4.47 ± 0.43 d | 4.31 ± 0.01 d | |
| EPM | 39.04 ± 4.17 a | 10.04 ± 0.84 c | 5.55 ± 0.20 bc | 6.21 ± 0.32 bc | 4.66 ± 0.01 c | |
| Control | Period 2 | 41.02 ± 0.40 a | 12.98 ± 1.22 ab | 7.43 ± 0.15 | 7.90 ± 0.82 a | 4.69 ± 0.01 b |
| SLP | 31.02 ± 2.14 b | 16.56 ± 1.36 a | 6.75 ± 0.40 | 4.57 ± 1.06 b | 4.78 ± 0.02 b | |
| SJA | 13.43 ± 0.23 e | 10.15 ± 0.79 b | 7.70 ± 0.67 | 6.10 ± 0.48 ab | 4.61 ± 0.03 c | |
| CQ | 16.85 ± 0.62 d | 12.61 ± 0.06 ab | 7.30 ± 0.87 | 5.10 ± 0.90 b | 4.26 ± 0.02 d | |
| EPM | 24.10 ± 0.21 c | 12.74 ± 2.61 ab | 7.34 ± 0.67 | 4.34 ± 1.06 b | 4.94 ± 0.04 a |
| Samples | Period 1 | Period 2 |
|---|---|---|
| SJA | 6.02 ± 2.02 | 6.39 ± 1.45 a |
| EPM | 5.52 ± 2.01 | 5.52 ± 1.88 ab |
| CQ | 5.37 ± 2.06 | 4.34 ± 2.27 c |
| SLP | 5.24 ± 2.18 | 5.61 ± 1.91 ab |
| Control | 5.27 ± 2.05 | 5.27 ± 2.05 bc |
| Descriptor | EPM | SJA | CQ | SLP | Control | p-Value |
|---|---|---|---|---|---|---|
| Acid | 5.46 ± 2.46 | 3.88 ± 3.00 | 4.00 ± 1.26 | 4.17 ± 2.93 | 5.86 ± 1.57 | NS |
| Apple | 4.60 ± 2.51 | 4.17 ± 2.22 | 2.33 ± 0.51 | 1.60 ± 0.54 | 3.71 ± 2.21 | NS |
| Bitter | 3.10 ± 1.10 | 3.91 ± 2.16 | 4.91 ± 2.73 | 4.50 ± 2.81 | 3.27 ± 1.95 | NS |
| Caramel | 6.59 ± 1.29 ab | 5.24 ± 1.99 bc | 7.47 ± 0.99 a | 4.69 ± 2.44 c | 5.47 ± 1.26 bc | 0.0001 |
| Chemical | 6.50 ± 1.51 | 3.75 ± 2.76 | 6.22 ± 2.27 | 5.29 ± 2.84 | 4.55 ± 3.47 | NS |
| Chocolate | 5.94 ± 1.18 a | 4.32 ± 2.26 b | 3.46 ± 1.26 bc | 6.23 ± 1.09 a | 1.86 ± 0.77 c | 0.0001 |
| Cocoa | 4.29 ± 1.89 a | 4.26 ± 1.85 a | 2.53 ± 1.24 b | 2.44 ± 0.81 b | 1.92 ± 0.66 b | 0.0001 |
| Cooked | 2.40 ± 2.07 | 1.80 ± 0.83 | 3.60 ± 2.41 | 3.33 ± 1.52 | 3.00 ± 2.00 | NS |
| Dairy | 4.00 ± 2.58 | 2.88 ± 1.55 | 3.00 ± 1.67 | 4.00 ± 1.41 | 3.00 ± 1.30 | NS |
| Dried fruit | 6.22 ± 1.30 a | 4.29 ± 2.39 bc | 3.00 ± 1.47 c | 3.00 ± 1.09 c | 5.33 ± 1.04 ab | 0.0001 |
| Dried leaf | 2.67 ± 1.07 ab | 3.20 ± 1.93 ab | 2.73 ± 1.84 ab | 1.94 ± 0.92 b | 3.63 ± 1.89 a | 0.043 |
| Fermented | 2.38 ± 1.02 c | 4.75 ± 2.43 b | 7.86 ± 1.09 a | 6.33 ± 1.23 ab | 6.05 ± 1.71 b | 0.0001 |
| Fresh | 6.00 ± 2.55 | 3.80 ± 2.30 | 2.60 ± 1.51 | 5.5 ± 3.42 | 5.38 ± 1.68 | NS |
| Fruity | 4.53 ± 2.47 | 3.89 ± 2.22 | 3.10 ± 1.59 | 2.71 ± 1.11 | 3.71 ± 1.89 | NS |
