Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions
Abstract
1. Introduction
| Legal Instrument | Description | Binding Nature | Examples | Ref |
|---|---|---|---|---|
| Regulation | Legislative acts that apply directly in all Member States without national implementation. | Legally binding in all Member States | Regulation (EC) No. 178/2002 (General Food Law) Regulation (EC) No. 1333/2008 (Food Additives) | [7,12] |
| Directive | Set objectives that Member States must achieve, but each MS chooses how to implement them. | Legally binding, but requires transposition into national law | Directive 2009/128/EC (Pesticide Sustainable Use) | [13] |
| Decision | Legally binding acts applicable to specific MSs, businesses, or individuals. Often used in crisis management. | Legally binding for the addressed parties | Decision 2002/657/EC (Performance of analytical methods and the interpretation of results) | [14] |
| Recommendation | Non-binding guidance to encourage best practices and policy direction. | Not legally binding | Commission Recommendation (EU) 2017/84 (Mineral Oil Hydrocarbons in Food) Commission Recommendation (EU) No. 2018/464 (Monitoring of metals and iodine in seaweed, halophytes and products based on seaweed) | [15,16] |
| Opinion | A formal non-binding instrument used by EU institutions to express views or provide guidance without imposing obligations. | Not legally binding | Opinion of the European Economic and Social Committee on ‘Towards a Fair Food Supply Chain’ (Exploratory opinion) EESC 2021/02472 | [17] |
| Report | Scientific assessments or policy evaluations that inform decision-making. | Not legally binding | Report From the EC to the EP and the Council on food and food ingredients treated with ionizing radiation for the years 2020–2021 COM/2023/676 | [18] |
| Others | Minutes, communication, staff working documents, proposal for a regulation, question. | Not legally binding | Several types of acts |
2. Regulatory Framework, Chemical Classes, and Regulations
2.1. General Food Law
2.2. Chemicals in Food
2.3. Controversies
3. Risk Assessment of Chemicals in Food
- -
- Reducing or replacing animal testing by using in vitro, in silico, and in chemico models;
- -
- Identifying, evaluating, and minimizing uncertainties in exposure assessments.
- -
- Filling knowledge gaps, particularly in mechanistic toxicology and exposure modeling;
- -
- Assessing the effects of exposure to chemical mixtures, including multiple chemicals and other stressors.
Cumulative Risk Assessment of Chemicals in Food
4. Actors of Chemical Food Safety and Role of Analytical Controls and Monitoring Studies
Analytical Controls and Monitoring Studies in Chemical Food Safety
- -
- Regulation (EU) No. 333/2007: Establishes criteria for the detection of heavy metals in foodstuffs [132].
- -
- Regulation (EU) No. 2017/625: Provides a framework for food safety controls [34].
- -
- Regulation (EU) No. 2019/627: Defines procedures for official laboratory testing [81].
- -
- Decision 2002/657/EC: Specifies criteria for the validation of analytical methods for veterinary drug residue detection [13].
- -
- Regulation (EU) No. 2021/808: Updates method performance criteria for residue analysis.
5. New Challenges in Chemical Food Safety
5.1. Emerging Contaminants
5.1.1. PFASs
5.1.2. Microplastics and Nanoplastics
5.1.3. Novel Maillard Reaction-Derived Chemical Contaminants
5.2. Artificial Intelligence
5.3. Multi-Source Data Fusion
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fung, F.; Wang, H.-S.; Menon, S. Food Safety in the 21st Century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, D.; Rietjens, I.M.; Zheng, L. Current and Emerging Issues in Chemical Food Safety. Curr. Opin. Food Sci. 2025, 62, 101284. [Google Scholar] [CrossRef]
- Food Safety–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=legissum:food_safety (accessed on 4 April 2025).
- European Commission. Farm to Fork Strategy—For a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Food: From Farm to Fork Statistics. Available online: https://ec.europa.eu/eurostat/web/products-pocketbooks/-/ks-30-08-339 (accessed on 13 February 2021).
- Smaoui, S.; Agriopoulou, S.; D’Amore, T.; Tavares, L.; Mousavi Khaneghah, A. The Control of Fusarium Growth and Decontamination of Produced Mycotoxins by Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 11125–11152. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. Off. J. Eur. Communities 2002, 31, 1–24. [Google Scholar]
- D’Amore, T.; Taranto, A.D.; Berardi, G.; Vita, V.; Iammarino, M. Nitrate as Food Additives: Reactivity, Occurrence, and Regulation. In Nitrate Handbook; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-0-429-32680-6. [Google Scholar]
- Types of Legislation|European Union. Available online: https://european-union.europa.eu/institutions-law-budget/law/types-legislation_en (accessed on 25 March 2025).
- Van der Meulen, B.M.J. The Structure of European Food Law. Laws 2013, 2, 69–98. [Google Scholar] [CrossRef]
- Gokani, N. Healthier Food Choices: From Consumer Information to Consumer Empowerment in EU Law. J. Consum. Policy 2024, 47, 271–296. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- European Commission. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Off. J. Eur. Union 2009, 309, 71–86. [Google Scholar]
- European Commission. Commission Decision of 14 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Notified under Document Number C(2002) 3044) (Text with EEA Relevance) (2002/657/EC)Text with EEA Relevance. Off. J. Eur. Union 2002, 221, 8–36. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2017/84 of 16 January 2017 on the Monitoring of Mineral Oil Hydrocarbons in Food and in Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2017, 12, 95–96. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2018/464 of 19 March 2018 on the Monitoring of Metals and Iodine in Seaweed, Halophytes and Products Based on Seaweed. Off. J. Eur. Union 2018, 78, 16–18. [Google Scholar]
- European Economic and Social Committee. Opinion of the European Economic and Social Committee on ‘Towards a Fair Food Supply Chain’ EESC 2021/02472 (Exploratory Opinion). Off. J. Eur. Union 2021, 517, 38–44. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament and the Council on Food and Food Ingredients Treated with Ionising Radiation for the Years 2020–2021; COM/2023/676; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Gensch, L.; Jantke, K.; Rasche, L.; Schneider, U.A. Pesticide Risk Assessment in European Agriculture: Distribution Patterns, Ban-Substitution Effects and Regulatory Implications. Environ. Pollut. 2024, 348, 123836. [Google Scholar] [CrossRef]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative Risk Assessment of Pesticide Residues in Food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int. J. Env. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef]
- Jin, J.; den Besten, H.M.W.; Rietjens, I.M.C.M.; Widjaja, F. Chemical and Microbiological Hazards Arising from New Plant-Based Foods, Including Precision Fermentation–Produced Food Ingredients. Annu. Rev. Food Sci. Technol. 2025, 16, 171–194. [Google Scholar] [CrossRef]
