Continuous Liquid–Liquid Extraction of High-Purity Lutein from Chlorella vulgaris via Centrifugal Partition Chromatography: Utilizing Limonene as Renewable Solvent for Microalgae Biomass Valorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. HPLC Analysis
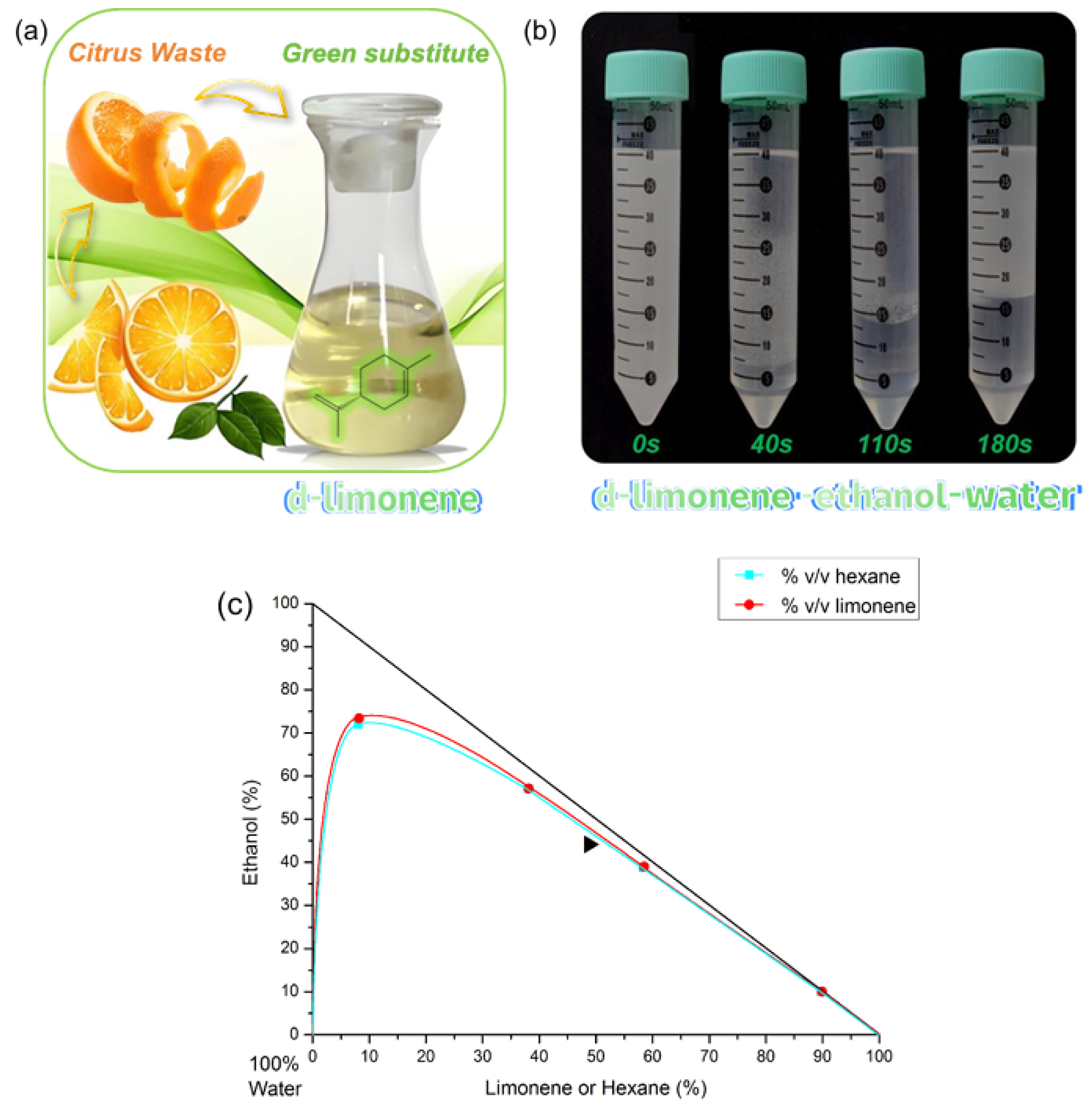
2.4. Limonene-Based Green Biphasic Liquid Systems
2.4.1. Ternary Phase Diagram
2.4.2. Phase Composition Determination
2.5. Determination of Partition Coefficient (KD)
2.6. HPCPC Separation
2.6.1. Instrument
2.6.2. Preparation of Biphasic Solvent System and Sample Solution
2.6.3. CPC Procedures
2.6.4. Elution–Extrusion Separation Mode
3. Results and Discussion
3.1. Physicochemical Properties of Limonene-Based Green Biphasic Liquid Systems
3.2. Comparison of the Ternary Phase Diagram
3.3. Partition Coefficient of Lutein in Limonene-Based Liquid System
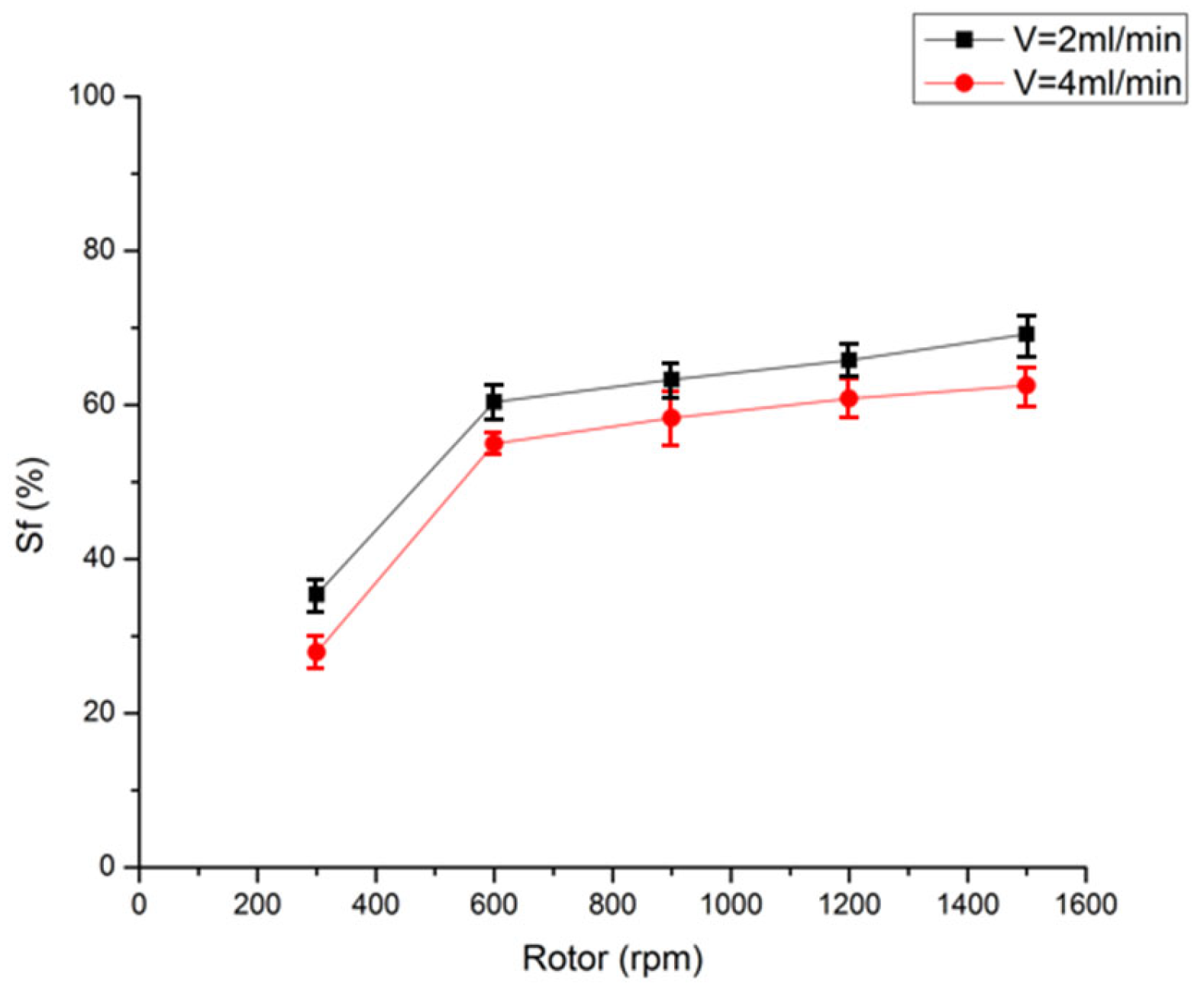
3.4. Stationary Phase Retention of Limonene-Based Liquid System
3.4.1. Effect of Rotation Speed
3.4.2. Impact of Flow Rate of Mobile Phase
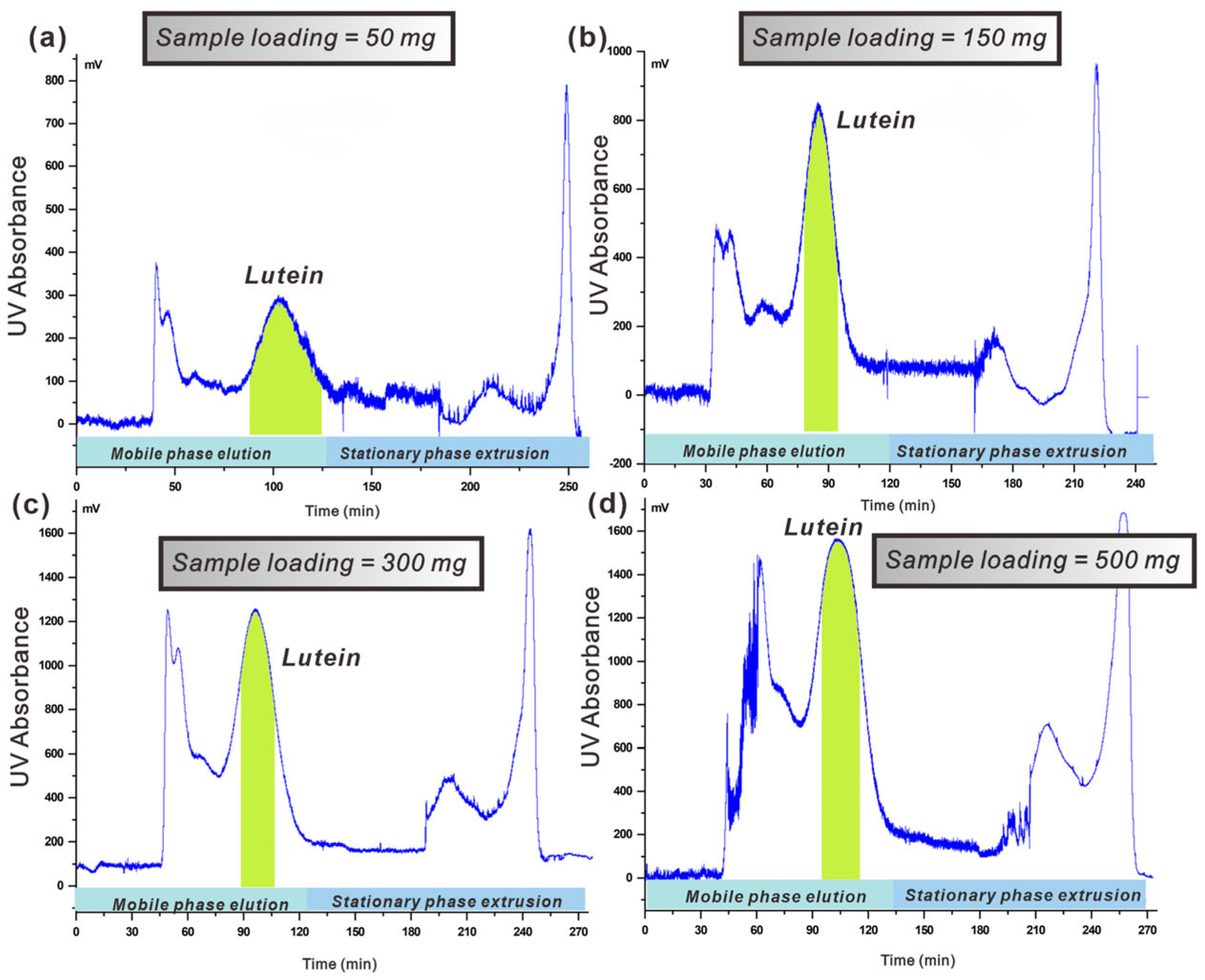
3.4.3. Effect of the Sample Loading Capacity
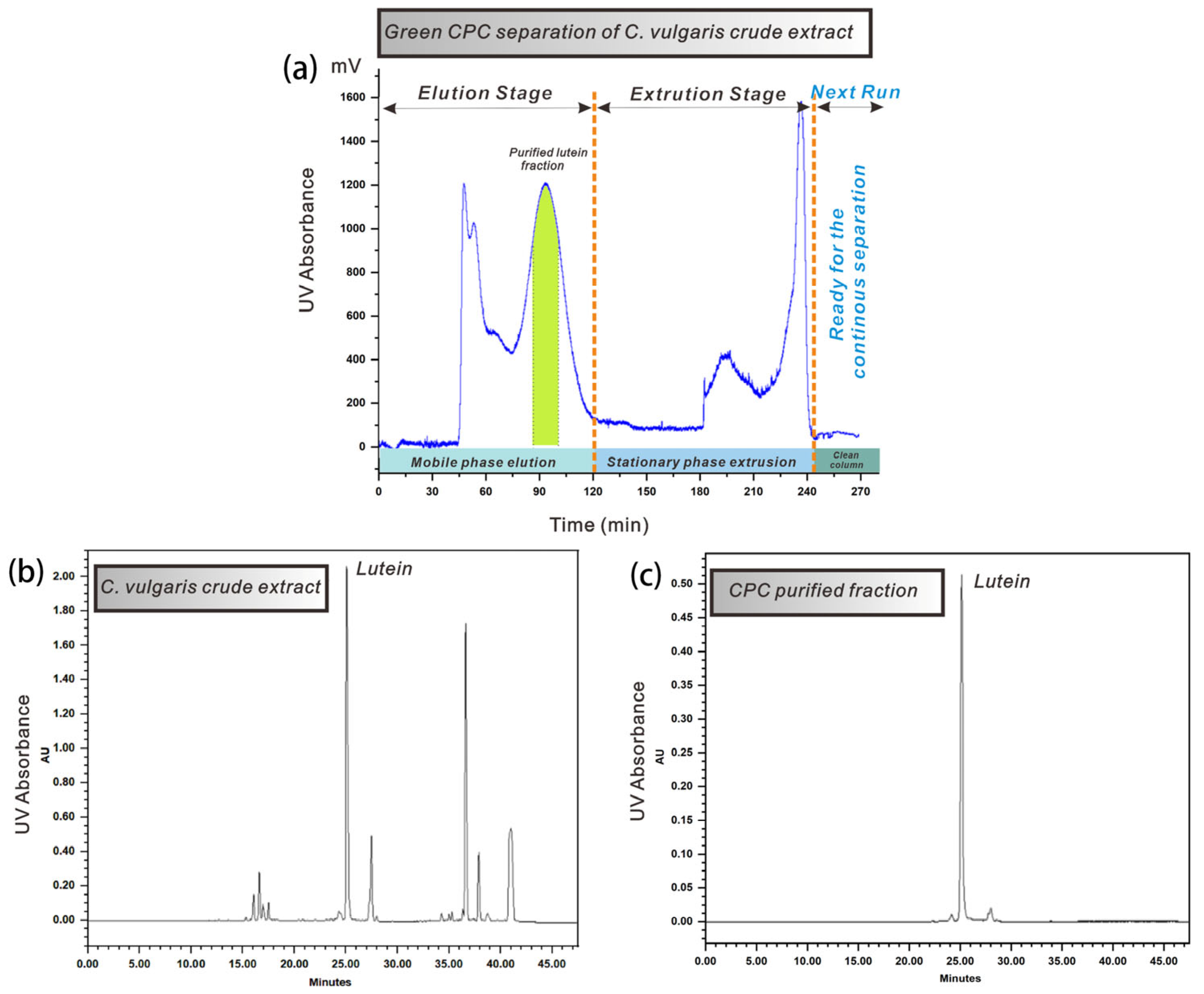
3.5. Elution–Extrusion CPC Separation of Lutein
3.5.1. Elution–Extrusion CPC Separation
3.5.2. Use of Limonene as “Green” Alternative Solvent
3.5.3. Elution–Extrusion Mode for Continuous and Sustainable Separation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.T.; Shariff, M.; Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of Microalga Chlorella vulgaris in Aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein Production from Microalgae: A Review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef] [PubMed]
