Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction Coupled with Green Supercritical Fluid Chromatography Methodology for Simultaneous Determination of Hindered Phenolic Antioxidant Migration from Food Contact Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments and Analytical Conditions
2.3. Preparation of Standard Solutions and Food Simulants
2.4. Simulated Migration Experiment
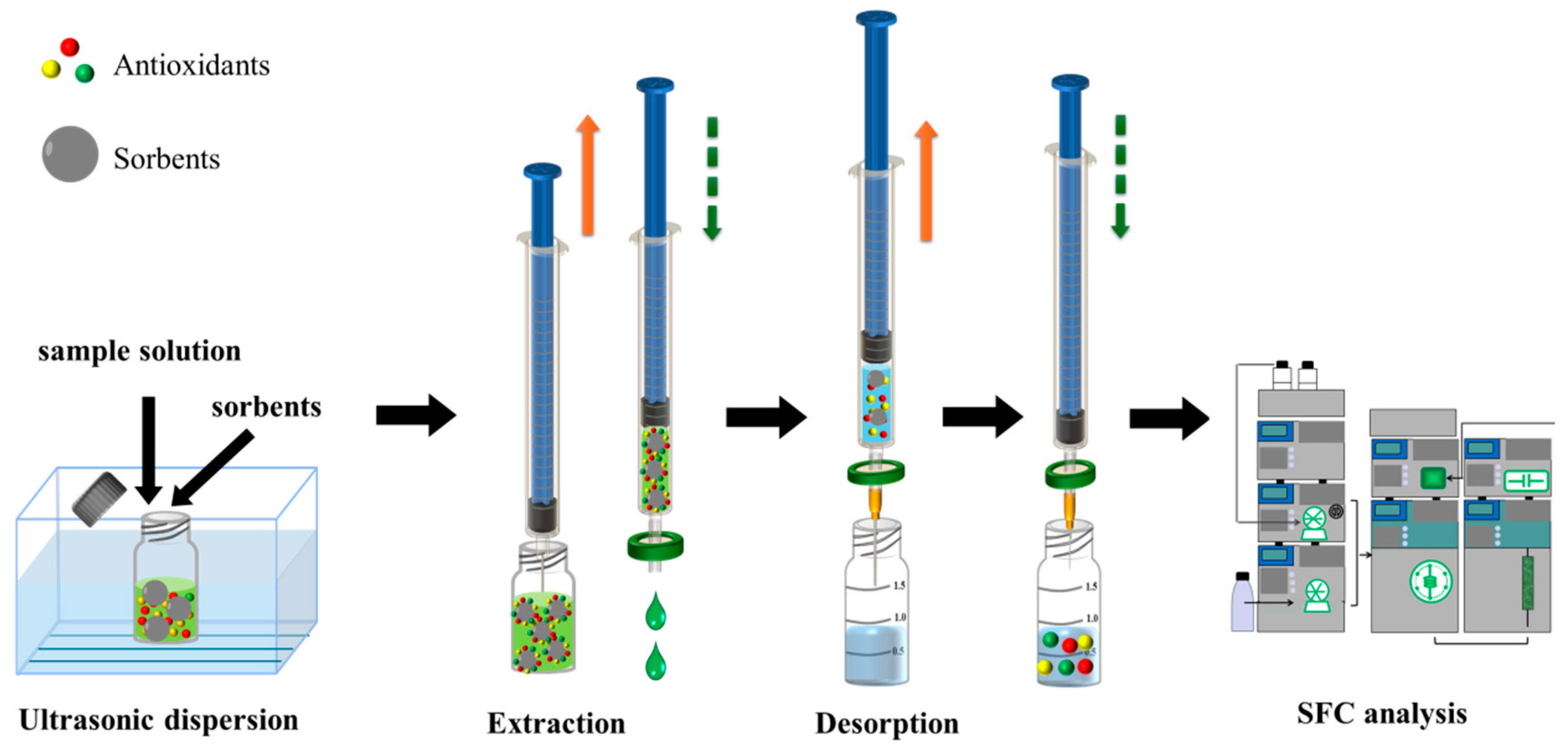
2.5. Operation of Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction
3. Results and Discussion
3.1. Investigation of d-SPFE Operating Parameters
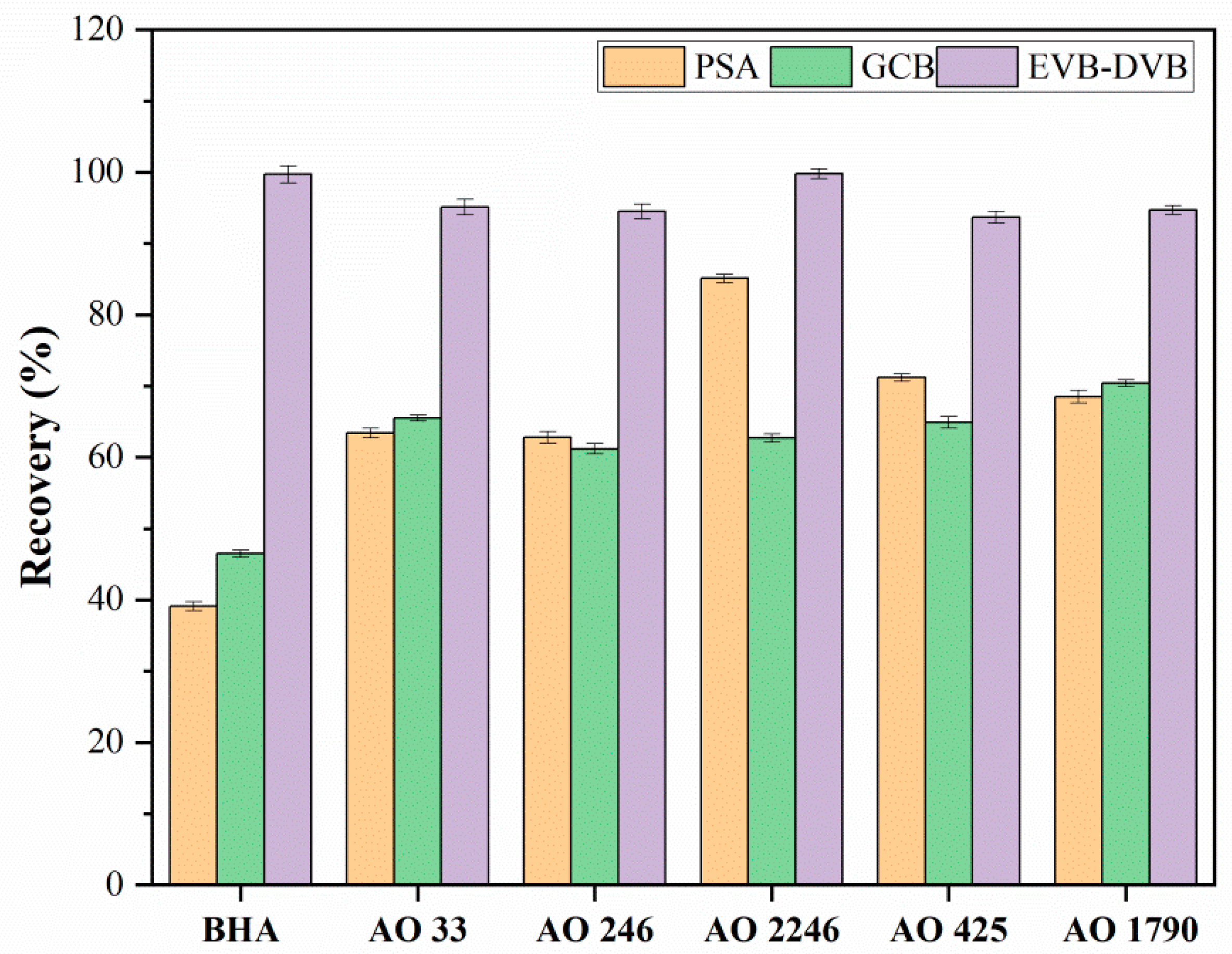