- Balkan, T.; Yılmaz, Ö. Method Validation, Residue and Risk Assessment of 260 Pesticides in Some Leafy Vegetables Using Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Chem. 2022, 384, 132516. [Google Scholar] [CrossRef]
- MacLachlan, D.J.; Hamilton, D. Estimation Methods for Maximum Residue Limits for Pesticides. Regul. Toxicol. Pharmacol. 2010, 58, 208–218. [Google Scholar] [CrossRef]
- Hyder; Travis, K.Z.; Welsh, Z.K.; Pate, I. Maximum Residue Levels: Fact or Fiction? Hum. Ecol. Risk Assess. Int. J. 2003, 9, 721–740. [Google Scholar] [CrossRef]
- Maxim, L.; Mazzocchi, M.; Van den Broucke, S.; Zollo, F.; Robinson, T.; Rogers, C.; Vrbos, D.; Zamariola, G.; Smith, A. Technical Assistance in the Field of Risk Communication. EFSA J. 2021, 19, e06574. [Google Scholar] [CrossRef] [PubMed]
- Coja, T.; Steinwider, J. The New European Transparency Regulation: A Panacea for EU Risk Assessment? J. Consum. Prot. Food Saf. 2022, 17, 1–3. [Google Scholar] [CrossRef]
- Attrey, D.P. Chapter 5—Role of Risk Analysis and Risk Communication in Food Safety Management. In Food Safety in the 21st Century; Gupta, R.K., Dudeja, Minhas, S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 53–68. ISBN 978-0-12-801773-9. [Google Scholar]
- European Commission. Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the Transparency and Sustainability of the EU Risk Assessment in the Food Chain and Amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. Off. J. Eur. Union 2019, 213, 1–28. [Google Scholar]
- Morvillo, M. Glyphosate Effect: Has the Glyphosate Controversy Affected the EU’s Regulatory Epistemology? Eur. J. Risk Regul. 2020, 11, 422–435. [Google Scholar] [CrossRef]
- Eissa, F.; Sebaei, A.S.; El Badry Mohamed, M. Food Additives and Flavourings: Analysis of EU RASFF Notifications from 2000 to 2022. J. Food Compos. Anal. 2024, 130, 106137. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products, Amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and Repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). Off. J. Eur. Union 2017, 95, 1–142. [Google Scholar]
- WHO; FAO. Principles and Methods for the Risk Assessment of Chemicals in Food (Environmental Health Criteria Series, 240)—International Programme on Chemical Safety (IPCS); WHO: Geneva, Switzerland, 2009; ISBN 978 92 4 157240 8. [Google Scholar]
- Gürtler, R. Risk Assessment of Food Additives. In Regulatory Toxicology; Reichl, F.-X., Schwenk, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1323–1337. ISBN 978-3-030-57499-4. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-Evaluation of Glutamic Acid (E 620), Sodium Glutamate (E 621), Potassium Glutamate (E 622), Calcium Glutamate (E 623), Ammonium Glutamate (E 624) and Magnesium Glutamate (E 625) as Food Additives. EFSA J. 2017, 15, e04910. [Google Scholar] [CrossRef]
- D’Amore, T.; Di Taranto, A.; Berardi, G.; Vita, V.; Marchesani, G.; Chiaravalle, A.E.; Iammarino, M. Sulfites in Meat: Occurrence, Activity, Toxicity, Regulation, and Detection. A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2701–2720. [Google Scholar] [CrossRef]
- D’Amore, T.; Di Taranto, A.; Berardi, G.; Vita, V.; Iammarino, M. Going Green in Food Analysis: A Rapid and Accurate Method for the Determination of Sorbic Acid and Benzoic Acid in Foods by Capillary Ion Chromatography with Conductivity Detection. LWT 2021, 141, 110841. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC (Text with EEA Relevance). Off. J. Eur. Union 2008, 354, 34–50. [Google Scholar]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, 70, 1–16. [Google Scholar]
- EFSA; Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2022 European Union Report on Pesticide Residues in Food. EFSA J. 2024, 22, e8753. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2024/989 of 2 April 2024 Concerning a Coordinated Multiannual Control Programme of the Union for 2025, 2026 and 2027 to Ensure Compliance with Maximum Residue Levels of Pesticides and to Assess the Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin and Repealing Implementing Regulation (EU) 2023/731. Off. J. Eur. Union 2024, 989. [Google Scholar]
- EU Multi-Annual Control Programmes—European Commission. Available online: https://food.ec.europa.eu/plants/pesticides/maximum-residue-levels/enforcement/eu-multi-annual-control-programmes_en (accessed on 25 March 2025).
- European Commission. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- Carisio, L.; Simon Delso, N.; Tosi, S. Beyond the Urgency: Pesticide Emergency Authorisations’ Exposure, Toxicity, and Risk for Humans, Bees, and the Environment. Sci. Total Environ. 2024, 947, 174217. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (Text with EEA Relevance)Text with EEA Relevance. Off. J. Eur. Union 2010, 15, 1–72. [Google Scholar]
- European Commission. Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Off. J. Eur. Union 2009, 152, 11–22. [Google Scholar]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food (Text with EEA Relevance). Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- European Commission. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, 338, 4–17. [Google Scholar]
- Neri, I.; Russo, G.; Grumetto, L. Bisphenol A and Its Analogues: From Their Occurrence in Foodstuffs Marketed in Europe to Improved Monitoring Strategies—A Review of Published Literature from 2018 to 2023. Arch. Toxicol. 2024, 98, 2441–2461. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Bisphenol A: Evaluation of a Study Investigating Its Neurodevelopmental Toxicity, Review of Recent Scientific Literature on Its Toxicity and Advice on the Danish Risk Assessment of Bisphenol A. EFSA J. 2010, 8, 1829. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2024/3190 of 19 December 2024 on the Use of Bisphenol A (BPA) and Other Bisphenols and Bisphenol Derivatives with Harmonised Classification for Specific Hazardous Properties in Certain Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) No 10/2011 and Repealing Regulation (EU) 2018/213. Off. J. Eur. Union 2024, 3190. [Google Scholar]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Crebelli, R. Towards a Harmonized Approach for Risk Assessment of Genotoxic Carcinogens in the European Union. Ann. Ist. Super. Sanita 2006, 42, 127–131. [Google Scholar] [PubMed]
- EFSA. Opinion of the Scientific Committee on a Request from EFSA Related to A Harmonised Approach for Risk Assessment of Substances Which Are Both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- Smaoui, S.; D’Amore, T.; Agriopoulou, S.; Mousavi Khaneghah, A. Mycotoxins in Seafood: Occurrence, Recent Development of Analytical Techniques and Future Challenges. Separations 2023, 10, 217. [Google Scholar] [CrossRef]
- Smaoui, S.; D’Amore, T.; Tarapoulouzi, M.; Agriopoulou, S.; Varzakas, T. Aflatoxins Contamination in Feed Commodities: From Occurrence and Toxicity to Recent Advances in Analytical Methods and Detoxification. Microorganisms 2023, 11, 2614. [Google Scholar] [CrossRef]
- Cendoya, E.; Chiotta, M.L.; Zachetti, V.; Chulze, S.N.; Ramirez, M.L. Fumonisins and Fumonisin-Producing Fusarium Occurrence in Wheat and Wheat by Products: A Review. J. Cereal Sci. 2018, 80, 158–166. [Google Scholar] [CrossRef]
