Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. WBA Extraction
2.2. Cell Cultures
2.2.1. Cell Lines
2.2.2. Cell Culture Condition
2.3. Cell Spheroids Harvesting and Dissociation
2.4. Growth Performance of Cancer Cells with WBA Induction
2.4.1. SW480 3D Culture, WBA Induction, and Harvesting
2.4.2. HepG2 Culture, WBA Induction, and Harvesting
2.4.3. HeLa Culture, WBA Induction, and Harvesting
2.5. Cell Morphology and Image Analysis
2.6. Cell Count and Viability Measurement
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Statistical Analysis
3. Results
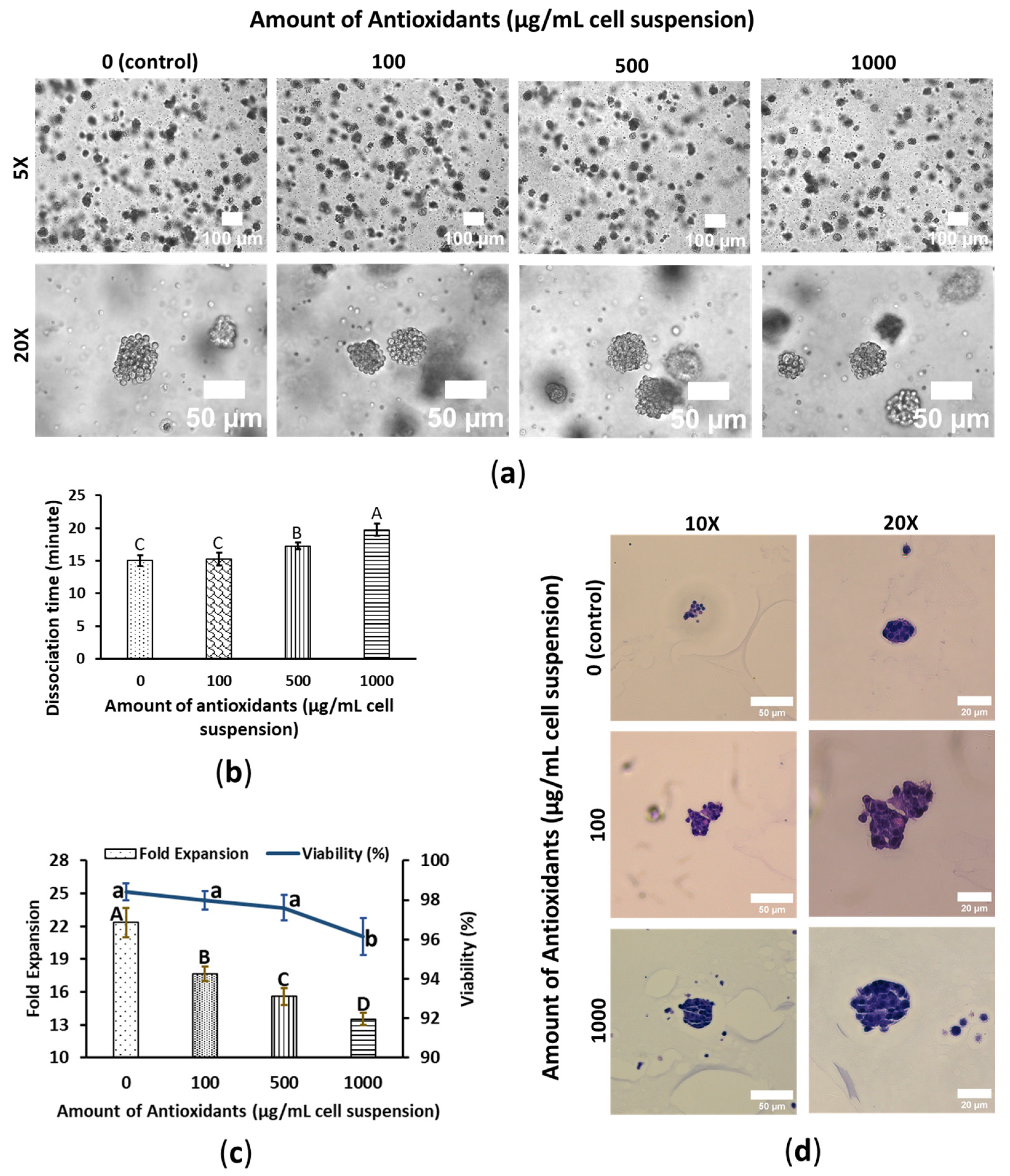
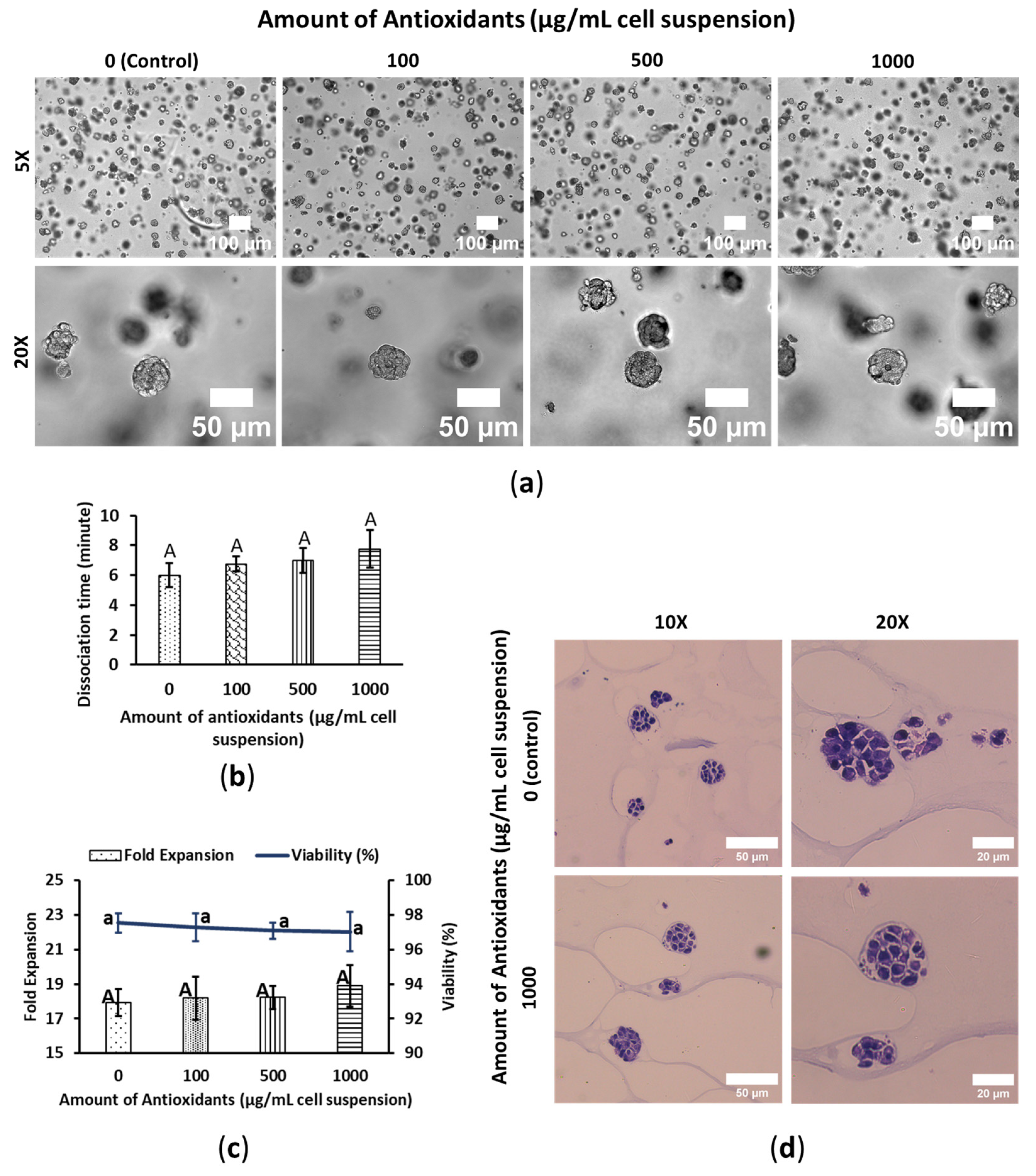
3.1. WBA Alleviates SW480′s Growth Performance
3.2. WBA Accelerates HepG2′s Growth Performance
3.3. WBA Does Not Interfere with HeLa’s Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Faraoni, P.; Laschi, S. Bioactive Compounds from Agrifood Byproducts: Their Use in Medicine and Biology. Int. J. Mol. Sci. 2024, 25, 5776. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, W.; Tilley, M.; Chen, R.; Sun, X.S.; Wang, W.; Li, Y. In Vitro Antioxidant Properties of Wheat Bran Extracts and Their Inhibitory Effects on Collagenase, Elastase, and Hyaluronidase. ACS Food Sci. Technol. 2024, 4, 1960–1966. [Google Scholar] [CrossRef]
- Shang, X.L.; Liu, C.Y.; Dong, H.Y.; Peng, H.H.; Zhu, Z.Y. Extraction, Purification, Structural Characterization, and Antioxidant Activity of Polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Kaur, A.; Yadav, M.P.; Singh, B.; Bhinder, S.; Simon, S.; Singh, N. Isolation and Characterization of Arabinoxylans from Wheat Bran and Study of Their Contribution to Wheat Flour Dough Rheology. Carbohydr. Polym. 2019, 221, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Dupoiron, S.; Lameloise, M.L.; Bedu, M.; Lewandowski, R.; Fargues, C.; Allais, F.; Teixeira, A.R.S.; Rakotoarivonina, H.; Rémond, C. Recovering Ferulic Acid from Wheat Bran Enzymatic Hydrolysate by a Novel and Non-Thermal Process Associating Weak Anion-Exchange and Electrodialysis. Sep. Purif. Technol. 2018, 200, 75–83. [Google Scholar] [CrossRef]