| Herbal | 2.30 ± 1.16 | 3.54 ± 2.02 | 3.33 ± 1.23 | 2.62 ± 1.04 | 3.10 ± 1.91 | NS |
| Honey | 5.63 ± 0.91 a | 4.48 ± 2.22 a | 3.25 ± 1.48 ab | 1.90 ± 0.87 b | 3.86 ± 2.59 ab | 0.002 |
| Maltol | 6.14 ± 1.67 a | 4.73 ± 2.15 ab | 3.17 ± 1.16 ab | 3.20 ± 1.13 b | 4.73 ± 2.15 ab | 0.019 |
| Moisture | 4.17 ± 2.32 b | 2.25 ± 1.48 b | 2.93 ± 1.71 b | 6.78 ± 1.20 a | 2.20 ± 0.78 b | 0.0001 |
| Pineapple | 2.14 ± 0.90 | 2.14 ± 1.06 | 4.00 ± 3.24 | 4.17 ± 2.40 | 3.22 ± 1.56 | NS |
| Prune | 6.88 ± 1.40 a | 5.19 ± 2.20 b | 2.64 ± 1.49 c | 6.60 ± 1.29 ab | 6.61 ± 1.33 a | 0.0001 |
| Olive | 2.88 ± 1.35 bc | 4.67 ± 2.34 b | 7.46 ± 1.36 a | 4.27 ± 2.90 bc | 1.86 ± 1.06 c | 0.0001 |
| Raisin | 6.53 ± 1.12 | 5.76 ± 2.30 | 5.27 ± 2.42 | 6.10 ± 1.19 | 6.83 ± 1.75 | NS |
| Rancid | 5.11 ± 2.61 a | 4.14 ± 2.11 ab | 5.09 ± 2.70 a | 1.78 ± 1.09 b | 3.40 ± 1.45 ab | 0.005 |
| Resin | 6.00 ± 1.26 a | 3.60 ± 1.43 ab | 2.75 ± 0.96 b | 5.13 ± 2.74 a | 4.15 ± 1.99 ab | 0.006 |
| Rum | 6.11 ± 1.26 a | 3.86 ± 2.10 b | 3.33 ± 1.72 b | 2.86 ± 1.06 b | 6.14 ± 1.29 a | 0.0001 |
| Smoked | 2.90 ± 0.99 ab | 3.88 ± 1.93 a | 3.30 ± 1.41 ab | 2.33 ± 1.23 b | 1.89 ± 0.78 b | 0.009 |
| Soil | 2.11 ± 1.26 | 3.46 ± 2.84 | 1.92 ± 1.24 | 2.29 ± 1.26 | 1.92 ± 0.76 | NS |
| Spices | 6.21 ± 1.57 a | 3.23 ± 2.18 b | 2.60 ± 1.35 b | 2.36 ± 1.15 b | 5.27 ± 1.61 a | 0.0001 |
| Spicy | 3.71 ± 2.36 | 3.43 ± 2.07 | 2.86 ± 1.21 | 2.67 ± 1.93 | 4.00 ± 2.65 | NS |
| Sweet | 6.77 ± 1.19 a | 4.89 ± 2.11 b | 7.20 ± 1.15 a | 6.77 ± 1.19 a | 6.43 ± 1.53 b | 0.0001 |
| Toasted | 2.75 ± 1.06 | 3.42 ± 1.92 | 2.94 ± 1.51 | 4.05 ± 2.32 | 4.00 ± 2.20 | NS |
| Tree bark | 4.68 ± 1.93 | 4.54 ± 2.04 ab | 2.88 ± 1.02 c | 2.92 ± 1.16 bc | 5.33 ± 1.07 a | 0.0001 |
| Vanilla | 6.36 ± 2.28 | 6.20 ± 2.33 | 5.47 ± 2.63 | 5.63 ± 2.24 | 5.46 ± 2.22 | NS |
| Vinegar | 7.00 ± 1.11 a | 4.44 ± 2.65 ab | 4.57 ± 3.18 ab | 5.13 ± 2.90 ab | 1.89 ± 0.78 b | 0.002 |
| Wood | 6.75 ± 1.48 a | 3.97 ± 2.17 b | 2.67 ± 1.35 c | 2.63 ± 1.14 bc | 5.83 ± 1.55 a | 0.0001 |
| Descriptor | EPM | SJA | CQ | SLP | Control | p-Value |
|---|---|---|---|---|---|---|
| Acid | 4.70 ± 1.49 | 4.25 ± 0.50 | 3.30 ± 1.82 | 3.71 ± 2.62 | 5.86 ± 1.57 | NS |
| Apple | 4.00 ± 2.45 | 2.50 ± 2.34 | 3.00 ± 1.41 | 2.75 ± 2.22 | 3.71 ± 2.21 | NS |
