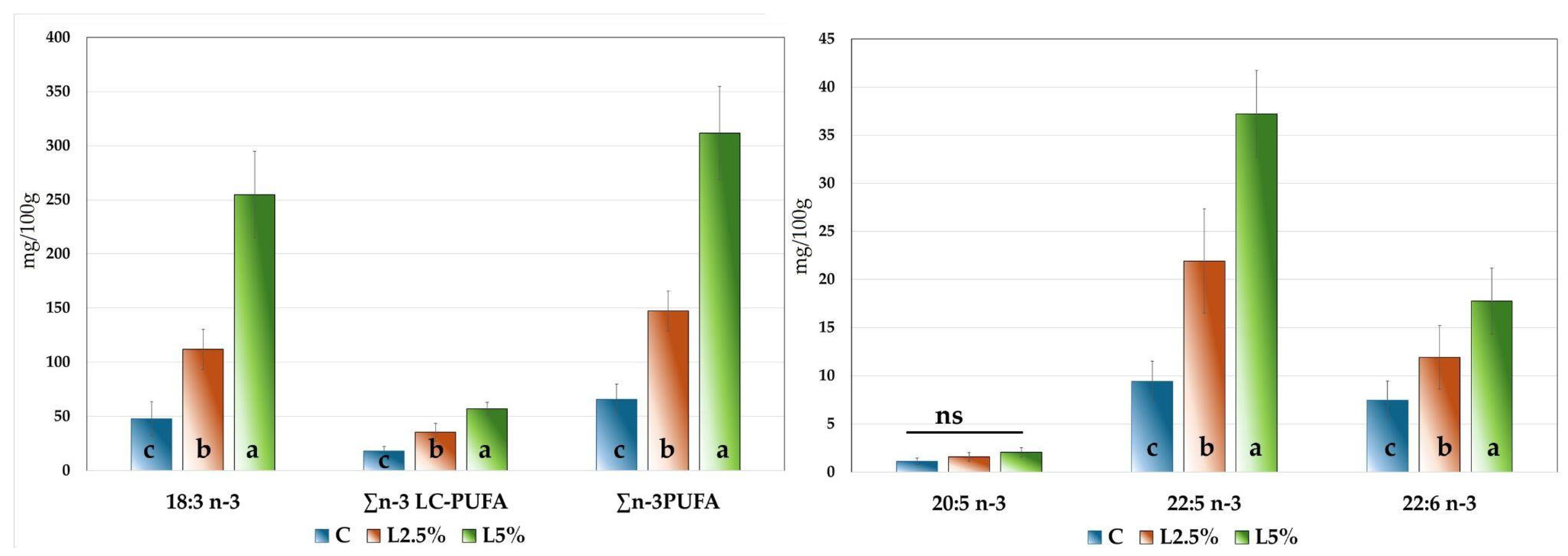
In contrast, ∑PUFA increased slightly with EL supplementation, with the highest value recorded in the L5% group (40.31%), which differed significantly from the C group (p = 0.011). These differences were primarily due to an increase in n-3 PUFA, as the meat of rabbits in the L5% group showed a marked enrichment in these fatty acids compared to the C group (9.11% vs. 3.18%, respectively), attributable to the supplementation with EL.
Among the n-6 PUFA, LA (18:2 n-6) was the most abundant, showing no significant differences among groups (p = 0.316), with a mean value of 28.01%. In contrast, significant differences (p < 0.05) were observed for long-chain n-6 PUFA (n-6 LC-PUFA). Specifically, eicosadienoic acid (20:2 n-6) decreased from 0.27% in the C group to 0.21% in L5%, and arachidonic acid (20:4 n-6), the second most abundant n-6 PUFA in rabbit meat, decreased from 4.46% to 3.25% between C and L5% groups, respectively.
