Enhancing the Textural Properties of Tibetan Pig Sausages via Zanthoxylum bungeanum Aqueous Extract: Polyphenol-Mediated Quality Improvements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Zanthoxylum bungeanum Aqueous Extract
2.3. Determination of Total Phenolic Content in ZBAE
2.4. Preparation of Tibetan Pig Sausage with ZBAE
2.5. Water Holding Capacity
2.6. Color
2.7. Texture Profile Analysis
2.8. Puncture Test
2.9. Intermolecular Forces
2.10. Moisture Distribution
2.11. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.12. Protein Secondary Structure
2.13. Scanning Electron Microscopy
2.14. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different ZBAE Concentrations on the Physicochemical Attributes of Tibetan Pig Sausages
3.1.1. WHC
3.1.2. Color
3.1.3. Textural Attributes Analysis
3.1.4. Puncture Test
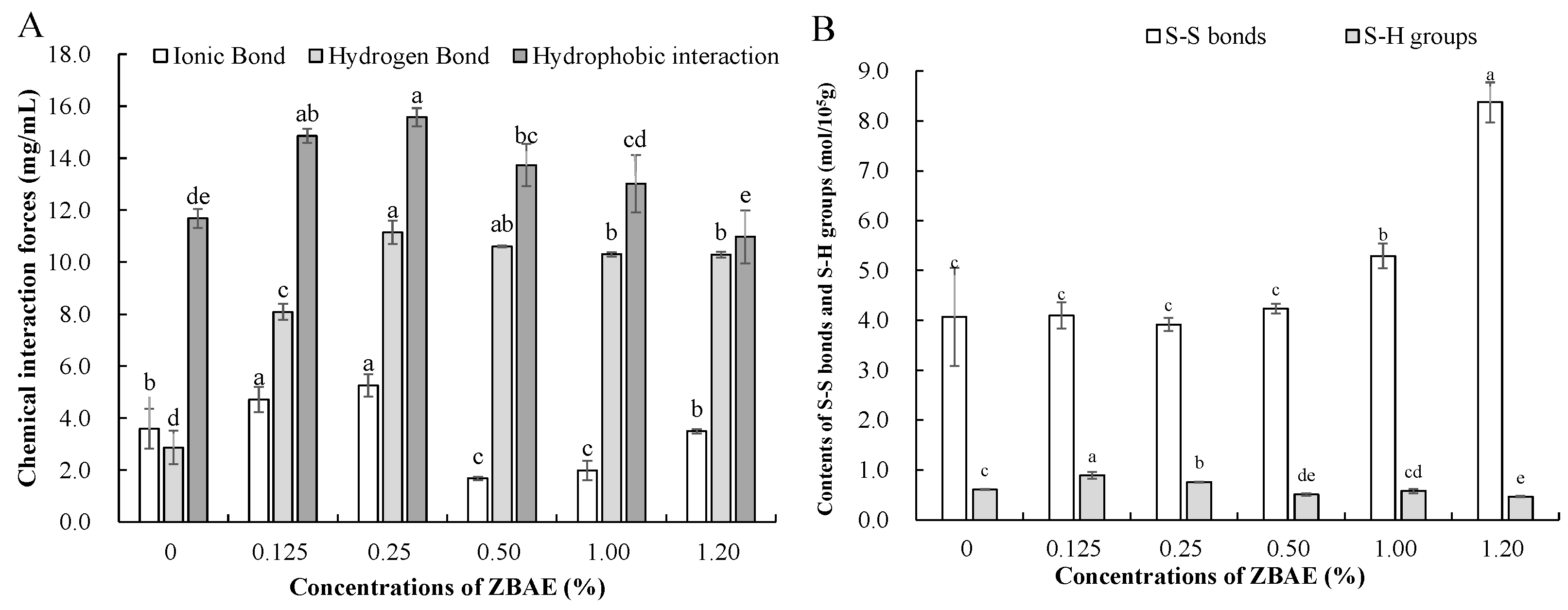
3.2. Effects of Different ZBAE Concentrations on the Intermolecular Forces of Tibetan Pig Sausages
3.2.1. Non-Covalent Interaction Forces
3.2.2. Sulfhydryl Group (SH) and Disulfide Bonds Contents
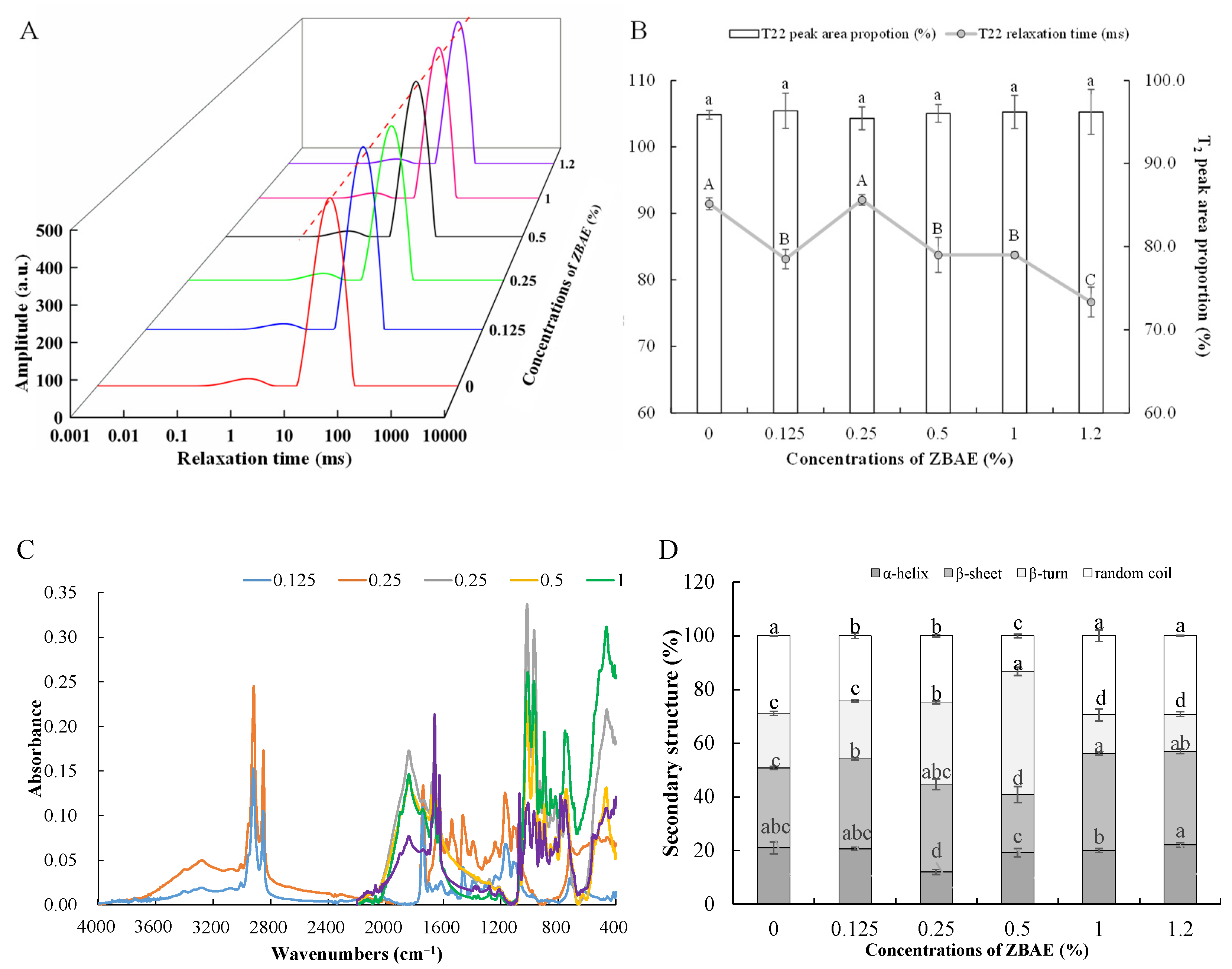
3.3. Effects of Different ZBAE Concentrations on the Water State of Tibetan Pig Sausages
3.4. Effects of Different ZBAE Concentrations on the Protein Structure of Tibetan Pig Sausages
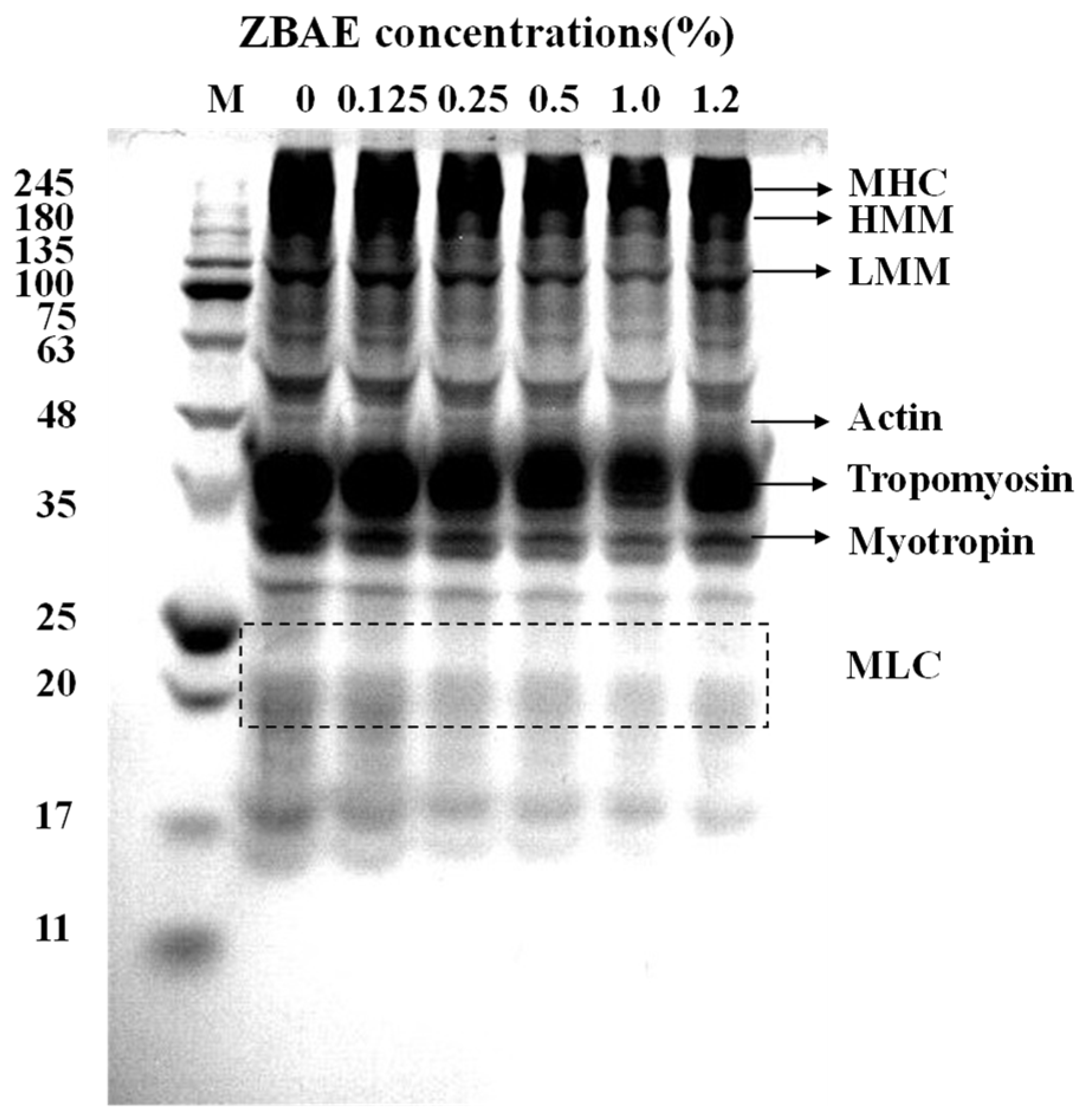
3.4.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.4.2. Protein Secondary Structure
3.4.3. Scanning Electron Microscope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZBAE | Zanthoxylum bungeanum aqueous extract |
| WHC | Water holding capacity |
| SH | Sulfhydryl |
| FTIR | Fourier transform infrared spectra |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| LF-NMR | Low-field nuclear magnetic resonance |
| MHC | Myosin heavy chain |
| MLC | Myosin light chain |
| Df | Fraction dimension |
References
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, F.; Qu, D.; Wan, T.; Xi, L.; Hu, C.Y. Aroma characterization of Sichuan and Cantonese sausages using electronic nose, gas chromatography–mass spectrometry, gas chromatography-olfactometry, odor activity values and metagenomic. Food Chem. X 2024, 24, 101924. [Google Scholar] [CrossRef]