- Goessens, T.; Mouchtaris-Michailidis, T.; Tesfamariam, K.; Truong, N.N.; Vertriest, F.; Bader, Y.; De Saeger, S.; Lachat, C.; De Boevre, M. Dietary Mycotoxin Exposure and Human Health Risks: A Protocol for a Systematic Review. Environ. Int. 2024, 184, 108456. [Google Scholar] [CrossRef]
- Chandravarnan, P.; Agyei, D.; Ali, A. The Prevalence and Concentration of Mycotoxins in Rice Sourced from Markets: A Global Description. Trends Food Sci. Technol. 2024, 146, 104394. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Arcella, D.; Gómez Ruiz, J.Á.; Innocenti, M.L.; Roldán, R. Human and Animal Dietary Exposure to Ergot Alkaloids. EFSA J. 2017, 15, e04902. [Google Scholar] [CrossRef]
- EFSA; Arcella, D.; Altieri, A.; Horváth, Z. Human Acute Exposure Assessment to Tropane Alkaloids. EFSA J. 2018, 16, e05160. [Google Scholar] [CrossRef]
- EFSA; Binaglia, M. Assessment of the Conclusions of the Joint FAO/WHO Expert Meeting on Tropane Alkaloids. EFSA J. 2022, 20, e07229. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [CrossRef]
- Pompa, C.; D’Amore, T.; Miedico, O.; Preite, C.; Chiaravalle, A.E. Evaluation and Dietary Exposure Assessment of Selected Toxic Trace Elements in Durum Wheat (Triticum Durum) Imported into the Italian Market: Six Years of Official Controls. Foods 2021, 10, 775. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of Exposure of Heavy Metals and Their Impact on Health Consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Miedico, O.; Pompa, C.; Preite, C.; Iammarino, M.; Nardelli, V. Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS. Life 2023, 13, 511. [Google Scholar] [CrossRef]
- Arcella, D.; Cascio, C.; Ruiz, J.Á.G. Chronic Dietary Exposure to Inorganic Arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef]
- Coelho, J.P. Arsenic Speciation in Algae: Case Studies in Europe. In Comprehensive Analytical Chemistry; Duarte, A.C., Reis, V., Eds.; Arsenic Speciation in Algae; Elsevier: Amsterdam, The Netherlands, 2019; Volume 85, pp. 179–198. [Google Scholar]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human Exposure to Dietary Inorganic Arsenic and Other Arsenic Species: State of Knowledge, Gaps and Uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef]
- EFSA. Panel on Contaminants in the Food Chain Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- EFSA. Results on Acrylamide Levels in Food from Monitoring Years 2007–2009 and Exposure Assessment. EFSA J. 2011, 9, 2133. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation (EU) 2019/1888 of 7 November 2019 on the Monitoring of the Presence of Acrylamide in Certain Foods. Off. J. Eur. Union 2019, 290, 31–33. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2022/553 of 5 April 2022 on Monitoring the Presence of Alternaria Toxins in Food. Off. J. Eur. Union 2022, 107, 90–92. [Google Scholar]
- EFSA. Dietary Exposure Assessment to Alternaria Toxins in the European Population. EFSA J. 2016, 14, e04654. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Verdini, E.; Sdogati, S.; Bibi, R.; Ciasca, B.; Pecorelli, I. Monitoring Alternaria Toxins in Italian Food to Support Upcoming Regulation. Food Addit. Contam Part B Surveill 2021, 15, 42–51. [Google Scholar] [CrossRef]
- Pacini, T.; D’Amore, T.; Sdogati, S.; Verdini, E.; Bibi, R.; Caporali, A.; Cristofani, E.; Maresca, C.; Orsini, S.; Pelliccia, A.; et al. Assessment of Alternaria Toxins and Pesticides in Organic and Conventional Tomato Products: Insights into Contamination Patterns and Food Safety Implications. Toxins 2025, 17, 12. [Google Scholar] [CrossRef]
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Off. J. Eur. Union 2002, 140, 10–22. [Google Scholar]
- Verstraete, F. Risk Management of Undesirable Substances in Feed Following Updated Risk Assessments. Toxicol. Appl. Pharmacol. 2013, 270, 230–247. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation No (EU) 2019/627 Of 15 March 2019 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation No (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation No (EC) No 2074/2005 as Regards Official Controls. Off. J. Eur. Union 2019, 131, 50. [Google Scholar]
- European Commission. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, 304, 24–44. [Google Scholar]
- European Commission. Commission Regulation (EU) No 231/2012 of 9 March 2012 Laying down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council (Text with EEA Relevance). Off. J. Eur. Union 2012, 83, 1–295. [Google Scholar]
- European Commission. Commission Regulation (EU) 2019/1871 of 7 November 2019 on Reference Points for Action for Non-Allowed Pharmacologically Active Substances Present in Food of Animal Origin and Repealing Decision 2005/34/EC (Text with EEA Relevance). Off. J. Eur. Union 2019, 289, 41–46. [Google Scholar]
- European Commission. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC (Text with EEA Relevance)Text with EEA Relevance. Off. J. Eur. Union 2019, 4, 43–167. [Google Scholar]
- Food Safety Regulation in Europe: A Comparative Institutional Analysis; Vos, E., Wendler, F., Eds.; Ius commune europaeum; Safe Foods; Intersentia: Cambridge, UK, 2006; ISBN 978-90-5095-636-9. [Google Scholar]
- Robinson, C.; Holland, N.; Leloup, D.; Muilerman, H. Conflicts of Interest at the European Food Safety Authority Erode Public Confidence. J. Epidemiol. Community Health 2013, 67, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Demortain, D. Regulatory Toxicology in Controversy. Sci. Technol. Hum. Values 2013, 38, 727–748. [Google Scholar] [CrossRef]
- Portier, C.J.; Armstrong, B.K.; Baguley, B.C.; Baur, X.; Belyaev, I.; Bellé, R.; Belpoggi, F.; Biggeri, A.; Bosland, M.C.; Bruzzi, P.; et al. Differences in the Carcinogenic Evaluation of Glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J. Epidemiol. Community Health 2016, 70, 741–745. [Google Scholar] [CrossRef]
- Hendlin, Y.; Arcuri, A. Assessing the Safety of Glyphosate: Comparing IARC, EFSA and EPA Procedures, Transparency, and Outcomes for Environmental Health. Environ. Epidemiol. 2019, 3, 158. [Google Scholar] [CrossRef]
- Rabesandratana, T. Europe’s Food Watchdog Embraces Transparency. Science 2015, 350, 368. [Google Scholar] [CrossRef]
- Nersesyan, A.; Knasmueller, S. Evaluation of the Scientific Quality of Studies Concerning Genotoxic Properties of Glyphosate; Institute of Cancer Research, Department of Medicine I, Medical University of Vienna: Vienna, Austria, 2021. [Google Scholar]
- Hilbeck, A.; Meyer, H.; Wynne, B.; Millstone, E. GMO Regulations and Their Interpretation: How EFSA’s Guidance on Risk Assessments of GMOs Is Bound to Fail. Environ. Sci. Eur. 2020, 32, 54. [Google Scholar] [CrossRef]
- Kalofiri, P.; Balias, G.; Tekos, F. The EU Endocrine Disruptors’ Regulation and the Glyphosate Controversy. Toxicol. Rep. 2021, 8, 1193–1199. [Google Scholar] [CrossRef]
- Millstone, E.P.; Dawson, E. EFSA’s Toxicological Assessment of Aspartame: Was It Even-Handedly Trying to Identify Possible Unreliable Positives and Unreliable Negatives? Arch. Public Health 2019, 77, 34. [Google Scholar] [CrossRef]
- Kawall, K.; Cotter, J.; Then, C. Broadening the GMO Risk Assessment in the EU for Genome Editing Technologies in Agriculture. Environ. Sci. Eur. 2020, 32, 106. [Google Scholar] [CrossRef]