- Canelli, G.; Murciano Martínez, P.; Austin, S.; Ambühl, M.E.; Dionisi, F.; Bolten, C.J.; Carpine, R.; Neutsch, L.; Mathys, A. Biochemical and Morphological Characterization of Heterotrophic Crypthecodinium cohnii and Chlorella vulgaris Cell Walls. J. Agric. Food Chem. 2021, 69, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of Nutraceutical Potential of the Microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- Ramasamy, S.; Arun, S.; Pugazhenthi, G.; Pakshirajan, K. Lutein as a High-Value Product from Anaerobic Digestate Using the Microalgae Chlorella Vulgaris: Downstream Processing and Application in Poultry. J. Water Process. Eng. 2024, 62, 105357. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; Yang, X.; Li, L.; Luo, D.; Liu, Z.; Jia, L. Transcriptome and Re-Sequencing Analyses Reveal Photosynthesis-Related Genes Involvement in Lutein Accumulation in Yellow Taproot Mutants of Carrot. Agronomy 2022, 12, 1866. [Google Scholar] [CrossRef]
- Jv, D.; Ji, T.; Xu, Z.; Li, A.; Chen, Z. The Remarkable Enhancement of Photo-Stability and Antioxidant Protection of Lutein Coupled with Carbon-Dot. Food Chem. 2023, 405, 134551. [Google Scholar] [CrossRef]
- Long, A.C.; Kuchan, M.; Mackey, A.D. Lutein as an Ingredient in Pediatric Nutritionals. J. AOAC Int. 2019, 102, 1034–1043. [Google Scholar] [CrossRef]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein Decreases Oxidative Stress and Inflammation in Liver and Eyes of Guinea Pigs Fed a Hypercholesterolemic Diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, X.; Wang, Z.-Q.; Yao, R.; Chen, M.-D.; Gao, B.-Y.; Zhang, C.-W.; Niu, J. Effects of Barranca Yajiagengensis Powder in the Diet of Trachinotus Ovatus on the Growth Performance, Antioxidant Capacity, Immunity and Morphology of the Liver and Intestine. Antioxidants 2022, 11, 1220. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Asaoka, R.; Gellermann, W.; Bernstein, P.S. Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants 2021, 10, 1857. [Google Scholar] [CrossRef]