- Ye, Z.W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative Stress, Redox Regulation and Diseases of Cellular Differentiation. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1607–1621. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Reactive Oxygen and Nitrogen Species in Carcinogenesis: Implications of Oxidative Stress on the Progression and Development of Several Cancer Types. Mini-Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Nurgali, K. The Emerging Antioxidant Paradigm of Mesenchymal Stem Cell Therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon Cancer and Colorectal Cancer: Prevention and Treatment by Potential Natural Products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef] [PubMed]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Cristani, M.; Citarella, A.; Carnamucio, F.; Micale, N. Nano-Formulations of Natural Antioxidants for the Treatment of Liver Cancer. Biomolecules 2024, 14, 1031. [Google Scholar] [CrossRef]
- Casas-Grajales, S. Antioxidants in Liver Health. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 59. [Google Scholar] [CrossRef]
- Silva, G.Á.F.; Nunes, R.A.L.; Morale, M.G.; Boccardo, E.; Aguayo, F.; Termini, L. Oxidative Stress: Therapeutic Approaches for Cervical Cancer Treatment. Clinics 2018, 73, e548s. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, S.T.; Chen, S.Y.; Chen, G.S.; Wu, C.C.; Huang, S.M. Mechanisms and Applications of the Anti-Cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Conklin, D.R.; Chen, H.; Wang, L.; Sang, S. 5-Alk(en)ylresorcinols as the major active components in wheat bran inhibit human colon cancer cell growth. Bioorg. Med. Chem. 2011, 19, 3973–3982. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoon, W.J.; Kim, S.S. Phytochemical compositions of immature wheat bran, and its antioxidant capacity, cell growth inhibition, and apoptosis induction through tumor suppressor gene. Molecules 2016, 21, 1292. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Moon, J.C.; Hah, Y.S.; Kim, W.Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W.; et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.S.; Lee, J.; Amarakoon, D.; Lou, Z.; Noronha, L.E.; Herald, T.J.; Perumal, R.; Smolensky, D. Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate. Int. J. Mol. Sci. 2021, 22, 8286. [Google Scholar] [CrossRef]
- García-Gutiérrez, N.; Maldonado-Celis, M.E.; Rojas-López, M.; Loarca-Piña, G.F.; Campos-Vega, R. The Fermented Non-Digestible Fraction of Spent Coffee Grounds Induces Apoptosis in Human Colon Cancer Cells (SW480). J. Funct. Foods 2017, 30, 237–246. [Google Scholar] [CrossRef]