- Jia, W.; Wang, X. Zanthoxylum bungeanum as a natural pickling spice alleviates health risks in animal-derived foods via up-regulating glutathione S-transferase, down-regulating cytochrome P450 1A. Food Chem. 2023, 411, 135535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, F.; He, L.; Wang, X.; Li, C. Effect of Zanthoxylum bungeanum extract on the quality and cathepsin L activity of Niuganba. Meat Sci. 2024, 217, 109594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, L.; Hei, M.; Zhao, Y.; Zhu, M.; Wang, H.; Zhou, H.; Ma, H. Conformation and functional modification of porcine myofibrillar protein by pepper leaf polyphenols under oxidative condition. LWT 2024, 198, 116017. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. The effect of linalool, limonene and sabinene on the thermal stability and structure of rabbit meat myofibrillar protein under malondialdehyde-induced oxidative stress. LWT 2021, 148, 111707. [Google Scholar] [CrossRef]
- Xu, B.; He, W.; Yi, Y.; Wang, H.; Xu, W.; Guo, D. The impacting mechanism of β-sanshool in Zanthoxylum bungeanum Maxim on the structure of myofibrillar protein in duck meat. LWT 2023, 182, 114838. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, W.; Qiang, Y.; Han, D.; Huang, F.; Chisoro, P.; Jia, W.; Fauconnier, M.L.; Purcaro, G.; Zhang, C. Deciphering Taste Endowment of Zanthoxylum bungeanum Maxim on Soy Sauce Marinated Beef: Binding of Key Taste Flavonols to Myofibrillar Protein. Available at SSRN 4828388. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4828388 (accessed on 2 May 2025).
- Jiang, J.; Watowita, P.; Chen, R.; Shi, Y.; Geng, J.-T.; Takahashi, K.; Li, L.; Osako, K. Multilayer gelatin/myofibrillar films containing clove essential oil: Properties, protein-phenolic interactions, and migration of active compounds. Food Packag. Shelf Life 2022, 32, 100842. [Google Scholar] [CrossRef]
- Cai, R.; Duan, M.; Wang, S.; Lu, F.; Wu, L.; Tan, Z.; Zhang, J.; Shang, P. Analysis on carcass performance and meat quality between the Tibetan pig and large white pig. J. Domest. Anim. Ecol. 2021, 42, 35–38. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S. High altitude adaptability and meat quality in Tibetan pigs: A reference for local pork processing and genetic improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, H.; Han, S.; Guo, C.; Wang, Y. A review on Tibetan swine (a)-carcass, meat quality, basic nutrition component, amino acids, fatty acids, inosine monophosphate and muscle fiber. Agric. Sci. Technol. 