- Yoshida, K.; Sakai, O.; Honda, T.; Kikuya, T.; Takeda, R.; Sawabe, A.; Inaba, M.; Koike, C. Effects of Astaxanthin, Lutein, and Zeaxanthin on Eye–Hand Coordination and Smooth-Pursuit Eye Movement after Visual Display Terminal Operation in Healthy Subjects: A Randomized, Double-Blind Placebo-Controlled Intergroup Trial. Nutrients 2023, 15, 1459. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Composition of Lutein Ester Regioisomers in Marigold Flower, Dietary Supplement, and Herbal Tea. J. Agric. Food Chem. 2015, 63, 9740–9746. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.-R.; Chen, C.-W.; Chang, J.S.; Dong, C.-D. Enhanced Mixotrophic Production of Lutein and Lipid from Potential Microalgae Isolate Chlorella Sorokiniana C16. Bioresour. Technol. 2023, 386, 129477. [Google Scholar] [CrossRef]
- Chen, H.; Lu, W. Advancements of Lutein Extraction from Microalgae Biomass: A Review. J. Biobased Mater. Bioenergy 2022, 16, 533–548. [Google Scholar] [CrossRef]
- Ito, Y.; Bowman, R.L. Countercurrent Chromatography with Flow-through Coil Planet Centrifuge. Science 1971, 173, 420–422. [Google Scholar] [CrossRef]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent Separation of Natural Products: An Update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef]
- Luca, S.V.; Bruesewitz, M.; Minceva, M. Evaluation of Advanced Liquid–Liquid Chromatography Operating Modes for the Tetrahydrocannabinol-Remediation of Hemp Extracts. Chem. Eng. Sci. 2023, 282, 119279. [Google Scholar] [CrossRef]