- Safrida, S.; Budijanto, S.; Nuraida, L.; Priosoeryanto, B.P. Potency of Bioactive Compound of Rice Bran for Colon Cancer Prevention. J. Kesehat. Masy. 2020, 16, 284–295. [Google Scholar] [CrossRef]
- Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnson, S.; Blanchard, C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules 2019, 24, 2465. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, V.R.M.; Carrera, I.; Corzo, L.; Cacabelos, R. Role of Bioactive Lipofishins in Prevention of Inflammation and Colon Cancer. Semin. Cancer Biol. 2019, 56, 175–184. [Google Scholar] [CrossRef]
- Malcomson, F.C.; Willis, N.D.; Mathers, J.C. Is resistant starch protective against colorectal cancer via modulation of the WNT signalling pathway? Proc. Nutr. Soc. 2015, 74, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Hashimoto, N.; Fukushima, M. Relationships among Alcoholic Liver Disease, Antioxidants, and Antioxidant Enzymes. World J. Gastroenterol. 2016, 22, 37–49. [Google Scholar] [CrossRef]
- Ham, H.; Yoon, S.W.; Kim, I.H.; Kwak, J.; Lee, J.S.; Jeong, H.s.; Lee, J. Protective Effects of Unsaponifiable Matter from Rice Bran on Oxidative Damage by Modulating Antioxidant Enzyme Activities in HepG2 Cells. LWT-Food Sci. Technol. 2015, 61, 602–608. [Google Scholar] [CrossRef]
- Alamri, Z.Z. The Role of Liver in Metabolism: An Updated Review with Physiological Emphasis. Int. J. Basic Clin. Pharmacol. 2018, 7, 2271–2276. [Google Scholar] [CrossRef]
- Blondet, N.M.; Messner, D.J.; Kowdley, K.V.; Murray, K.F. Mechanisms of hepatocyte detoxification. In Physiology of the Gastrointestinal Tract; Academic Press: Cambridge, MA, USA, 2018; pp. 981–1001. [Google Scholar]
- Jauković, A.; Abadjieva, D.; Trivanović, D.; Stoyanova, E.; Kostadinova, M.; Pashova, S.; Kestendjieva, S.; Kukolj, T.; Jeseta, M.; Kistanova, E.; et al. Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties. Stem Cell Rev. Rep. 2020, 16, 853–875. [Google Scholar] [CrossRef]
- Yildiz-Ozturk, E.; Saglam-Metiner, P.; Yesil-Celiktas, O. Lung Carcinoma Spheroids Embedded in a Microfluidic Platform. Cytotechnology 2021, 73, 457–471. [Google Scholar] [CrossRef]