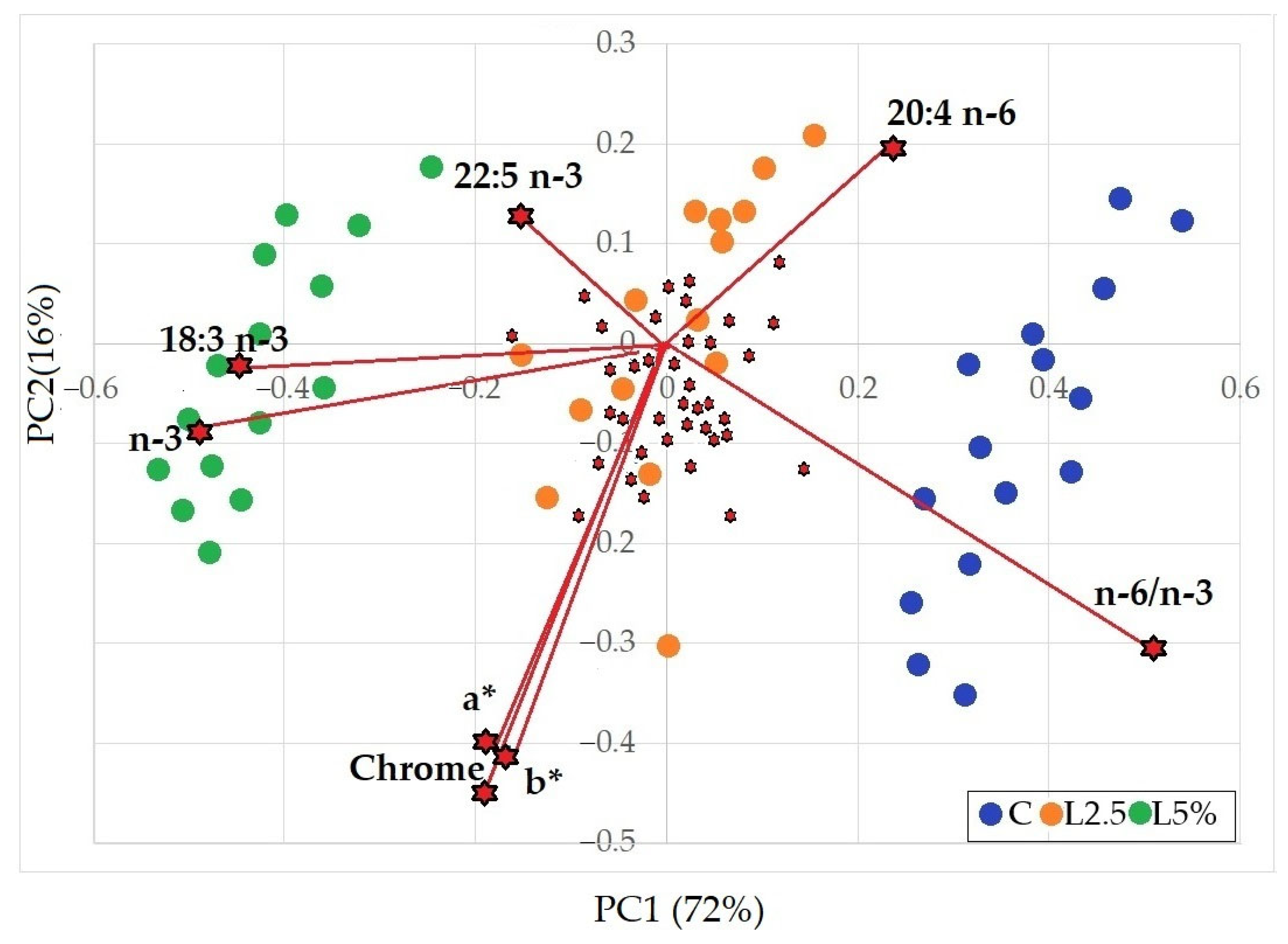
The experimental intervention, i.e., dietary inclusion of n-3 PUFA via EL, significantly affected (p < 0.001) the composition of ALA (18:3 n-3), the predominant n-3 PUFA in rabbit meat. Its content increased with the linseed level in the diet, from 2.27% in the C group, to 4.13% in L2.5%, and up to 7.42% in L5%, with all three groups differing significantly from one another. This represented an approximate +45 percentage point (p.p.) increase among groups.
The proportion of n-3 PUFAs in meat affects the values of certain nutritional indices, as reported in
Table 7. The n-6/n-3 ratio ranged from 10.79 in group C to 3.34 in the L5% group. The L2.5% group showed an intermediate value, which nevertheless differed significantly (
p < 0.001) from those of the other two groups. The SFA/PUFA ratio exhibited significant differences (
p = 0.004), only between the C and L5% groups, with a difference of −11 p.p. The lowest values for both the TI and AI were reported in the L5% group (
p < 0.01) compared to the other two groups. In detail TI was 0.576 for L5% vs. 0.827 as the average of the other two groups, and the AI index 0.451 for L5% vs. 0.504 as the average of the other two. For PI index, only group C differed significantly from L5% (+11 p.p.). The amount of malondialdehyde in the meat was analysed to assess the extent of lipid oxidation, the two EL-supplemented groups showed TBARS values that did not differ significantly from each other but were significantly higher than those of the C group (0.057 mg/kg as the average of the EL-supplemented groups vs. 0.045 mg/kg in the C group).
Notably, in the control group, DPA represented 52% of the total n-3 LC-PUFAs, whereas in the supplemented groups this proportion increased to 62%, highlighting the significant impact of flaxseed supplementation on the long-chain fatty acid profile of rabbit meat.