- Friesen, J.B.; Ahmed, S.; Pauli, G.F. Qualitative and Quantitative Evaluation of Solvent Systems for Countercurrent Separation. J. Chromatogr. A 2015, 1377, 55–63. [Google Scholar] [CrossRef]
- Almeida, M.R.; Ferreira, F.; Domingues, P.; Coutinho, J.A.P.; Freire, M.G. Towards the Purification of IgY from Egg Yolk by Centrifugal Partition Chromatography. Sep. Purif. Technol. 2022, 299, 121697. [Google Scholar] [CrossRef]
- Soares, B.P.; Santos, J.H.P.M.; Martins, M.; Almeida, M.R.; Santos, N.V.; Freire, M.G.; Santos-Ebinuma, V.C.; Coutinho, J.A.P.; Pereira, J.F.B.; Ventura, S.P.M. Purification of Green Fluorescent Protein Using Fast Centrifugal Partition Chromatography. Sep. Purif. Technol. 2021, 257, 117648. [Google Scholar] [CrossRef]
- Santos, J.H.P.M.; Almeida, M.R.; Martins, C.I.R.; Dias, A.C.R.V.; Freire, M.G.; Coutinho, J.A.P.; Ventura, S.P.M. Separation of Phenolic Compounds by Centrifugal Partition Chromatography. Green Chem. 2018, 20, 1906–1916. [Google Scholar] [CrossRef]
- Sun, C.; Theodoropoulos, C.; Scrutton, N.S. Techno-Economic Assessment of Microbial Limonene Production. Bioresour. Technol. 2020, 300, 122666. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus Waste as Feedstock for Bio-Based Products Recovery: Review on Limonene Case Study and Energy Valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Faure, K.; Bouju, E.; Suchet, P.; Berthod, A. Use of Limonene in Countercurrent Chromatography: A Green Alkane Substitute. Anal. Chem. 2013, 85, 4644–4650. [Google Scholar] [CrossRef]
- Faure, K.; Bouju, E.; Suchet, P.; Berthod, A. Limonene in Arizona Liquid Systems Used in Countercurrent Chromatography. I Physicochemical Properties. Anal. Bioanal. Chem. 2014, 406, 5909–5917. [Google Scholar] [CrossRef]