3.1.1. Selection of Sorbent Types
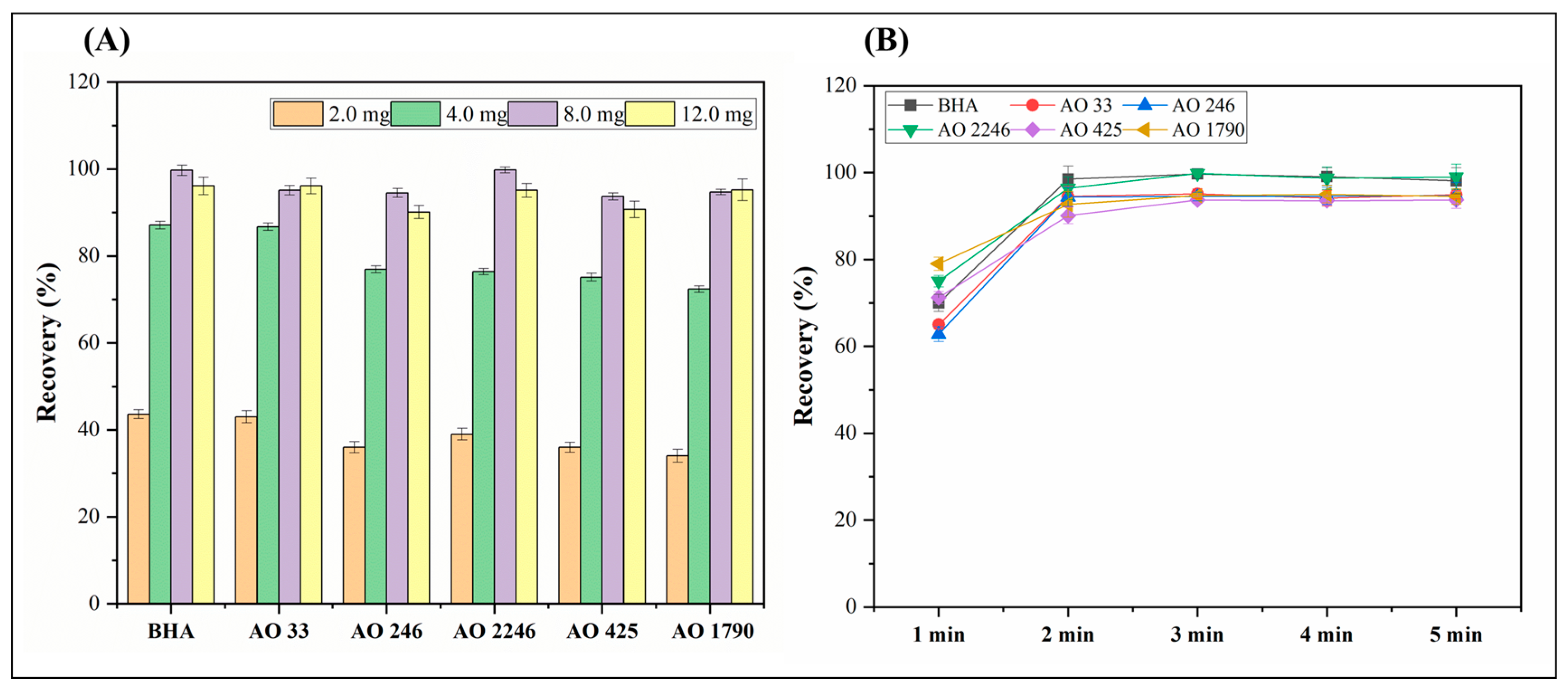
3.1.2. Study of Absorption Conditions
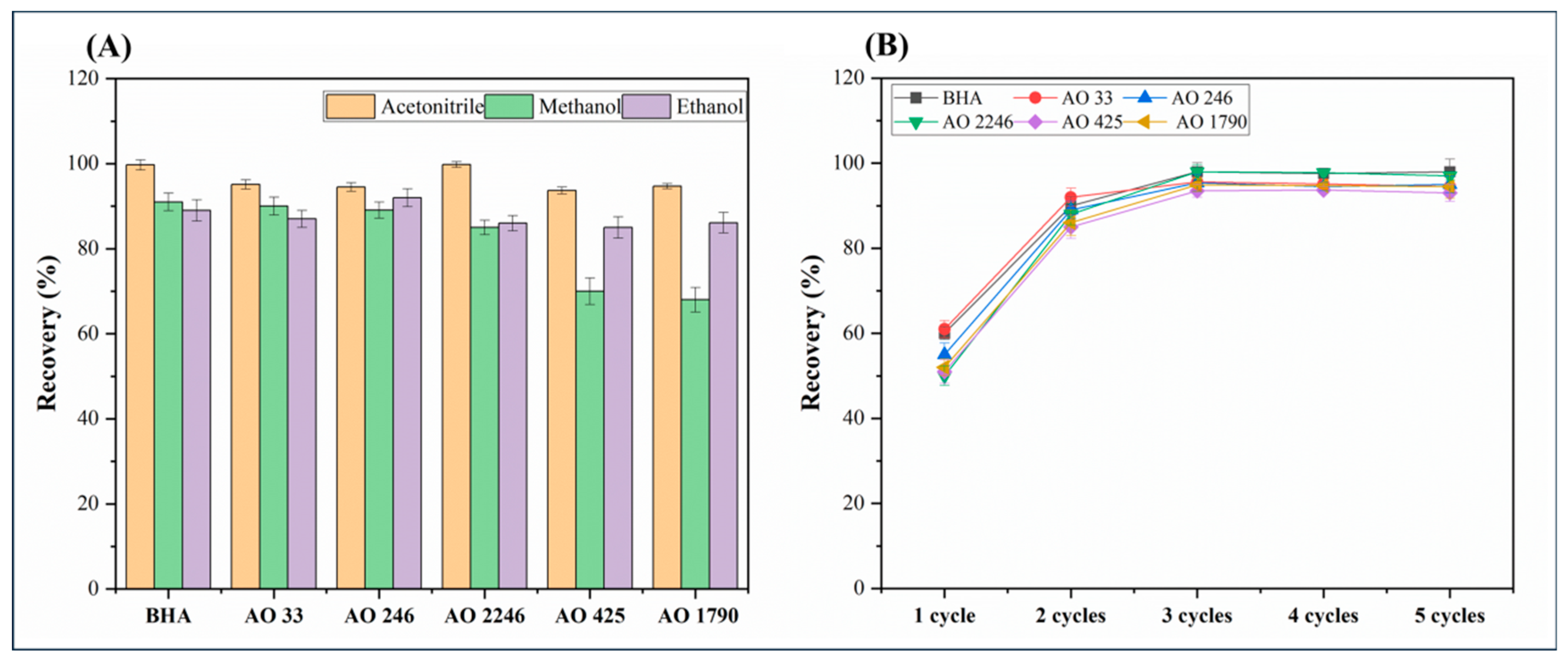
3.1.3. Study of Desorption Conditions
3.2. Optimization of Supercritical Fluid Chromatography Conditions
3.3. Methodological Validation and Evaluation
3.4. Method Application in Migration Analysis
3.5. Preliminary Migration Study of Hindered Phenolic Antioxidants
3.6. Comparison with Reported Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pipliya, S.; Karunanithi, S.; Eswaran, U.G.M.; Kumar, S.; Mandliya, S.; Srivastav, P.P.; Suthar, T.; Shaikh, A.M.; Harsányi, E.; et al. Migration of Chemical