2013, 14, 1369. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. The comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F. Handbook of Meat Processing; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Poklar Ulrih, N. Analytical techniques for the study of polyphenol–protein interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 2144–2161. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Zheng, J.; Yao, X.; Wang, W.; Tomasevic, I.; Sun, W. Effect of NaCl replacement by other salt mixtures on myofibrillar proteins: Underlining protein structure, gel formation, and chewing properties. J. Food Sci. 2024, 89, 9060–9072. [Google Scholar] [CrossRef]
- Bae, S.M.; Jeong, J.Y. Investigating the Effects of Pink-Generating Ligands on Enhancing Color Stability and Pigment Properties in Pork Sausage Model Systems Cured with Sodium Nitrite or White Kimchi Powder. Foods 2024, 13, 2872. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Yin, J.; Huang, J.; Yan, Y.; Zhang, F.; Xie, N. Physicochemical characteristics and gel-forming properties of mandarin fish (Siniperca chuatsi) protein during the fish fermentation with Lactobacillus sake SMF-L5: The formation of garlic-cloves shaped protein gel. Food Chem. 2023, 409, 135282. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Meng, L.; Jiao, X.; Yan, B.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Effect of fish mince size on physicochemical and gelling properties of silver carp (Hypophthalmichthys molitrix) surimi gel. LWT 2021, 149, 111912. [Google Scholar] [CrossRef]
- Zhao, X.; Han, G.; Wen, R.; Xia, X.; Chen, Q.; Kong, B. Influence of lard-based diacylglycerol on rheological and physicochemical properties of thermally induced gels of porcine myofibrillar protein at different NaCl concentrations. Food Res. Int. 2020, 127, 108723. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Xue, Y.; Xue, C. Changes of structural and physical properties of semi-gel from Alaska pollock surimi during 4 °C storage. Food Hydrocoll. 2019, 87, 772–782. [Google Scholar] [CrossRef]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crops Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yang, H. Gelation properties of silver carp (Hypophthalmichthys molitrix) surimi as affected by phenolic compounds in lotus root knot extract. Int. J. Food Prop. 2023, 26, 991–1004. [Google Scholar] [CrossRef]