| Bitter | 3.18 ± 1.77 ab | 3.40 ± 2.51 ab | 2.90 ± 1.66 b | 5.38 ± 1.30 a | 3.27 ± 1.95 ab | 0.056 |
| Caramel | 6.50 ± 1.09 a | 6.10 ± 1.29 a | 2.79 ± 1.42 b | 6.00 ± 1.41 a | 5.47 ± 1.26 a | 0.0001 |
| Chemical | 7.00 ± 1.41 ab | 4.14 ± 2.97 b | 7.93 ± 0.82 a | 3.90 ± 2.80 b | 4.55 ± 3.47 b | 0.0001 |
| Chocolate | 2.56 ± 1.01 ab | 3.97 ± 2.44 a | 2.64 ± 1.50 ab | 4.24 ± 2.16 a | 1.86 ± 0.77 b | 0.002 |
| Cocoa | 4.13 ± 1.64 a | 3.62 ± 2.30 ab | 2.80 ± 1.31 ab | 3.58 ± 2.57 ab | 1.91 ± 0.66 b | 0.044 |
| Cooked | 2.67 ± 1.52 | 3.14 ± 1.54 | 3.00 ± 1.63 | 3.29 ± 2.49 | 3.00 ± 2.00 | NS |
| Dairy | 4.75 ± 2.87 ab | 1.80 ± 0.83 b | 6.00 ± 2.94 a | 3.75 ± 0.95 ab | 3.00 ± 1.30 ab | 0.026 |
| Dried fruit | 4.44 ± 2.31 ab | 6.08 ± 1.49 a | 3.25 ± 1.48 b | 5.55 ± 1.21 a | 5.33 ± 1.04 ab | 0.003 |
| Dried leaf | 3.00 ± 1.85 b | 2.00 ± 0.66 b | 3.00 ± 1.37 b | 5.82 ± 1.07 a | 3.63 ± 1.89 b | 0.0001 |
| Fermented | 3.00 ± 1.82 c | 2.00 ± 0.86 c | 7.62 ± 1.19 a | 6.08 ± 1.56 ab | 6.05 ± 1.71 b | 0.0001 |
| Fresh | 3.00 ± 1.78 | 4.00 ± 2.44 | 4.75 ± 2.87 | 3.83 ± 2.25 | 5.38 ± 1.68 | NS |
| Fruity | 3.90 ± 2.80 | 5.82 ± 1.16 | 3.20 ± 2.04 | 3.82 ± 2.35 | 3.71 ± 1.89 | NS |
| Herbal | 2.17 ± 1.03 b | 4.00 ± 2.09 a | 3.00 ± 1.61 ab | 2.38 ± 0.51 ab | 3.10 ± 1.91 ab | 0.048 |
| Honey | 4.21 ± 2.52 | 5.24 ± 1.34 | 2.80 ± 1.47 | 3.95 ± 2.24 | 3.86 ± 2.59 | NS |
| Maltol | 4.80 ± 1.75 ab | 7.00 ± 2.00 a | 2.85 ± 1.34 b | 4.31 ± 1.93 b | 4.09 ± 2.38 b | 0.001 |
| Moisture | 2.21 ± 1.62 | 1.67 ± 0.65 | 2.14 ± 1.21 | 1.75 ± 0.70 | 2.20 ± 0.78 | NS |
| Pineapple | 4.13 ± 3.09 | 2.25 ± 0.95 | 1.50 ± 0.70 | 3.40 ± 2.61 | 3.22 ± 1.56 | NS |
| Prune | 2.00 ± 0.93 b | 6.73 ± 1.28 a | 2.4 ± 1.35 b | 2.40 ± 1.35 b | 6.61 ± 1.33 a | 0.0001 |
| Olive | 3.90 ± 2.07 | 3.75 ± 1.90 | 3.67 ± 1.65 | 2.00 ± 0.89 | 1.86 ± 1.06 | 0.044 |
| Raisin | 6.88 ± 1.45 a | 5.53 ± 1.66 a | 3.23 ± 1.64 b | 5.81 ± 1.51 a | 6.83 ± 1.75 a | 0.0001 |
| Rancid | 3.64 ± 2.33 | 3.50 ± 2.07 | 2.80 ± 1.31 | 4.00 ± 2.13 | 3.40 ± 1.45 | NS |
| Resin | 1.82 ± 0.87 b | 4.50 ± 2.55 a | 3.50 ± 1.22 ab | 2.00 ± 0.92 b | 4.15 ± 1.99 a | 0.001 |
| Rum | 6.00 ± 1.09 a | 4.75 ± 0.70 a | 2.93 ± 1.22 b | 5.80 ± 1.61 a | 6.14 ± 1.29 a | 0.0001 |