- Faure, K.; Bouju, E.; Doby, J.; Berthod, A. Limonene in Arizona Liquid Systems Used in Countercurrent Chromatography. II Polarity and Stationary-Phase Retention. Anal. Bioanal. Chem. 2014, 406, 5919–5926. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Lu, Y. Preparative Separation of Phytosterol Analogues from Green Alga Chlorella vulgaris Using Recycling Counter-current Chromatography. J. Sep. Sci. 2017, 40, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, Y.; Hu, D.; Ye, J.; Lu, Y.; Dai, Z. One-Step Preparative Separation of Fucoxanthin from Three Edible Brown Algae by Elution-Extrusion Countercurrent Chromatography. Mar. Drugs 2022, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Berthod, A.; Hu, R.; Ma, W.; Pan, Y. Screening of Complex Natural Extracts by Countercurrent Chromatography Using a Parallel Protocol. Anal. Chem. 2009, 81, 4048–4059. [Google Scholar] [CrossRef] [PubMed]
- Buthmann, F.; Hohlmann, J.; Volpert, S.; Neuwald, M.; Hamza, D.; Schembecker, G. Counteracting Bleeding in Centrifugal Partition Chromatography: Redosing of the Stationary Phase. Separations 2024, 11, 98. [Google Scholar] [CrossRef]
- Buthmann, F.; Hohlmann, J.; Neuwald, M.; Schembecker, G. Optimizing Chromatographic Separation with Redosing: Effects on Separation Efficiency of a Model System in Centrifugal Partition Chromatography. Separations 2024, 11, 111. [Google Scholar] [CrossRef]
- Oshiro, A.A.; Jorge, A.M.S.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Evaluation of Ethanol-Based Aqueous Two-Phase Systems Performance for Application in Centrifugal Partition Chromatography (CPC). Sep. Purif. Technol. 2024, 329, 125114. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Elution−extrusion Countercurrent Chromatography. Use of the Liquid Nature of the Stationary Phase to Extend the Hydrophobicity Window. Anal. Chem. 2003, 75, 5886–5894. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Developments in the Application of Counter-Current Chromatography to Plant Analysis. J. Chromatogr. A 2006, 1112, 181–194. [Google Scholar] [CrossRef]
- Yang, F.; Quan, J.; Zhang, T.Y.; Ito, Y. Multidimensional Counter-Current Chromatographic System and Its Application. J. Chromatogr. A 1998, 803, 298–301. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, Y.; Berthod, A. Using the Liquid Nature of the Stationary Phase in Counter-Current Chromatography V. The Back-Extrusion Method. J. Chromatogr. A 2008, 1189, 10–18. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, W.; Hu, R.; Berthod, A.; Pan, Y. Rapid and Preparative Separation of Traditional Chinese Medicine Evodia Rutaecarpa Employing Elution-Extrusion and Back-Extrusion Counter-Current Chromatography: Comparative Study. J. Chromatogr. A 2009, 1216, 4140–4146. [Google Scholar] [CrossRef]

| Parameter | Unit | n-Hexane | Limonene | Ethanol |
|---|---|---|---|---|
| Molecular weight | Dalton | 86 | 136 | 46 |
| Density | g/cm3 | 0.659 | 0.841 | 0.790 |
| Water solubility a | % | 0.00024 | 0.08 | miscible |
| Ethanol solubility a | % | 33.3 | 38 | miscible |
| Boiling point | °C | 68.9 | 178 | 78.4 |
| Viscosity 25 °C | mPa·s or cP | 0.307 | 0.923 | 1.096 |
| UV cut-off wavelength | nm | 210 | 250 | 210 |
| studied biphasic liquid system: oil/ethanol/water (10/9/1, v/v) | ||||
| Density difference b | g/cm3 | +0.115 (n-hexane upper phase) | −0.009 (limonene lower phase) | / |
| Oil phase | % v/v | 42 | 43 | |
| Polar phase | % v/v | 58 | 57 | |
| Ethanol in oil phase | % v/v | 9.9 | 12.2 | |