- Djenane, D.; Khaled, B.M.; Ben Miri, Y.; Metahri, M.S.; Montañés, L.; Aider, M.; Ariño, A. Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient. Foods 2024, 14, 59. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, J.; Yun, X.; Dong, T. Effect of Artemisia sphaerocephala Krasch gum on the gel properties of myofibrillar protein and its application in cooked sheep sausage. Food Hydrocoll. 2023, 142, 108752. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Zhang, H.; Wang, H.; Kong, B. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef]
- Ingenbosch, K.N.; Vieyto-Nuñez, J.C.; Ruiz-Blanco, Y.B.; Mayer, C.; Hoffmann-Jacobsen, K.; Sanchez-Garcia, E. Effect of organic solvents on the structure and activity of a minimal lipase. J. Org. Chem. 2021, 87, 1669–1678. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT 2021, 142, 110984. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, X.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, H.; Wang, M. Microwave irradiation promotes aggregation behavior of myosin through conformation changes. Food Hydrocoll. 2019, 96, 11–19. [Google Scholar] [CrossRef]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Wu, Y.; Sun, Q.; Pan, J.; Dong, X.; Li, S. Effects of Eucommia ulmoides Leaf Extract on the Technological Quality, Protein Oxidation, and Lipid Oxidation of Cooked Pork Sausage During Refrigerated Storage. Foods 2025, 14, 441. [Google Scholar] [CrossRef]
- Diao, X.; Zhu, J.; Huang, L.; Li, S.; Mao, X.; Li, C.; Ke, W. Effect of citrus fiber on physicochemical quality of Frankfurter sausages: A study on lipid oxidation and protein gel characteristics. LWT 2024, 209, 116778. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Wang, Y.; Wang, R.; Zeng, M. Effect of κ-carrageenan on the gelation properties of oyster protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Sharma, S.; Majumdar, R.K.; Mehta, N.K. Gelling properties and microstructure of the silver carp surimi treated with pomegranate (Punica granatum L.) peel extract. J. Food Sci. Technol. 