Compounds from Packaging Materials into Packaged Foods: Interaction, Mechanism, Assessment, and Regulations. Foods 2024, 13, 3125. [Google Scholar] [CrossRef]
- Li, J.; Si, Z.; Wang, S.; Li, S.; Zhou, H.; Liu, J. Effect of hindered phenolic antioxidants on crosslinking characteristics of low-density polyethylene initiated by peroxide. Energy Rep. 2023, 9, 159–166. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.Y.; Jung, J.S.; Sin, H.S.; Lee, H.G.; Jang, D.Y.; Lee, S.H.; Lim, K.M.; Choi, D. Comparison of migration and cumulative risk assessment of antioxidants, antioxidant degradation products, and other non-intentionally added substances from plastic food contact materials. Food Packag. Shelf Life 2023, 35, 101037. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Han, B.; Shang, Y.; Wang, H.; Shen, Y.; Li, R.; Wang, M.; Zhuang, Z.; Wang, Z.; Fang, M.; Jing, T. Prevalence of synthetic phenolic antioxidants in food contact materials from China and their implications for human dietary exposure through take-away food. J. Hazard. Mater. 2024, 473, 134599. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2023, 25, 13–33. [Google Scholar] [CrossRef]
- GB 9685-2016; Standard for Uses of Additives in Food Contact Materials and Their Products. SAC: Beijing, China, 2016.