| Smoked | 2.14 ± 1.34 ab | 3.88 ± 2.36 a | 2.85 ± 1.46 ab | 2.22 ± 0.66 ab | 1.89 ± 0.78 b | 0.023 |
| Soil | 2.36 ± 1.36 ab | 2.09 ± 0.94 ab | 3.22 ± 1.35 ab | 3.73 ± 2.5 a | 1.92 ± 0.76 b | 0.015 |
| Spices | 2.56 ± 1.20 b | 3.95 ± 2.24 ab | 2.81 ± 1.51 b | 3.65 ± 2.56 ab | 5.27 ± 1.61 a | 0.007 |
| Spicy | 4.50 ± 2.44 | 1.00 ± 0 | 3.80 ± 1.64 | 2.80 ± 1.78 | 4.00 ± 2.65 | NS |
| Sweet | 6.80 ± 1.32 a | 6.19 ± 1.44 a | 3.23 ± 1.34 b | 6.32 ± 1.64 a | 6.43 ± 1.53 a | 0.0001 |
| Toasted | 6.10 ± 1.28 a | 2.64 ± 0.50 bc | 3.00 ± 1.73 bc | 2.08 ± 0.76 c | 4.00 ± 2.20 b | 0.0001 |
| Tree bark | 2.78 ± 1.06 c | 6.93 ± 1.28 a | 3.65 ± 1.16 c | 5.62 ± 1.32 b | 5.33 ± 1.07 b | 0.0001 |
| Vanilla | 5.35 ± 2.39 | 5.88 ± 2.28 | 4.70 ± 2.55 | 5.55 ± 2.21 | 5.46 ± 2.22 | N.S. |
| Vinegar | 2.75 ± 1.48 | 2.75 ± 0.95 | 2.50 ± 1.58 | 4.14 ± 2.97 | 1.89 ± 0.78 | N.S. |
| Wood | 6.77 ± 1.30 ab | 6.58 ± 1.50 abc | 7.33 ± 0.90 a | 5.41 ± 1.59 c | 5.83 1.55 bc | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Viveros, I.J.; García-Barrón, S.E.; Hernández-Rodríguez, B.E.; Barrera-Rodríguez, A.I.; Acero-Ortega, C.A.; Espejel-García, A. Influence of Traditional Vanilla Curing on Its Physicochemical Properties and Aromatic Profile. Foods 2025, 14, 1652. https://doi.org/10.3390/foods14091652
Perez-Viveros IJ, García-Barrón SE, Hernández-Rodríguez BE, Barrera-Rodríguez AI, Acero-Ortega CA, Espejel-García A. Influence of Traditional Vanilla Curing on Its Physicochemical Properties and Aromatic Profile. Foods. 2025; 14(9):1652. https://doi.org/10.3390/foods14091652
Chicago/Turabian StylePerez-Viveros, Isabel Janid, Sergio Erick García-Barrón, Blanca Elizabeth Hernández-Rodríguez, Ariadna Isabel Barrera-Rodríguez, Claudia Ariadna Acero-Ortega, and Anastacio Espejel-García. 2025. "Influence of Traditional Vanilla Curing on Its Physicochemical Properties and Aromatic Profile" Foods 14, no. 9: 1652. https://doi.org/10.3390/foods14091652
APA StylePerez-Viveros, I. J., García-Barrón, S. E., Hernández-Rodríguez, B. E., Barrera-Rodríguez, A. I., Acero-Ortega, C. A., & Espejel-García, A. (2025). Influence of Traditional Vanilla Curing on Its Physicochemical Properties and Aromatic Profile. Foods, 14(9), 1652. https://doi.org/10.3390/foods14091652