- Leone, L. EFSA under Revision: Transparency and Sustainability in the Food Chain. Yearb. Eur. Law. 2020, 39, 536–568. [Google Scholar] [CrossRef]
- Klintman, M.; and Kronsell, A. Challenges to Legitimacy in Food Safety Governance? The Case of the European Food Safety Authority (EFSA). J. Eur. Integr. 2010, 32, 309–327. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Guidance on Protocol Development for EFSA Generic Scientific Assessments. EFSA J. 2023, 21, e08312. [Google Scholar] [CrossRef]
- Committee, E.S.; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; et al. Scientific Motivations and Criteria to Consider Updating EFSA Scientific Assessments. EFSA J. 2017, 15, e04737. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Chemicals Strategy for Sustainability Towards a Toxic-Free Environment; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Ingenbleek, L.; Lautz, L.S.; Dervilly, G.; Darney, K.; Astuto, M.C.; Tarazona, J.; Liem, A.K.D.; Kass, G.E.N.; Leblanc, J.C.; Verger, P.; et al. Risk Assessment of Chemicals in Food and Feed: Principles, Applications and Future Perspectives. In Environmental Pollutant Exposures and Public Health; Harrison, R.M., Ed.; The Royal Society of Chemistry: London, UK, 2020; pp. 1–38. [Google Scholar] [CrossRef]
- Tarazona, J.V. Use of New Scientific Developments in Regulatory Risk Assessments: Challenges and Opportunities. Integr. Environ. Assess. Manag. 2013, 9, e85–e91. [Google Scholar] [CrossRef]
- Terron, A.; Marx-Stoelting, P.; Braeuning, A. The Use of NAMs and Omics Data in Risk Assessment. EFSA J. 2022, 20, e200908. [Google Scholar] [CrossRef]
- Moné, M.J.; Pallocca, G.; Escher, S.E.; Exner, T.; Herzler, M.; Bennekou, S.H.; Kamp, H.; Kroese, E.D.; Leist, M.; Steger-Hartmann, T.; et al. Setting the Stage for Next-Generation Risk Assessment with Non-Animal Approaches: The EU-ToxRisk Project Experience. Arch. Toxicol. 2020, 94, 3581–3592. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Guidance on Harmonised Methodologies for Human Health, Animal Health and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [CrossRef]
- EFSA. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA J. 2018, 16, e05123. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; et al. Update: Use of the Benchmark Dose Approach in Risk Assessment. EFSA J. 2017, 15, e04658. [Google Scholar] [CrossRef] [PubMed]
- Long, A.S.; Wills, J.W.; Krolak, D.; Guo, M.; Dertinger, S.D.; Arlt, V.M.; White, P.A. Benchmark Dose Analyses of Multiple Genetic Toxicity Endpoints Permit Robust, Cross-Tissue Comparisons of MutaMouse Responses to Orally Delivered Benzo[a]Pyrene. Arch. Toxicol. 2018, 92, 967–982. [Google Scholar] [CrossRef]
- Cao, X.; Mittelstaedt, R.A.; Pearce, M.G.; Allen, B.C.; Soeteman-Hernández, L.G.; Johnson, G.E.; Bigger, C.A.H.; Heflich, R.H. Quantitative Dose-Response Analysis of Ethyl Methanesulfonate Genotoxicity in Adult Gpt-Delta Transgenic Mice. Env. Mol. Mutagen. 2014, 55, 385–399. [Google Scholar] [CrossRef]
- Gollapudi, B.B.; Johnson, G.E.; Hernandez, L.G.; Pottenger, L.H.; Dearfield, K.L.; Jeffrey, A.M.; Julien, E.; Kim, J.H.; Lovell, D.P.; Macgregor, J.T.; et al. Quantitative Approaches for Assessing Dose-Response Relationships in Genetic Toxicology Studies. Env. Mol. Mutagen. 2013, 54, 8–18. [Google Scholar] [CrossRef]
- Johnson, G.E.; Soeteman-Hernández, L.G.; Gollapudi, B.B.; Bodger, O.G.; Dearfield, K.L.; Heflich, R.H.; Hixon, J.G.; Lovell, D.P.; MacGregor, J.T.; Pottenger, L.H.; et al. Derivation of Point of Departure (PoD) Estimates in Genetic Toxicology Studies and Their Potential Applications in Risk Assessment. Env. Mol. Mutagen. 2014, 55, 609–623. [Google Scholar] [CrossRef]
- White, P.A.; Long, A.S.; Johnson, G.E. Quantitative Interpretation of Genetic Toxicity Dose-Response Data for Risk Assessment and Regulatory Decision-Making: Current Status and Emerging Priorities. Env. Mol. Mutagen. 2020, 61, 66–83. [Google Scholar] [CrossRef]
- Gollapudi, B.B.; Su, S.; Li, A.A.; Johnson, G.E.; Reiss, R.; Albertini, R.J. Genotoxicity as a Toxicologically Relevant Endpoint to Inform Risk Assessment: A Case Study with Ethylene Oxide. Env. Mol. Mutagen. 2020, 61, 852–871. [Google Scholar] [CrossRef]
- Guérard, M.; Johnson, G.; Dertinger, S.; Duran-Pacheco, G.; Funk, J.; Zeller, A. Dose-Response Relationship of Temozolomide, Determined by the Pig-a, Comet, and Micronucleus Assay. Arch. Toxicol. 2017, 91, 2443–2453. [Google Scholar] [CrossRef]
- Doménech, E.; Martorell, S. Review of the Terminology, Approaches, and Formulations Used in the Guidelines on Quantitative Risk Assessment of Chemical Hazards in Food. Foods 2024, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Akoto, O.; Oppong-Otoo, J.; Osei-Fosu, P. Carcinogenic and Non-Carcinogenic Risk of Organochlorine Pesticide Residues in Processed Cereal-Based Complementary Foods for Infants and Young Children in Ghana. Chemosphere 2015, 132, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.P.; Vaillancourt, E.; Thomas, R.S.; Carmichael, P.L.; Ouedraogo, G.; Kojima, H.; Barroso, J.; Ansell, J.; Barton-Maclaren, T.S.; Bennekou, S.H.; et al. Paving the Way for Application of next Generation Risk Assessment to Safety Decision-Making for Cosmetic Ingredients. Regul. Toxicol. Pharmacol. 2021, 125, 105026. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Existing Approaches Incorporating Replacement, Reduction and Refinement of Animal Testing: Applicability in Food and Feed Risk Assessment. EFSA J. 2009, 7, 1052. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Development of a Roadmap for Action on New Approach Methodologies in Risk Assessment. EFS3 2022, 19, 7341E. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Guidance Document on Scientific Criteria for Grouping Chemicals into Assessment Groups for Human Risk Assessment of Combined Exposure to Multiple Chemicals. EFS2 2021, 19, e07033. [Google Scholar] [CrossRef]
- Grosssteiner, I.; Mienne, A.; Lucas, L.; L-Yvonnet, P.; Trenteseaux, C.; Fontaine, K.; Sarda, X. Cumulative Risk Assessment with Pesticides in the Framework of MRL Setting. EFSA J. 2023, 21, e211009. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, A.; Christ, F.; Silva, V.; Vested, A.; Schlünssen, V.; González, N.; Gai, L.; Abrantes, N.; Baldi, I.; Bureau, M.; et al. Identifying Pesticides of High Concern for Ecosystem, Plant, Animal, and Human Health: A Comprehensive Field Study across Europe and Argentina. Sci. Total Environ. 2024, 948, 174671. [Google Scholar] [CrossRef]
- EFSA. Cumulative Dietary Risk Characterisation of Pesticides That Have Chronic Effects on the Thyroid. EFSA J. 2020, 18, e06088. [Google Scholar] [CrossRef]
- EFSA. Cumulative Dietary Risk Characterisation of Pesticides That Have Acute Effects on the Nervous System. EFSA J. 2020, 18, e06087. [Google Scholar] [CrossRef]
- EFSA; Anagnostopoulos, C.; Anastassiadou, M.; Castoldi, A.F.; Cavelier, A.; Coja, T.; Crivellente, F.; Dujardin, B.; Hart, A.; Hooghe, W.; et al. Retrospective Cumulative Dietary Risk Assessment of Craniofacial Alterations by Residues of Pesticides. EFSA J. 2022, 20, e07550. [Google Scholar] [CrossRef]
- Assessment Tools and Resources|EFSA. Available online: https://www.efsa.europa.eu/en/science/tools-and-resources (accessed on 28 April 2025).