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trac-Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Nicácio, A.E.; Boeing, J.S.; Garcia, F.P.; Nakamura, C.V.; Visentainer, J.V.; Maldaner, L. Rapid extraction method followed by a d-SPE clean-up step for determination of phenolic composition and antioxidant and antiproliferative activities from berry fruits. Food Chem. 2020, 309, 125694. [Google Scholar] [CrossRef] [PubMed]
- Phalipat, P.; Bunkoed, O.; Llompart, M.; Hongyok, S.; Nurerk, P. Covalent organic framework composite hydrogel sorbent beads for vortex-assisted dispersive miniaturized solid phase extraction of parabens and synthetic phenolic antioxidants in foodstuffs. Microchem. J. 2024, 207, 111873. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Liu, S.; Li, Y.; Zhao, H.; Chen, Q.; Hou, X. Dissolution-precipitation method concatenated sodium alginate/MOF-derived magnetic multistage pore carbon magnetic solid phase extraction for determination of antioxidants and ultraviolet stabilizers in polylactic acid food contact plastics. Talanta 2024, 270, 125487. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Wei, Y. Magnetic solid-phase extraction based on magnetic zeolitic imazolate framework-8 coupled with high performance liquid chromatography for the determination of polymer additives in drinks and foods packed with plastic. Food Chem. 2018, 256, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Gao, H.; Gao, H.; Yan, G.; Wang, F.; Wang, Y.; Chen, M. Dummy molecularly imprinted solid phase extraction in a nylon membrane filter for analysis of vardenafil in health care products. Microchem. J. 2021, 165, 106157. [Google Scholar] [CrossRef]
- Liang, R.; Hu, Y.; Li, G. Monodisperse pillar [5]arene-based polymeric sub-microsphere for on-line extraction coupling with high-performance liquid chromatography to determine antioxidants in the migration of food contact materials. J. Chromatogr. A 2020, 1625, 461276. [Google Scholar] [CrossRef]
- Shi, S.; Guo, K.; Tong, R.; Liu, Y.; Tong, C.; Peng, M. Online extraction–HPLC–FRAP system for direct identification of antioxidants from solid Du-zhong brick tea. Food Chem. 2019, 288, 215–220. [Google Scholar] [CrossRef]
- Shen, Q.-H.; Huang, Q.; Xie, J.-Y.; Wang, K.; Qian, Z.-M.; Li, D.-Q. A rapid analysis of antioxidants in Sanghuangporus baumii by online extraction-HPLC-ABTS. RSC Adv. 2021, 11, 25646–25652. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef]
- Guldberg, T.S.; Sandrød, M.; Øiaas, J.B.; Holten, T.; Zahlsen, K.; Kvitvang, H.F. Analysis of synthetic antioxidants in salmon silage using QuEChERS extraction method followed by determination by LC-MS/MS; a single-laboratory validation study. J. Chromatogr. B 2021, 1174, 122715. [Google Scholar] [CrossRef]
- He, S.; Choi, D.; Tang, W.; Ho Row, K. ZIF-8@SiO2 based novel dispersive solid-phase filter extraction technique for the purification of laminarin and fucoidan from undaria pinnatifida. Microchem. J. 2022, 180, 107552. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Han, Y.; Han, D.; Yan, H. Rapid and inexpensive nylon-66-filter solid-phase extraction followed by gas chromatography tandem mass spectrometry for analyzing perfluorinated carboxylic acids in milk. J. Chromatogr. A 2022, 1677, 463288. [Google Scholar] [CrossRef]
- Zhu, W.; Jin, P.; Yang, H.; Li, F.; Wang, C.; Li, T.; Fan, J. A green extraction strategy for the detection of antioxidants in food simulants and beverages migrated from plastic packaging materials. Food Chem. 2023, 406, 135060. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cheng, C.; Xu, L.; Pan, L.; Xia, H.F.; Lu, L. Migration of antioxidants from food-contact rubber materials to food simulants. J. Food Eng. 2022, 318, 110904. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, H.; Lu, L.; Lv, X.; Ju, G.; Zhao, J.; Sun, F.; Wang, Y.; Yu, W. Simultaneous Determination and Exposure Assessment of Antioxidants in Food-Contact Plastic Materials by HPLC-MS/MS. J. Food Prot. 2023, 86, 100121. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Jiang, W.-C.; Wu, W.; Xu, L.-Y.; Cheng, H.-L.; Zeng, J.; Wang, S.-Y.; Zhao, Y.; Xu, Z.-H.; Zhang, G.-Q. Colorimetric sensor array for identifying antioxidants based on pyrolysis-free synthesis of Fe–N/C single-atom nanozymes. Talanta 2024, 279, 126621. [Google Scholar] [CrossRef]
- West, C. Supercritical fluid chromatography is not (only) normal-phase chromatography. J. Chromatogr. A 2024, 1713, 464546. [Google Scholar] [CrossRef]
- Si-Hung, L.; Bamba, T. Current state and future perspectives of supercritical fluid chromatography. TrAC Trends Anal. Chem. 2022, 149, 116550. [Google Scholar] [CrossRef]
- Gigi, A.A.; Praveena, U.; Pillai, P.S.; Ragavan, K.V.; Anandharamakrishnan, C. Advances and challenges in the fractionation of edible oils and fats through supercritical fluid processing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70017. [Google Scholar] [CrossRef]
- Zhang, K.; Lou, C.; Zhu, Y.; Zhi, M.; Zeng, X. Hyperbranched anion exchangers prepared from thiol-ene modified polymeric substrates for suppressed ion chromatography. Talanta 2018, 184, 491–498. [Google Scholar] [CrossRef]
- EN 13130-1:2004; Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to food Simulants. CEN: Brussels, Belgium, 2004.