- Baster, Z.; Li, L.; Kukkurainen, S.; Chen, J.; Pentikäinen, O.; Győrffy, B.; Hytönen, V.P.; Zhu, H.; Rajfur, Z.; Huang, C. Cyanidin-3-Glucoside Binds to Talin and Modulates Colon Cancer Cell Adhesions and 3D Growth. FASEB J. 2020, 34, 2227–2237. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Dey, T.; Kumar, L.; Kar, S.; Sarkar, R.; Ghorai, M.; Malik, S.; Jha, N.K.; Vellingiri, B.; Kesari, K.K.; et al. Cellular Landscaping of Cisplatin Resistance in Cervical Cancer. Biomed. Pharmacother. 2022, 153, 113345. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA Damage Protection Potentials of Selected Phenolic Acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Moreno, K.; Chávez-García, D.; Hirata, G.; Vazquez-Duhalt, R. Monolayer (2D) or Spheroids (3D) Cell Cultures for Nanotoxicological Studies? Comparison of Cytotoxicity and Cell Internalization of Nanoparticles. Toxicol. Vitr. 2022, 85, 105461. [Google Scholar] [CrossRef]
- Chai, Y.W.; Lee, E.H.; Gubbe, J.D.; Brekke, J.H. 3D Cell Culture in a Self-Assembled Nanofiber Environment. PLoS ONE 2016, 11, e0162853. [Google Scholar] [CrossRef] [PubMed]

| SN | WBA Extract Concentration (µg/mL Cell Suspension) 1 | In 24-WP | 2% WBA Extract and Fresh Medium Addition |
|---|---|---|---|
| 01 | 100 | 200 µg/2 mL/well | 10 uL 2% WBA extract with 1500 uL fresh medium |
| 02 | 500 | 1000 µg/2 mL/well | 50 uL 2% WBA extract with 1500 uL fresh medium |
| 03 | 1000 | 2000 µg/2 mL/well | 100 uL 2% WBA extract with 1500 uL fresh medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.S.; Qi, G.; Li, C.; Li, Y.; Wang, W.; Atala, A.; Sun, X.S. Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells. Foods 2025, 14, 1633. https://doi.org/10.3390/foods14091633
Rahman MS, Qi G, Li C, Li Y, Wang W, Atala A, Sun XS. Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells. Foods. 2025; 14(9):1633. https://doi.org/10.3390/foods14091633
Chicago/Turabian StyleRahman, Md Sharifur, Guangyan Qi, Cheng Li, Yonghui Li, Weiqun Wang, Anthony Atala, and Xiuzhi Susan Sun. 2025. "Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells" Foods 14, no. 9: 1633. https://doi.org/10.3390/foods14091633
APA StyleRahman, M. S., Qi, G., Li, C., Li, Y., Wang, W., Atala, A., & Sun, X. S. (2025). Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells. Foods, 14(9), 1633. https://doi.org/10.3390/foods14091633