- EFSA—European Food Safety Authority. Modern Methodologies and Tools for Human Hazard Assessment of Chemicals. EFSA J. 2014, 12, 3638. [Google Scholar] [CrossRef]
- Chemical Hazards Database (OpenFoodTox)|EFSA. Available online: https://www.efsa.europa.eu/en/data-report/chemical-hazards-database-openfoodtox (accessed on 28 April 2025).
- Rossi, A.; Rossi, G.; Rosamilia, A.; Micheli, M.R. Official Controls on Food Safety: Competent Authority Measures. Ital. J. Food Saf. 2020, 9, 8607. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Lo Magro, S.; Vita, V.; Di Taranto, A. Optimization and Validation of a High Throughput UHPLC-MS/MS Method for Determination of the EU Regulated Lipophilic Marine Toxins and Occurrence in Fresh and Processed Shellfish. Mar. Drugs 2022, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 333/2007 of 28 March 2007 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)Pyrene in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2007, 88, 29–38. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, 180, 84–109. [Google Scholar]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization—ISO: Geneva, Switzerland, 2017.
- Cantwell, H. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics; Eurachem Publications: Bucharest, Romania, 2025. [Google Scholar]
- Bianchi, F.; Giannetto, M.; Careri, M. Analytical Systems and Metrological Traceability of Measurement Data in Food Control Assessment. TrAC Trends Anal. Chem. 2018, 107, 142–150. [Google Scholar] [CrossRef]
- DG-SANTE. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; SANTE 11312/2021; EURL: Geel, Belgium, 2010. [Google Scholar]
- Hooda, A.; Vikranta, U.; Duary, R.K. Principles of Food Dairy Safety: Challenges and Opportunities. In Engineering Solutions for Sustainable Food and Dairy Production: Innovations and Techniques in Food Processing and Dairy Engineering; Chandra Deka, S., Nickhil, C., Haghi, A.K., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 35–65. ISBN 978-3-031-75834-8. [Google Scholar]
- Das, R.; Raj, D. Sources, Distribution, and Impacts of Emerging Contaminants—A Critical Review on Contamination of Landfill Leachate. J. Hazard. Mater. Adv. 2025, 17, 100602. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Khan, N.A.; López-Maldonado, E.A.; Majumder, A.; Singh, S.; Varshney, R.; López, J.R.; Méndez, P.F.; Ramamurthy, P.C.; Khan, M.A.; Khan, A.H.; et al. A State-of-Art-Review on Emerging Contaminants: Environmental Chemistry, Health Effect, and Modern Treatment Methods. Chemosphere 2023, 344, 140264. [Google Scholar] [CrossRef]
- Kirkeli, C.; Valdersnes, S.; Ali, A.M. Target and Non-Target Screening of Poly- and Perfluoroalkyl Substances (PFAS) in Fish Liver Samples from the River Nile in Sudan: A Baseline Assessment. Mar. Pollut. Bull. 2025, 211, 117388. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per- and Polyfluoroalkyl Substances (PFAS) in PubChem: 7 Million and Growing. Env. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef]
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Higgins, C.P.; Alarif, W.M.; Al-Lihaibi, S.S.; Ghandourah, M.; Kallenborn, R. Per- and Polyfluoroalkyl Substances (PFASs) in Contaminated Coastal Marine Waters of the Saudi Arabian Red Sea: A Baseline Study. Env. Sci. Pollut. Res. Int. 2021, 28, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef]
- Arinaitwe, K.; Koch, A.; Taabu-Munyaho, A.; Marien, K.; Reemtsma, T.; Berger, U. Spatial Profiles of Perfluoroalkyl Substances and Mercury in Fish from Northern Lake Victoria, East Africa. Chemosphere 2020, 260, 127536. [Google Scholar] [CrossRef]
- Giesy, J.P.; Mabury, S.A.; Martin, J.W.; Kannan, K.; Jones, P.D.; Newsted, J.L.; Coady, K. Perfluorinated Compounds in the Great Lakes. In Persistent Organic Pollutants in the Great Lakes; Hites, R.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 391–438. ISBN 978-3-540-32990-9. [Google Scholar]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Env. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Rüdel, H.; Radermacher, G.; Fliedner, A.; Lohmann, N.; Koschorreck, J.; Duffek, A. Tissue Concentrations of Per- and Polyfluoroalkyl Substances (PFAS) in German Freshwater Fish: Derivation of Fillet-to-Whole Fish Conversion Factors and Assessment of Potential Risks. Chemosphere 2022, 292, 133483. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Bordon, I.C.; Kannan, K.; Moreira, I.; Quinete, N. Perfluoroalkyl Substances Associations with Morphometric Health Indices in Three Fish Species from Differentially Contaminated Water Bodies in Southeastern Brazil. Environ. Technol. Innov. 2021, 21, 101198. [Google Scholar] [CrossRef]
- Omoike, O.E.; Pack, R.P.; Mamudu, H.M.; Liu, Y.; Strasser, S.; Zheng, S.; Okoro, J.; Wang, L. Association between per and Polyfluoroalkyl Substances and Markers of Inflammation and Oxidative Stress. Env. Res. 2021, 196, 110361. [Google Scholar] [CrossRef]
- Lemos, L.; Gantiva, L.; Kaylor, C.; Sanchez, A.; Quinete, N. American Oysters as Bioindicators of Emerging Organic Contaminants in Florida, United States. Sci. Total Environ. 2022, 835, 155316. [Google Scholar] [CrossRef] [PubMed]
- Ogunbiyi, O.D.; Lemos, L.; Brinn, R.P.; Quinete, N.S. Bioaccumulation Potentials of Per-and Polyfluoroalkyl Substances (PFAS) in Recreational Fisheries: Occurrence, Health Risk Assessment and Oxidative Stress Biomarkers in Coastal Biscayne Bay. Environ. Res. 2024, 263, 120128. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; et al. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef] [PubMed]
- Kasmuri, N.; Tarmizi, N.A.A.; Mojiri, A. Occurrence, Impact, Toxicity, and Degradation Methods of Microplastics in Environment-a Review. Env. Sci. Pollut. Res. Int. 2022, 29, 30820–30836. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Env. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Microplastics in Our Diet: A Growing Concern for Human Health. Sci. Total Environ. 2025, 968, 178882. [Google Scholar] [CrossRef]