2022, 59, 4210–4220. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Hei, M.; Zhao, Y.; Li, J.; Kang, Z.; Ma, H.; Xiong, G. An Evaluation of the Effects of Pepper (Zanthoxylum bungeanum Maxim.) Leaf Extract on the Physiochemical Properties and Water Distribution of Chinese Cured Meat (Larou) During Storage. Foods 2024, 13, 3972. [Google Scholar] [CrossRef]
- Xu, Q.-D.; Yu, Z.-L.; Zeng, W.-C. Structural and functional modifications of myofibrillar protein by natural phenolic compounds and their application in pork meatball. Food Res. Int. 2021, 148, 110593. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Dong, K.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Mulberry fruit powder enhanced the antioxidant capacity and gel properties of hammered minced beef: Oxidation degree, rheological, and structure. LWT 2022, 154, 112648. [Google Scholar] [CrossRef]
- Cheng, J.-R.; Liu, X.-M.; Zhang, Y.-S.; Zhang, Y.-H.; Chen, Z.-Y.; Tang, D.-B.; Wang, J.-Y. Protective effects of Momordica grosvenori extract against lipid and protein oxidation-induced damage in dried minced pork slices. Meat Sci. 2017, 133, 26–35. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, K.; Wang, Y.; Xie, Y.; Wang, Z.; Li, P.; Xu, B. Insight into the mechanism of textural deterioration of myofibrillar protein gels at high temperature conditions. Food Chem. 2020, 330, 127186. [Google Scholar] [CrossRef]
- Zhao, X.; Han, G.; Sun, Q.; Liu, H.; Liu, Q.; Kong, B. Influence of lard-based diacylglycerol on the rheological and physicochemical properties of thermally induced pork myofibrillar protein gels at different pH levels. LWT 2020, 117, 108708. [Google Scholar] [CrossRef]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Double-crosslinked effect of TGase and EGCG on myofibrillar proteins gel based on physicochemical properties and molecular docking. Food Chem. 2021, 345, 128655. [Google Scholar] [CrossRef]
- Wasinnitiwong, N.; Benjakul, S.; Hong, H. Effects of κ-carrageenan on gel quality of threadfin bream (Nemipterus spp.) surimi containing salted duck egg white powder. Int. J. Biol. Macromol. 2022, 221, 61–70. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef]