- GB/T 23296.1-2009; Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants. SAC: Beijing, China, 2009.
- Petrulionienė, T.; Murauskas, T.; Naujalis, E. Validation and Application of an LC–MS/MS Method for the Determination of Antioxidants Originating from Commercial Polyethylene Packages and their Migration into Food Simulants. Food Anal. Methods 2024, 17, 1087–1099. [Google Scholar] [CrossRef]
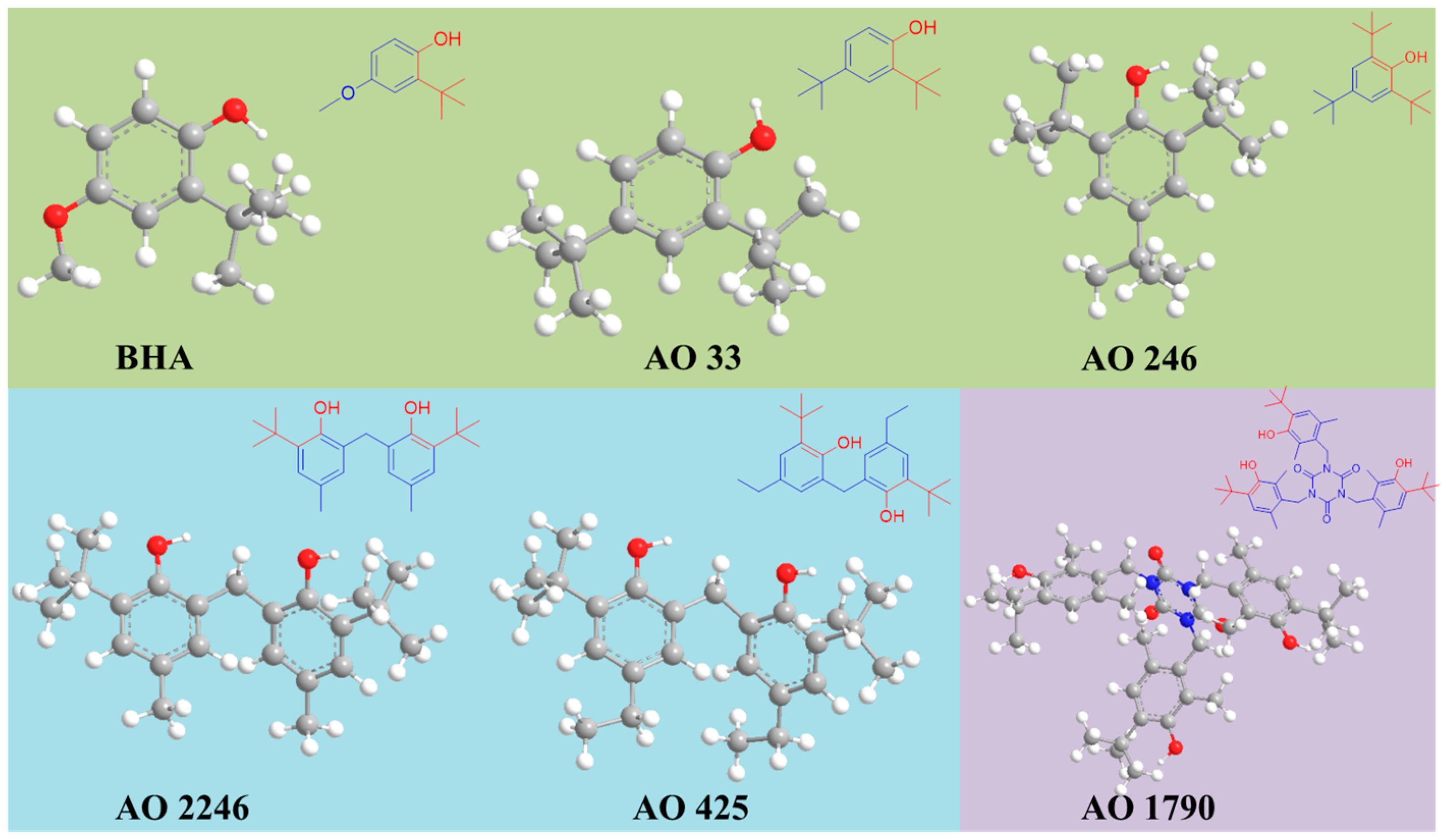


| Hindered Phenolic Antioxidant | Chemical Compound | Abbreviation | CAS No. | Molecular Formula | Molecular Weight | Classification |
|---|---|---|---|---|---|---|
| BHA | Butylated hydroxyanisole | BHA | 25013-16-5 | C11H16O2 | 180.2 | Monophenolic antioxidant |
| Antioxidant 33 | 2,4-Di-tert-butylphenol | AO 33 | 96-76-4 | C14H22O | 206.3 | Monophenolic antioxidant |
| Antioxidant 246 | 2,4,6-Tri-tert-butylphenol | AO 246 | 732-26-3 | C18H30O | 262.4 | Monophenolic antioxidant |
| Antioxidant 2246 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) | AO 2246 | 119-47-1 | C23H32O2 | 340.5 | Bisphenolic antioxidant |
| Antioxidant 425 | 2,2′-Methylenebis(4-ethyl-6-tert-butylphenol) | AO 425 | 88-24-4 | C25H36O2 | 368.6 | Bisphenolic antioxidant |
| Antioxidant 1790 | Tris(4-tert-butyl-3-hydroxy- 2,6-dimethylbenzyl) isocyanurate | AO 1790 | 40601-76-1 | C42H57N3O6 | 699.9 | Polyphenolic antioxidant |
| Antioxidant | Linear Range (μg/mL) | Slope ± SE * (×103) | Intercept ± SE | R2 | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| BHA | 0.02–2.0 | 32.75 ± 0.36 | 268.3 ± 5.2 | 0.9996 | 3.6 | 12 |
| AO 33 | 0.02–2.0 | 34.65 ± 0.38 | −526.6 ± 8.3 | 0.9998 | 3.5 | 12 |
| AO 246 | 0.02–2.0 | 31.28 ± 0.35 | 400.3 ± 6.1 | 0.9996 | 3.4 | 11 |
| AO 2246 | 0.02–2.0 | 56.91 ± 0.58 | −292.8 ± 5.7 | 0.9998 | 2.7 | 9 |
| AO 425 | 0.02–2.0 | 60.59 ± 0.71 | 503.3 ± 9.0 | 0.9997 | 2.4 | 8 |
| AO 1790 | 0.02–2.0 | 38.05 ± 0.43 | −180.9 ± 5.6 | 0.9998 | 3.6 | 12 |