- Gupta, P.; Mahapatra, A.; Manna, B.; Suman, A.; Ray, S.S.; Singhal, N.; Singh, R.K. Sorption of PFOS onto Polystyrene Microplastics Potentiates Synergistic Toxic Effects during Zebrafish Embryogenesis and Neurodevelopment. Chemosphere 2024, 366, 143462. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Ali, W.; Zhuang, T.; Sun, J.; Wang, T.; Song, J.; Ma, Y.; Yuan, Y.; Bian, J.; et al. Co-Exposure of Polyvinyl Chloride Microplastics with Cadmium Promotes Nonalcoholic Fatty Liver Disease in Female Ducks through Oxidative Stress and Glycolipid Accumulation. Poult. Sci. 2024, 103, 104152. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polym. (Basel) 2022, 14, 2700. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Wei, H.; Xia, Y.; Luo, Y. Pigment Microparticles and Microplastics Found in Human Thrombi Based on Raman Spectral Evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Env. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of Microplastics in Human Colectomy Specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; Grotta, R.L.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, H.; Shi, L.; Jia, Y.; Sheng, H. Detection and Quantification of Microplastics in Various Types of Human Tumor Tissues. Ecotoxicol. Environ. Saf. 2024, 283, 116818. [Google Scholar] [CrossRef]
- Kuai, Y.; Chen, Z.; Xie, K.; Chen, J.; He, J.; Gao, J.; Yu, C. Long-Term Exposure to Polystyrene Microplastics Reduces Macrophages and Affects the Microbiota–Gut–Brain Axis in Mice. Toxicology 2024, 509, 153951. [Google Scholar] [CrossRef]
- Liang, B.; Deng, Y.; Zhong, Y.; Chen, X.; Huang, Y.; Li, Z.; Huang, X.; Yang, X.; Du, J.; Ye, R.; et al. Gastrointestinal Incomplete Degradation Exacerbates Neurotoxic Effects of PLA Microplastics via Oligomer Nanoplastics Formation. Adv. Sci. 2024, 11, e2401009. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The Global Threat from Plastic Pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- EFSA. EFSA Scientific Colloquium 25—A Coordinated Approach to Assess the Human Health Risks of Micro- and Nanoplastics in Food. EFSA Support. Publ. 2021, 18, 6815E. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, X.; Tian, Y.; Zeng, J.; Zhuang, P.; Jia, W.; Zhang, Y. Joint Control of Multiple Food Processing Contaminants in Maillard Reaction: A Comprehensive Review of Health Risks and Prevention. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70138. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Liu, D.; Wu, X.; Zeng, Y.; Sun, X.; Zhang, Y.; Li, Y.; Wu, Y.; Han, M.; Qie, R.; et al. Fried-Food Consumption and Risk of Overweight/Obesity, Type 2 Diabetes Mellitus, and Hypertension in Adults: A Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 6809–6820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Han, J.; Khan, Z.A.; Fang, Q.; Jin, Y.; Chen, X.; Zhang, Y.; Wang, M.; Qian, J.; et al. MD2 Activation by Direct AGE Interaction Drives Inflammatory Diabetic Cardiomyopathy. Nat. Commun. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, P.; Jiao, J.; Wang, J.; Zhang, Y. Association of Acrylamide Hemoglobin Biomarkers with Obesity, Abdominal Obesity and Overweight in General US Population: NHANES 2003-2006. Sci. Total Env. 2018, 631–632, 589–596. [Google Scholar] [CrossRef]
- Chiang, V.S.-C.; and Quek, S.-Y. The Relationship of Red Meat with Cancer: Effects of Thermal Processing and Related Physiological Mechanisms. Crit. Rev. Food Sci. Nutr. 2017, 57, 1153–1173. [Google Scholar] [CrossRef]
- Mariussen, E.; Alexander, J.; Bukhvalova, B.A.; Dahl, L.; Hardie Olsen, A.; Kvalem, H.E.; Schlabach, M.; Amlund, H.; Hannisdal, R.; Ruus, A.; et al. Risk Assessment of Grilled and Barbecued Food. Food Risk Assess Europe 2024, 2. [Google Scholar] [CrossRef]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 100 C, Arsenic, Metals, Fibres, and Dusts; IARC—International Agency for Research on Cancer, Ed.; World Health Organization WHO-Press: Lyon, France, 2012; Volume 100C, ISBN 978-92-832-0135-9. [Google Scholar]
- IARC. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal; IARC: Lyon, France, 1991; ISBN 978-92-832-1251-5. [Google Scholar]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; ISBN 978-92-832-1256-0. [Google Scholar]
- IARC. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water; IARC: Lyon, France, 2013; ISBN 978-92-832-1324-6. [Google Scholar]
- IARC. Some Naturally Occurring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation; IARC: Lyon, France, 1986; ISBN 978-92-832-1240-9. [Google Scholar]
- Food, Nutrition and Agriculture—31/2002. Available online: https://www.fao.org/4/Y4267M/y4267m10.htm (accessed on 7 April 2025).
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Scientific Opinion on Flavouring Group Evaluation 13 Revision 3 (FGE.13Rev3): Furfuryl and Furan Derivatives with and without Additional Side-Chain Substituents and Heteroatoms from Chemical Group 14. EFSA J. 2021, 19, e06386. [Google Scholar] [CrossRef]
- Ortu, E.; and Caboni, P. Levels of 5-Hydroxymethylfurfural, Furfural, 2-Furoic Acid in Sapa Syrup, Marsala Wine and Bakery Products. Int. J. Food Prop. 2017, 20, S2543–S2551. [Google Scholar] [CrossRef]
- Vella, C.; Attard, E. Consumption of Minerals, Toxic Metals and Hydroxymethylfurfural: Analysis of Infant Foods and Formulae. Toxics 2019, 7, 33. [Google Scholar] [CrossRef]
- Chongwatpol, J. A Technological, Data-Driven Design Journey for Artificial Intelligence (AI) Initiatives. Educ. Inf. Technol. 2024, 29, 15933–15963. [Google Scholar] [CrossRef]
- Navigating Ethical Challenges in an AI-Enabled Food Industry. Food Sci. Technol. 2024, 38, 40–43. [CrossRef]
- Kim, D.; Kim, S.-Y.; Yoo, R.; Choo, J.; Yang, H. Innovative AI Methods for Monitoring Front-of-Package Information: A Case Study on Infant Foods. PLoS ONE 2024, 19, e0303083. [Google Scholar] [CrossRef] [PubMed]