- Guan, A.; Mei, K.; Lv, M.; Lu, J.; Lou, Q.; Yang, W. The effect of electron beam irradiation on IgG binding capacity and conformation of tropomyosin in shrimp. Food Chem. 2018, 264, 250–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Yuan, X.; Zhao, S.; Kang, Z.; Zhu, M.; He, H.; Ma, H. Physicochemical, conformational and functional changes of quinoa protein affected by high-pressure homogenization. LWT 2023, 173, 114343. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Hao, R.; Wang, Y.; Ju, H.; Li, S.; Dong, X. Dose affected the role of gallic acid on mediating gelling properties of oxidatively stressed Japanese seerfish myofibrillar protein. LWT 2020, 118, 108849. [Google Scholar] [CrossRef]
- Jiang, D.; Shen, P.; Pu, Y.; Jin, M.; Yu, C.; Qi, H. Enhancement of gel properties of Scomberomorus niphonius myofibrillar protein using phlorotannin extracts under UVA irradiation. J. Food Sci. 2020, 85, 2050–2059. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel properties of myofibrillar protein as affected by gelatinization and retrogradation behaviors of modified starches with different crosslinking and acetylation degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]
- Manzoor, A.; Haque, A.; Ahmad, S.; Hopkins, D.L. Incorporation of betel leaf extract provides oxidative stability and improves phytochemical, textural, sensory and antimicrobial activities of buffalo meat sausages. Meat Sci. 2023, 200, 109157. [Google Scholar] [CrossRef]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin–Gum arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]

| Concentrations of ZBAE (%) | 0 | 0.125 | 0.25 | 0.50 | 1.00 | 1.20 |
|---|---|---|---|---|---|---|
| Water holding capacity (%) | 94.40 ± 0.16 d | 94.87 ± 0.09 c | 96.07 ± 0.09 a | 95.13 ± 0.09 b | 94.53 ± 0.09 d | 94.00 ± 0.16 e |
| L* | 42.27 ± 0.29 a | 41.01 ± 0.68 abc | 41.50 ± 0.17 b | 40.46 ± 0.54 cd | 39.61 ± 0.43 de | 37.75 ± 1.11 e |
| a* | 11.20 ± 0.25 a | 11.17 ± 0.08 a | 11.41 ± 0.43 a | 10.53 ± 0.56 ab | 9.72 ± 0.96 ab | 9.07 ± 1.04 b |
| b* | 9.42 ± 0.49 c | 10.11 ± 0.32 bc | 10.24 ± 0.23 b | 10.07 ± 0.61 bc | 11.31 ± 0.41 a | 10.10 ± 0.74 bc |
| Hardness (g) | 3449.94 ± 25.70 e | 4480.61 ± 15.44 c | 8275.25 ± 19.13 a | 7400.55 ± 3.88 b | 4501.26 ± 3.58 c | 4270.16 ± 31.97 d |