Evaluation of Proximate Composition and Fatty Acid Profile in Relation to Dietary and Metabolic Factors
As shown in
Table 1, although the diets were isoenergetic, the EL-supplemented groups showed a higher lipid content, which may contribute to greater fat accumulation in the meat [
63]. Several studies [
10,
16,
40,
57] have reported lower fat percentages, with no significant differences attributable to linseed supplementation. However, in those studies, chemical analyses were performed on the LTL muscle, which is typically the leanest muscle in the carcass. In contrast, the samples analysed in the present study were derived from mixed meat portions taken from various carcass regions. Other authors have even reported higher fat values in thigh meat than those observed in our study, particularly when animals were slaughtered at older ages or reached heavier final weights [
29,
58].
Rabbit meat is naturally characterised by a relatively high PUFA content, ranging from 35% to 42%, levels typically found only in game meats [
7,
64]. In contrast, MUFA levels remain low, even in rabbits fed diets rich in oleic acid [
9,
16]. When compared to other types of meat, rabbit meat shows a similar SFA content (approximately 38.5%) to pork, beef, and veal, but higher than that of chicken (29.5%) [
6,
65].
However, recent feeding strategies aimed at improving the health value of rabbit meat have led to modifications in dietary composition. Recommendations for rabbit nutrition emphasise a higher fibre intake [
66], which has promoted a shift from concentrated feeds to greater inclusion of alfalfa and oilseeds. This approach, as shown by multiple studies [
11,
17,
57,
58,
67], has successfully lowered the SFA content of rabbit meat while increasing its PUFA proportion. Nevertheless, this reduction has mainly affected stearic acid (18:0), while palmitic acid (16:0) remained at levels comparable to those observed in other meats [
8,
10,
15,
57]. In our study, the inclusion of extruded linseed (EL) effectively reduced palmitic acid levels in meat, confirming results reported by other authors [
17,
49,
57,
68], and contributing to a more favourable lipid profile for consumers [
69].
However, particular attention should be given to the quality of PUFA, promoting the accumulation of n-3 PUFA in meat. Diets enriched with linseed effectively address this nutritional goal [
12].
Although the primary aim of our work was to enhance n-3 PUFA content, it is important to underline that EL supplementation induced broader compositional changes. In the EL groups, energy derived from NFE in the control diet was partially replaced by lipids, modifying the balance of SFA, MUFA, and PUFA. These adjustments, adopted in many experiments to correct integration of EL in the diet [
15,
17,
29,
49,
58], must be considered when interpreting the effects of EL supplementation, as differences observed among groups cannot be attributed solely to increased n-3 intake.
In this context, particular attention should be given not just to the total PUFA content, but to its quality. Linseed-based diets effectively promote ALA (18:3n-3) enrichment in rabbit meat, as confirmed by the close alignment between our data and predictive models proposed by Petracci et al. [
23]. However, the increase in ALA does not follow a strictly linear response to the inclusion level; it is also affected by the duration of supplementation, as shown by Matics et al. [
17]. Their study demonstrated a progressive rise in ALA and n-3 LC-PUFAs with longer feeding periods.
Rabbits, like all mammals, are unable to synthesise essential fatty acids de novo, such as linoleic acid (LA, 18:2n-6) and alpha-linolenic acid (ALA, 18:3n-3), which must be supplied through the diet [
70]. Once ingested, PUFAs are emulsified in the intestinal lumen and incorporated into chylomicrons following enterocyte absorption. They are then transported via the lymphatic and circulatory systems to various tissues [
71].
The balance between n-6 and n-3 fatty acids is critical, as these compounds compete for the same enzymatic pathways (desaturases and elongases) involved in the biosynthesis of LC-PUFAs [
72,
73]. These reactions form a complex cascade that converts short-chain fatty acids into long- and very-long-chain PUFAs, a process crucial for PUFA accumulation in meat.
The two main precursors, LA and ALA, undergo a sequence of reactions catalysed by desaturases and elongases. Δ6-desaturase is the first enzyme in this cascade, introducing a double bond at the Δ6 position of both LA and ALA. Elongation by ELOVL5 extends the carbon chain from C18 to C20, and Δ5-desaturase subsequently adds another double bond at the Δ5 position, leading to the formation of ARA (20:4n-6) and EPA (20:5n-3) [
71,
74,
75]. To produce DHA (22:6n-3), additional steps are required: further elongation by ELOVL2, a second Δ6-desaturation, and a peroxisomal β-oxidation step that shortens the chain. Alternatively, DHA may be synthesised from DPA via the action of Δ4-desaturase, which introduces an additional double bond [
26,
27,
75].