- Nfor, K.A.; Theodore Armand, T.P.; Ismaylovna, K.P.; Joo, M.-I.; Kim, H.-C. An Explainable CNN and Vision Transformer-Based Approach for Real-Time Food Recognition. Nutrients 2025, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, K.B. Applications of Artificial Intelligence and Machine Learning in Food Quality Control and Safety Assessment. Food Eng. Rev. 2024, 16, 1–21. [Google Scholar] [CrossRef]
- Kudashkina, K.; Corradini, M.G.; Thirunathan, P.; Yada, R.Y.; Fraser, E.D.G. Artificial Intelligence Technology in Food Safety: A Behavioral Approach. Trends Food Sci. Technol. 2022, 123, 376–381. [Google Scholar] [CrossRef]
- Qian, C.; Murphy, S.I.; Orsi, R.H.; Wiedmann, M. How Can AI Help Improve Food Safety? Annu. Rev. Food Sci. Technol. 2023, 14, 517–538. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Samaei, M.R.; Baghapour, M.A.; Alipour, H.; Isazadeh, S.; Azhdarpoor, A.; Mousavi Khaneghah, A. Nitrate Concentrations and Health Risks in Cow Milk from Iran: Insights from Deterministic, Probabilistic, and AI Modeling. Environ. Pollut. 2024, 341, 122901. [Google Scholar] [CrossRef] [PubMed]
- Rivas, D.; Vilas, C.; Alonso, A.A.; Varas, F. Derivation of Postharvest Fruit Behavior Reduced Order Models for Online Monitoring and Control of Quality Parameters During Refrigeration. J. Food Process Eng. 2013, 36, 480–491. [Google Scholar] [CrossRef]
- Kannapinn, M.; Schäfer, M.; Weeger, O. TwinLab: A Framework for Data-Efficient Training of Non-Intrusive Reduced-Order Models for Digital Twins. Eng. Comput. 2024. ahead-of-print. [Google Scholar] [CrossRef]
- Tao, F.; Qi, Q. Make More Digital Twins. Nature 2019, 573, 490–491. [Google Scholar] [CrossRef]
- Grieves, M.; Vickers, J. Digital Twin: Mitigating Unpredictable, Undesirable Emergent Behavior in Complex Systems. In Transdisciplinary Perspectives on Complex Systems: New Findings and Approaches; Kahlen, F.-J., Flumerfelt, S., Alves, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–113. ISBN 978-3-319-38756-7. [Google Scholar]
- Adade, S.Y.-S.S.; Lin, H.; Johnson, N.A.N.; Nunekpeku, X.; Aheto, J.H.; Ekumah, J.-N.; Kwadzokpui, B.A.; Teye, E.; Ahmad, W.; Chen, Q. Advanced Food Contaminant Detection through Multi-Source Data Fusion: Strategies, Applications, and Future Perspectives. Trends Food Sci. Technol. 2025, 156, 104851. [Google Scholar] [CrossRef]
- Jin, Y.; Li, C.; Huang, Z.; Jiang, L. Simultaneous Quantitative Determination of Low-Concentration Preservatives and Heavy Metals in Tricholoma Matsutakes Based on SERS and FLU Spectral Data Fusion. Foods 2023, 12, 4267. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, W.; Wang, Z.; Wang, J. Rapid Determination of Potential Aflatoxigenic Fungi Contamination on Peanut Kernels during Storage by Data Fusion of HS-GC-IMS and Fluorescence Spectroscopy. Postharvest Biol. Technol. 2021, 171, 111361. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.M.; Goicoechea, H.C. Multi-Level Data Fusion Strategies for Modeling Three-Way Electrophoresis Capillary and Fluorescence Arrays Enhancing Geographical and Grape Variety Classification of Wines. Anal. Chim. Acta 2020, 1126, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ni, L.; Bai, X.; Jiang, H.; Xu, L. Simultaneous Analysis of Mildew Degree and Aflatoxin B1 of Wheat by a Multi-Task Deep Learning Strategy Based on Microwave Detection Technology. LWT 2023, 184, 115047. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data Fusion Methodologies for Food and Beverage Authentication and Quality Assessment—A Review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; D’Amore, T.; Tarapoulouzi, M.; Varzakas, T.; Smaoui, S. Chemometrics in Mycotoxin Detection by Mass Spectrometry. In Mass Spectrometry in Food Analysis; World Scientific: Singapore, 2024; pp. 277–303. ISBN 9789811296239. [Google Scholar]
- Miedico, O.; Nardelli, V.; D’Amore, T.; Casale, M.; Oliveri, P.; Malegori, C.; Paglia, G.; Iammarino, M. Identification of Mechanically Separated Meat Using Multivariate Analysis of 43 Trace Elements Detected by Inductively Coupled Mass Spectrometry: A Validated Approach. Food Chem. 2022, 397, 133842. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
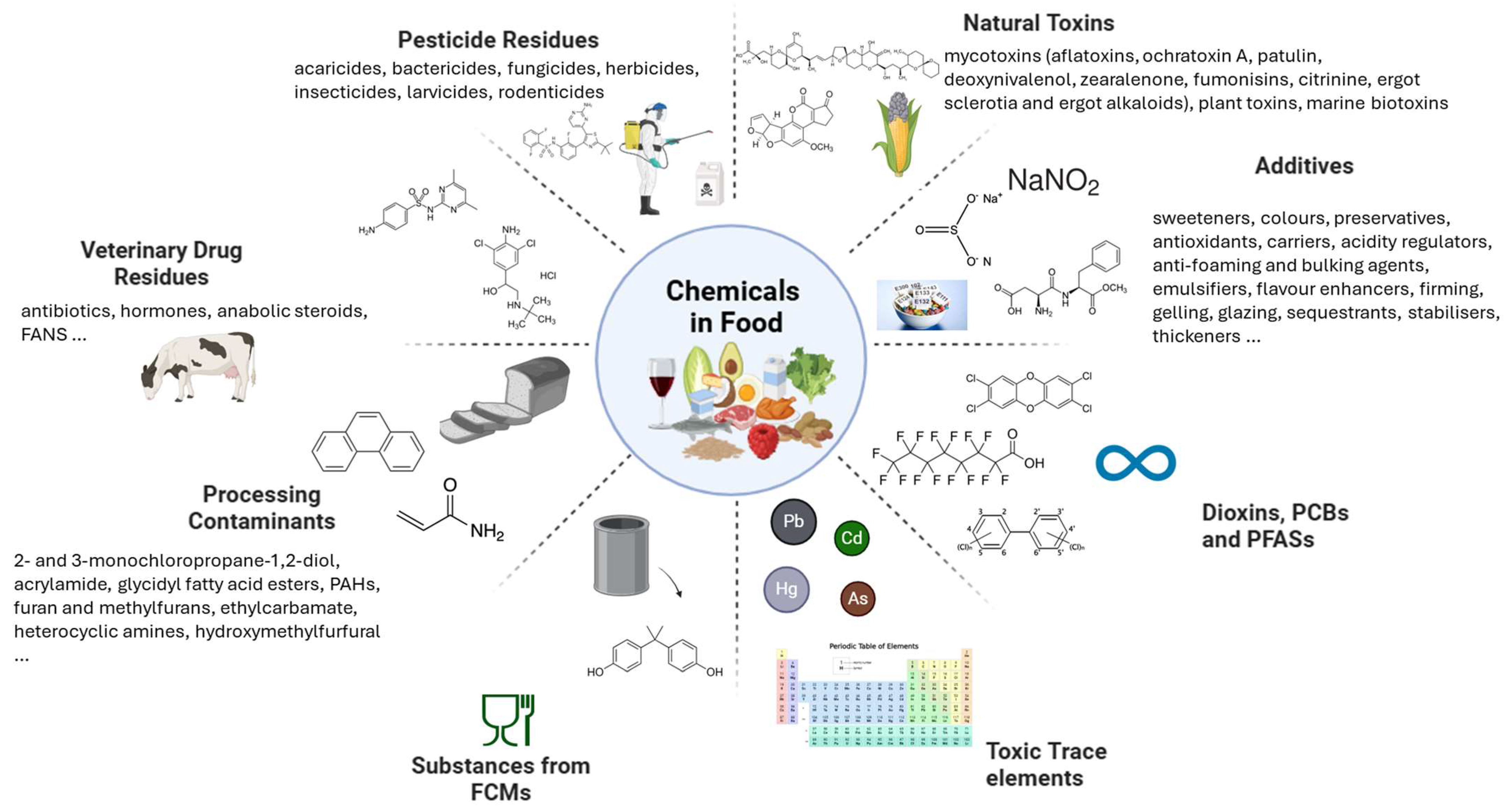



| Chemical Class | Subclasses | Key Regulations | Notes | Ref |
|---|---|---|---|---|
| Chemical Contaminants | Mycotoxins (aflatoxins, ochratoxin A, patulin, deoxynivalenol, zearalenone, fumonisins, citrinin, ergot sclerotia, and ergot alkaloids) Plant toxins (erucic acid, tropane alkaloids, hydrocyanic acid, pyrrolizidine alkaloids, opium alkaloids, Δ9-THC) Metals and other elements (lead, cadmium, mercury, arsenic, inorganic tin) PCBs and Dioxins Perfluoroalkyl substances Processing contaminants (polycyclic aromatic hydrocarbons (PAH): benzo(a)pyrene, sum of 4 PAHs; 3-monochloropropane-1,2-diol (3-MCPD), glycidyl fatty acid esters) Others (nitrates, melamine, perchlorate) | Regulation (EU) No. 2023/915 | Establishes maximum levels for contaminants in food | [54] |
| Marine Biotoxins | paralytic shellfish poison (PSP), amnesic shellfish poison (ASP), okadaic acid and dinophysistoxins, yessotoxins, azaspiracids | Regulation (EC) No. 627/2019 | Establish maximum levels and control plans | [81] |
| Acrylamide | Regulation (EU) No. 2017/2158 | Implementation of acrylamide reduction measures | [82] | |
| Recommendation (EU) No. 2019/1888 | Monitoring the presence of acrylamide in certain foods | [74] | ||
| Alternaria Toxins | alternariol, alternariol monomethyl ether, and tenuazonic acid | Recommendation (EU) No. 2022/553 | Monitoring the presence of Alternaria toxins in food | [75] |