| Antioxidant | Spiked Level (ng/mL) | Simulant A Ultrapure Water | Simulant B 3% Acetic Acid Aqueous Solution | Simulant C 10% Ethanol Aqueous Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Intra-Day RSD (%) | Inter-Day RSD (%) | Recovery (%) | Intra-Day RSD (%) | Inter-Day RSD (%) | Recovery (%) | Intra-Day RSD (%) | Inter-Day RSD (%) | ||
| BHA | 20 | 90.5 ± 5.3 | 5.8 | 6.3 | 94.8 ± 6.8 | 7.2 | 2.7 | 100.7 ± 5.3 | 5.3 | 3.4 |
| 100 | 95.0 ± 2.2 | 2.3 | 2.7 | 96.7 ± 2.9 | 3.0 | 7.5 | 95.8 ± 6.5 | 6.8 | 4.7 | |
| 500 | 91.8 ± 4.0 | 4.4 | 7.4 | 97.1 ± 2.7 | 2.8 | 2.1 | 98.4 ± 2.1 | 2.1 | 3.5 | |
| AO 33 | 20 | 90.8 ± 3.7 | 4.1 | 1.3 | 96.1 ± 4.7 | 4.9 | 6.8 | 97.1 ± 5.7 | 5.9 | 4.9 |
| 100 | 92.9 ± 2.9 | 3.1 | 6.4 | 90.7 ± 3.1 | 3.4 | 3.5 | 94.9 ± 5.2 | 5.5 | 3.6 | |
| 500 | 89.4 ± 2.3 | 2.6 | 0.9 | 93.3 ± 4.8 | 5.1 | 4.8 | 97.2 ± 5.1 | 5.2 | 5.3 | |
| AO 246 | 20 | 90.0 ± 5.9 | 6.5 | 3.4 | 101.6 ± 8.1 | 8.0 | 6.2 | 93.8 ± 3.8 | 4.0 | 2.6 |
| 100 | 90.6 ± 1.8 | 2.0 | 7.1 | 98.3 ± 1.0 | 1.0 | 5.6 | 93.1 ± 2.7 | 2.9 | 4.2 | |
| 500 | 92.4 ± 2.9 | 3.1 | 1.8 | 97.1 ± 2.0 | 2.1 | 3.5 | 96.3 ± 7.1 | 7.4 | 3.7 | |
| AO 2246 | 20 | 93.4 ± 5.3 | 5.7 | 6.2 | 95.7 ± 5.0 | 5.2 | 6.9 | 97.6 ± 3.0 | 3.1 | 7.4 |
| 100 | 94.1 ± 4.6 | 4.9 | 7.9 | 92.4 ± 3.6 | 3.9 | 6.4 | 93.2 ± 4.0 | 4.3 | 6.1 | |
| 500 | 93.6 ± 3.2 | 3.4 | 3.6 | 96.8 ± 5.2 | 5.4 | 1.2 | 98.4 ± 3.1 | 3.1 | 5.6 | |
| AO 425 | 20 | 89.7 ± 5.2 | 5.8 | 4.2 | 92.7 ± 2.6 | 2.8 | 4.2 | 96.3 ± 3.3 | 3.4 | 3.9 |
| 100 | 90.0 ± 2.2 | 2.4 | 6.4 | 91.8 ± 3.0 | 3.3 | 5.4 | 95.1 ± 2.3 | 2.4 | 2.8 | |
| 500 | 91.3 ± 3.5 | 3.8 | 4.1 | 93.2 ± 2.2 | 2.4 | 5.6 | 97.6 ± 7.6 | 7.8 | 8.2 | |
| AO 1790 | 20 | 89.4 ± 2.2 | 2.4 | 4.3 | 91.8 ± 5.4 | 5.9 | 6.2 | 94.2 ± 4.9 | 5.2 | 7.7 |
| 100 | 95.3 ± 3.0 | 3.1 | 3.0 | 97.5 ± 4.1 | 4.2 | 2.1 | 95.0 ± 3.6 | 3.8 | 3.2 | |
| 500 | 91.0 ± 2.3 | 2.5 | 3.5 | 96.4 ± 2.6 | 2.7 | 4.2 | 95.9 ± 2.3 | 2.4 | 3.5 | |
| Method | Matrix | Analytes | Sorbent Dosage | Total Time Spent | Sensitivity (LOD) | Recovery | Reference |
|---|---|---|---|---|---|---|---|
| d-SPFE-SFC | Simulants | 6 | 8 mg | 18 min | 2.4–3.6 ng/g | 89.4–101.6% | This research |