In rabbits, the enzymatic conversion of ALA to its long-chain derivatives, such as EPA and DHA, is limited due to relatively low Δ6-desaturase activity. As a result, the majority of ALA remains unmetabolised and is directly deposited in muscle tissue, leading to a measurable increase in total n-3 PUFA levels [
10]. This metabolic complexity is reflected in our results. While DPA levels increased significantly in both EL-supplemented groups, DHA levels showed only moderate increases. Furthermore, the L2.5% group did not consistently exhibit intermediate values across all measured fatty acids. Notably, its SFA content and levels of arachidonic acid (ARA) were statistically similar to those of the control group. This suggests that the response to EL supplementation depends not only on the intake of omega-3 fatty acids derived from linseed, but also on the relative availability of other dietary components that modulate fatty acid metabolism.
Meat, particularly that rich in PUFAs, such as chicken, rabbit, and fish, is a source of LC-PUFAs, which cannot be obtained from plant-based foods and are essential for various metabolic functions. Jayaprakash et al. [
22], in fact, described these compounds as bioactive lipids. Unlike n-6 LC-PUFAs, which are abundantly available in animal-derived products and certain plant seeds, n-3 LC-PUFAs are present in high amounts only in fish and in some algae [
25,
76]. Meat represents the main dietary source of DPA (22:5 n-3), which accumulates in the tissues of mammals and poultry [
21], while fish, although rich in EPA and DHA [
24,
76], contains comparatively lower levels of DPA [
24,
25,
26]. Although research on the health significance of DPA is limited [
26,
27], it has been suggested that DPA may play an important role in preventing cardiovascular and metabolic diseases, potentially with greater efficiency than EPA and DHA, due to its higher oxidative stability and consequently greater bioavailability [
26].
Western diets typically exhibit an n-6/n-3 ratio more than 10:1, and such elevated ratios are hypothesised to contribute to the development of chronic diseases [
72]. A ratio of 4:1 has been associated with a significant reduction in the risk of various metabolic and cardiovascular disorders [
67,
77]. The World Health Organization (WHO) recommends an intake of LA ranging from 2.5% to 9% of total energy in adults, and a minimum intake of 0.5% to 2% for ALA [
78]. The European Food Safety Authority (EFSA), in its 2010 guidelines [
79], established reference intake values of 4% of total energy for LA and 0.5% for ALA. Both the WHO and EFSA recommend a minimum daily intake of 250 mg of long-chain n-3 fatty acids.
The SFA/PUFA ratio in rabbits approaches unity, as the high content of PUFA only partially reduces the accumulation of SFA in the meat [
65,
80]. Excessive dietary intake of SFA is associated with cardiovascular diseases, metabolic disorders, and cancer; therefore, SFA consumption should be limited according to nutritional guidelines issued by health organisations, such as the WHO and EFSA [
78,
79].
The AI and TI indices, calculated from fatty acid composition, were developed by Ulbricht and Southgate [
45]. These indices represent ratios comparing certain SFA to MUFA and PUFA. The AI assigns equal coefficients to MUFA and PUFA but gives a fourfold higher weight to myristic acid due to its strong cholesterol-raising effect, while stearic acid, considered neutral, is excluded from this calculation. A higher AI value indicates that the fatty acid profile of the meat promotes greater cholesterol accumulation. Conversely, a higher TI value suggests that fat has a greater tendency to induce thrombosis, with stearic acid also regarded negatively in this index [
45].
In rabbit meat, both indices (AI and TI) are notably lower compared to other meat types [
81]. Nevertheless, supplementation with flaxseed further reduces these index values, as reported in some experiments [
10,
18].
Additionally, PUFAs that are not incorporated into membrane phospholipids may be stored in muscle as triglycerides or catabolised via β-oxidation, particularly in the mitochondria, where they are used to generate energy. Among all fatty acids, n-3 PUFAs are those most readily oxidised in oxidative muscle fibres [
44].
The PI, which was associated with the presence of PUFAs in the meat, is characterised by greater instability due to the number of double bonds in FAs. It showed a trend opposite to that of the SFA/PUFA ratio.
The peroxidation index closely correlates with TBARS values, indicating the limited stability of polyunsaturated fatty acids, which increase when animals are supplemented with EL. Additionally, both parameters are consistent with the myoglobin oxidation index (measured as the absorbance ratio at 580 nm/630 nm; see
Table 4). The impact of dietary inclusion of linseed on lipid instability has also been highlighted by several authors [
10,
15,
23,
56,
68]. However, as shown in
Figure 2, it is important to highlight that the sum of n-3 PUFAs exceeded 300 mg/100 g of meat, the threshold defined by EFSA [
82,
83] for a food product to be labelled as a source of omega-3 fatty acids. These findings support the potential of EL-enriched diets to produce functional meat products