| Water in oil phase | % v/v | 0 | 0.06 | |
| Oil in polar phase | % v/v | 20.7 | 21.4 | |
| Water in polar phase | % v/v | 8.6 | 8.8 | |
| Solvent Systems (v/v) | Partition Coefficient (KD) |
|---|---|
| n-Hexane–ethanol–water (15:9:1) | 0.13 |
| n-Hexane–ethanol–water (10:9:1) | 0.17 |
| n-Hexane–ethanol–water (10:7:3) | 1.49 |
| Limonene–ethanol–water (15:9:1) | 0.47 |
| Limonene–ethanol–water (10:9:1) | 0.75 |
| Limonene–ethanol–water (10:7:3) | 10.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Lin, X.; Ye, J.; Lu, Y. Continuous Liquid–Liquid Extraction of High-Purity Lutein from Chlorella vulgaris via Centrifugal Partition Chromatography: Utilizing Limonene as Renewable Solvent for Microalgae Biomass Valorization. Foods 2025, 14, 1637. https://doi.org/10.3390/foods14091637
Kong W, Lin X, Ye J, Lu Y. Continuous Liquid–Liquid Extraction of High-Purity Lutein from Chlorella vulgaris via Centrifugal Partition Chromatography: Utilizing Limonene as Renewable Solvent for Microalgae Biomass Valorization. Foods. 2025; 14(9):1637. https://doi.org/10.3390/foods14091637
Chicago/Turabian StyleKong, Weiheng, Xianjiang Lin, Jing Ye, and Yanbin Lu. 2025. "Continuous Liquid–Liquid Extraction of High-Purity Lutein from Chlorella vulgaris via Centrifugal Partition Chromatography: Utilizing Limonene as Renewable Solvent for Microalgae Biomass Valorization" Foods 14, no. 9: 1637. https://doi.org/10.3390/foods14091637
APA StyleKong, W., Lin, X., Ye, J., & Lu, Y. (2025). Continuous Liquid–Liquid Extraction of High-Purity Lutein from Chlorella vulgaris via Centrifugal Partition Chromatography: Utilizing Limonene as Renewable Solvent for Microalgae Biomass Valorization. Foods, 14(9), 1637. https://doi.org/10.3390/foods14091637