| d-SPE-HPLC-DAD | Tomato paste, etc. | 4 | 500 mg | 20 min | 0.25–0.50 ng/g | >80% | [13] |
| M-SPE-UPLC-MS/MS | Plastics | 2 | 15 mg | 25 min | 0.023–3.105 ng/g | 70.6–102.3% | [14] |
| QuEChERS-LC-MS/MS | Salmon silage | 3 | 42.5 g | 27 min | 12–15 ng/g | 97–101% | [21] |
| DES-HPLC-UV | Simulants | 3 | 0.15 g | 30 min | 0.15–0.25 μg/L | / | [24] |
| Ultrasonic extraction-LC-MS/MS | Simulants | 2 | / | 35 min | 0.0006–0.0012 mg/kg | 96.66–98.05% | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Lou, C.; Yu, X.; Zhang, K.; Zhang, K.; Jiang, L.; Zhu, Y. Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction Coupled with Green Supercritical Fluid Chromatography Methodology for Simultaneous Determination of Hindered Phenolic Antioxidant Migration from Food Contact Materials. Foods 2025, 14, 2301. https://doi.org/10.3390/foods14132301
Pan S, Lou C, Yu X, Zhang K, Zhang K, Jiang L, Zhu Y. Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction Coupled with Green Supercritical Fluid Chromatography Methodology for Simultaneous Determination of Hindered Phenolic Antioxidant Migration from Food Contact Materials. Foods. 2025; 14(13):2301. https://doi.org/10.3390/foods14132301
Chicago/Turabian StylePan, Shaojie, Chaoyan Lou, Xiaolin Yu, Kaidi Zhang, Kai Zhang, Lei Jiang, and Yan Zhu. 2025. "Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction Coupled with Green Supercritical Fluid Chromatography Methodology for Simultaneous Determination of Hindered Phenolic Antioxidant Migration from Food Contact Materials" Foods 14, no. 13: 2301. https://doi.org/10.3390/foods14132301
APA StylePan, S., Lou, C., Yu, X., Zhang, K., Zhang, K., Jiang, L., & Zhu, Y. (2025). Ultrasound-Assisted Dispersive Solid-Phase Filter Extraction Coupled with Green Supercritical Fluid Chromatography Methodology for Simultaneous Determination of Hindered Phenolic Antioxidant Migration from Food Contact Materials. Foods, 14(13), 2301. https://doi.org/10.3390/foods14132301