| Food Additives | 26 functional classes (sweeteners, colors, preservatives, antioxidants, carriers, acids, acidity regulators, anti-caking, anti-foaming and bulking agents, emulsifiers, emulsifying salts, flavor enhancers, firming, gelling, glazing, raising and foaming agents, humectants, modified starches, packaging gases, propellants, sequestrants, stabilizers, thickeners, flour treatment agents) | Regulation (EC) No. 1333/2008 | Defines approved food additives, their conditions of use, and maximum levels | [11] |
| Regulation (EU) No. 231/2012 | Purity criteria of food additives | [83] | ||
| Flavorings | flavoring substances, flavoring preparations, thermal process flavorings, smoke flavorings, flavor precursors, or other flavorings or mixtures | Regulation (EC) No. 1334/2008 | [40] | |
| Pesticide Residues | acaricides, bactericides, fungicides, herbicides, insecticides, larvicides, rodenticides | Regulation (EC) No. 396/2005; | Sets MRLs for pesticides | [41] |
| Regulation (EC) No. 1107/2009 | placing of plant protection products on the market | [45] | ||
| Directive No. 2009/128/EC | promotes sustainable pesticide use | [12] | ||
| Veterinary Drug Residues | antibiotics, hormones, anabolic steroids, FANS (…) | Regulation (EU) No. 37/2010 | Establishes MRLs for veterinary medicinal products in food-producing animals. | [47] |
| Regulation (EC) No. 470/2009 | Outlines the process for determining MRLs for veterinary medicinal products in food. | [48] | ||
| Regulation (EU) No. 2019/1871 | Establishes reference limits for unauthorized pharmacologically active substances detected in food of animal origin | [84] | ||
| Regulation (EU) No. 2019/6 | Specifies the rules governing the approval and use of veterinary medicinal products | [85] | ||
| Food Contact Materials | monomers, other starting substances, macromolecules obtained from microbial fermentation, additives, and polymer production aids contaminants | Regulation (EC) No. 1935/2004 | Establishes safety requirements and migration limits for materials in contact with food. | [50] |
| Regulation (EU) No. 10/2011 | Criteria and authorization of plastic materials and articles intended to come into contact with food | [49] |
| Substance | Study Model | Endpoint | Tissue | BMDL | Ref |
|---|---|---|---|---|---|
| Benzo[a]pyrene | Mouse | GM | liver and small intestine | 8.47 and 0.75, respectively | [109] |
| Ethyl methanesulfonate | Mouse | GM | liver and spleen | 2.3 and 0.35, respectively | [110] |
| Ethyl methanesulfonate | Mouse | GM | liver and bone marrow | 41 and 9.3, respectively | [111] |
| 1-Methyl-1-nitrosourea | Rat | GM | peripheral blood | 0.2 | [112] |
| Aristolochic acids | Rat | GM | peripheral blood | 5.3 | [113] |
| Ethylene oxide | Mouse | CA | peripheral blood | 20.4 | [114] |
| Temozolomide | Rat | MN | peripheral blood and liver | 0.3 and 2.5, respectively | [115] |
| Parameter | Description | Main Acceptance Criteria |
|---|---|---|
| Selectivity/Specificity | The ability of the method to distinguish the analyte from the possible interferences | No interferences near the analyte signal (e.g., ±5% retention time in chromatographic methods) |
| Limit of Detection (LOD) | The minimum reliably detectable amount of an analyte | Method-specific LOD/LOQ thresholds |
| Limit of Quantification (LOQ) | The lowest concentration that can be reliably quantified | Method-specific LOD/LOQ thresholds |
| Linearity | The ability to obtain test results that are directly proportional to the concentration of the analyte in the sample | R² > 0.98–0.99 |
| Accuracy | The closeness of an analytical measurement to the true or accepted reference value; it is described in ISO 5725-1 as the sum of precision and trueness | It is described in ISO 5725-1 as the sum of precision and trueness |
| Precision | The closeness of agreement between the measured values obtained by the replicate measurements on the same or similar objects under specified conditions; generally estimated as (relative) standard deviation (RSD) or coefficient of variation (CV). Precision can vary depending on the level of variability considered: (1) repeatability refers to closeness of results when the same sample is measured under the same conditions within a short period (usually one day); (2) intermediate precision involves precision over a longer period in a single lab, accounting for more variables like different analysts or reagents; (3) reproducibility refers to precision of results across different laboratories (important when methods are standardized or used in multiple labs). | Intermediate precision (n ≥ 6) CV(%) < 5–25 RSD < 15% |
| Trueness | The agreement between a reasonably large number of measurements and true value (reference value), generally estimated as recovery (R) | R(%) = 70–120 |
| Robustness | Stability of method performance under varying conditions | minor changes (e.g., pH, mobile phases) major changes (matrix) |
| Matrix effect | An influence of one or more co-extracted compounds from the sample on the measurement of the analyte concentration or mass. It may be observed as an increased or decreased detector response compared with that produced by solvent solutions of the analyte | ME(%) ≤ 20 |
| Uncertainty | A range around the reported result within which the true value is expected to fall with a specified level of confidence, typically 95% | U ≤ 50% of MRL for contaminants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, T.; Smaoui, S.; Varzakas, T. Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions. Foods 2025, 14, 1628. https://doi.org/10.3390/foods14091628
D’Amore T, Smaoui S, Varzakas T. Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions. Foods. 2025; 14(9):1628. https://doi.org/10.3390/foods14091628
Chicago/Turabian StyleD’Amore, Teresa, Slim Smaoui, and Theodoros Varzakas. 2025. "Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions" Foods 14, no. 9: 1628. https://doi.org/10.3390/foods14091628
APA StyleD’Amore, T., Smaoui, S., & Varzakas, T. (2025). Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions. Foods, 14(9), 1628. https://doi.org/10.3390/foods14091628