| Springiness (%) | 0.561 ± 0.008 d | 0.543 ± 0.025 d | 0.689 ± 0.004 b | 0.771 ± 0.005 a | 0.667 ± 0.004 bc | 0.655 ± 0.005 c |
| Cohesiveness | 0.427 ± 0.005 d | 0.382 ± 0.007 e | 0.476 ± 0.002 b | 0.536 ± 0.002 a | 0.445 ± 0.002 c | 0.434 ± 0.005 d |
| Gumminess | 1452.80 ± 21.27 e | 1788.61 ± 48.37 d | 3894.61 ± 59.06 a | 3957.68 ± 24.99 a | 1976.94 ± 26.81 c | 2067.92 ± 19.44 b |
| Chewiness | 780.70 ± 3.85 d | 885.10 ± 11.83 c | 3054.23 ± 18.95 a | 3081.40 ± 13.57 a | 1359.00 ± 5.92 b | 1359.15 ± 27.06 b |
| Resilience (%) | 0.138 ± 0.001 c | 0.093 ± 0.002 e | 0.148 ± 0.002 b | 0.170 ± 0.002 a | 0.118 ± 0.001 d | 0.116 ± 0.004 d |
| Gel strength (g·cm) | 1101.01 ± 5.66 c | 1106.04 ± 1.84 c | 1607.87 ± 9.73 a | 1180.42 ± 9.36 b | 1071.28 ± 8.48 d | 1056.02 ± 9.84 d |
| Concentrations of ZBAE (%) | 0 | 0.125 | 0.25 | 0.50 | 1.00 | 1.20 |
|---|---|---|---|---|---|---|
| Fraction dimension | 2.748 ± 0.003 cd | 2.814 ± 0.001 a | 2.769 ± 0.006 b | 2.757 ± 0.000 c | 2.749 ± 0.005 cd | 2.747 ± 0.002 d |
| Porosity (%) | 37.597 ± 0.438 b | 38.48 ± 0.090 a | 38.350 ± 0.333 a | 37.670 ± 0.204 b | 35.873 ± 0.109 c | 34.350 ± 0.151 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wei, H.; Luo, Z.; Li, L.; Liu, Z.; Xie, N. Enhancing the Textural Properties of Tibetan Pig Sausages via Zanthoxylum bungeanum Aqueous Extract: Polyphenol-Mediated Quality Improvements. Foods 2025, 14, 1639. https://doi.org/10.3390/foods14091639
Huang J, Wei H, Luo Z, Li L, Liu Z, Xie N. Enhancing the Textural Properties of Tibetan Pig Sausages via Zanthoxylum bungeanum Aqueous Extract: Polyphenol-Mediated Quality Improvements. Foods. 2025; 14(9):1639. https://doi.org/10.3390/foods14091639
Chicago/Turabian StyleHuang, Jingjing, Haiqiu Wei, Zhang Luo, Liang Li, Zhendong Liu, and Ningning Xie. 2025. "Enhancing the Textural Properties of Tibetan Pig Sausages via Zanthoxylum bungeanum Aqueous Extract: Polyphenol-Mediated Quality Improvements" Foods 14, no. 9: 1639. https://doi.org/10.3390/foods14091639
APA StyleHuang, J., Wei, H., Luo, Z., Li, L., Liu, Z., & Xie, N. (2025). Enhancing the Textural Properties of Tibetan Pig Sausages via Zanthoxylum bungeanum Aqueous Extract: Polyphenol-Mediated Quality Improvements. Foods, 14(9), 1639. https://doi.org/10.3390/foods14091639









