Preparation of Rice Bran-Enriched Sweet Rice Wine and Its Quality Improvement Through Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
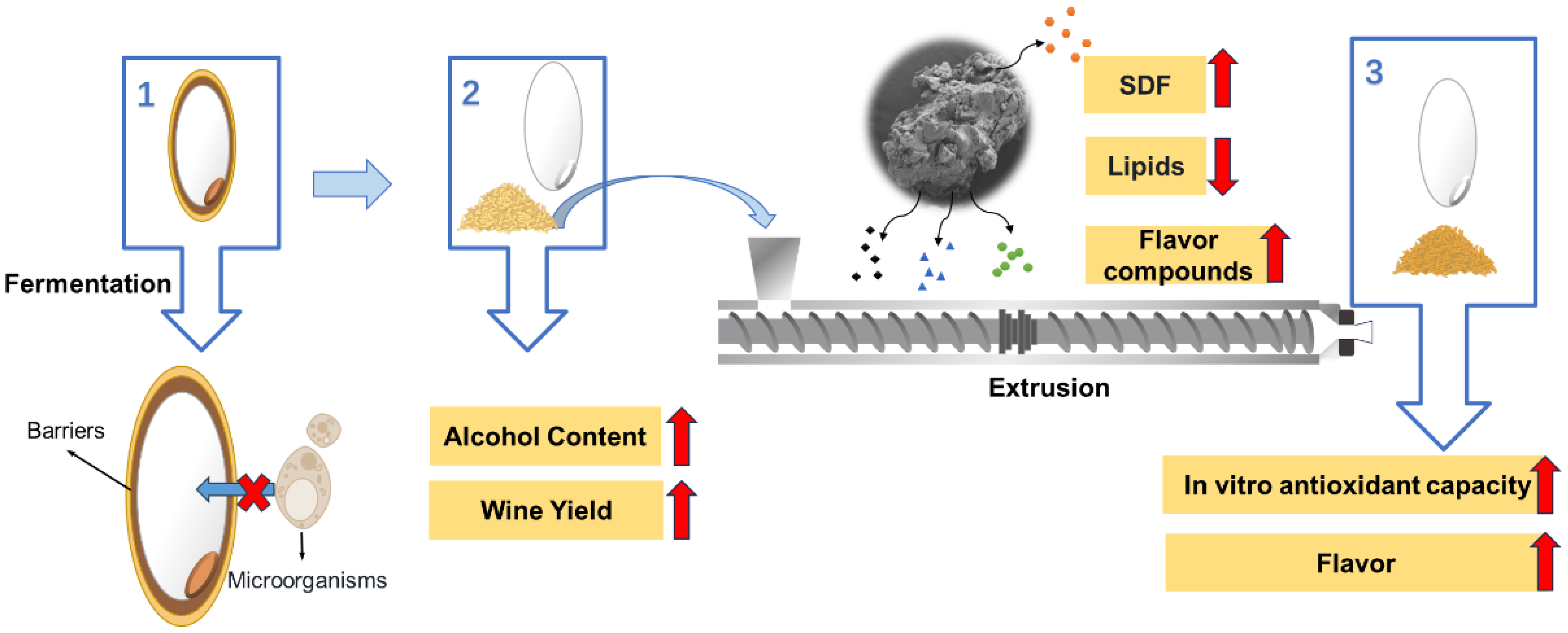
2.2. Rice Bran (RB) Extrusion
2.3. Rice Wine Fermentation
2.4. Characterization of Extruded RB
2.4.1. Basic Component Determination
2.4.2. Color Measurement
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.4.5. Water Solubility Index and Water Absorption Index Determination
2.5. Characterization of Sweet Rice Wine
2.5.1. Determination of Physicochemical Properties
2.5.2. Antioxidant Activity Determination
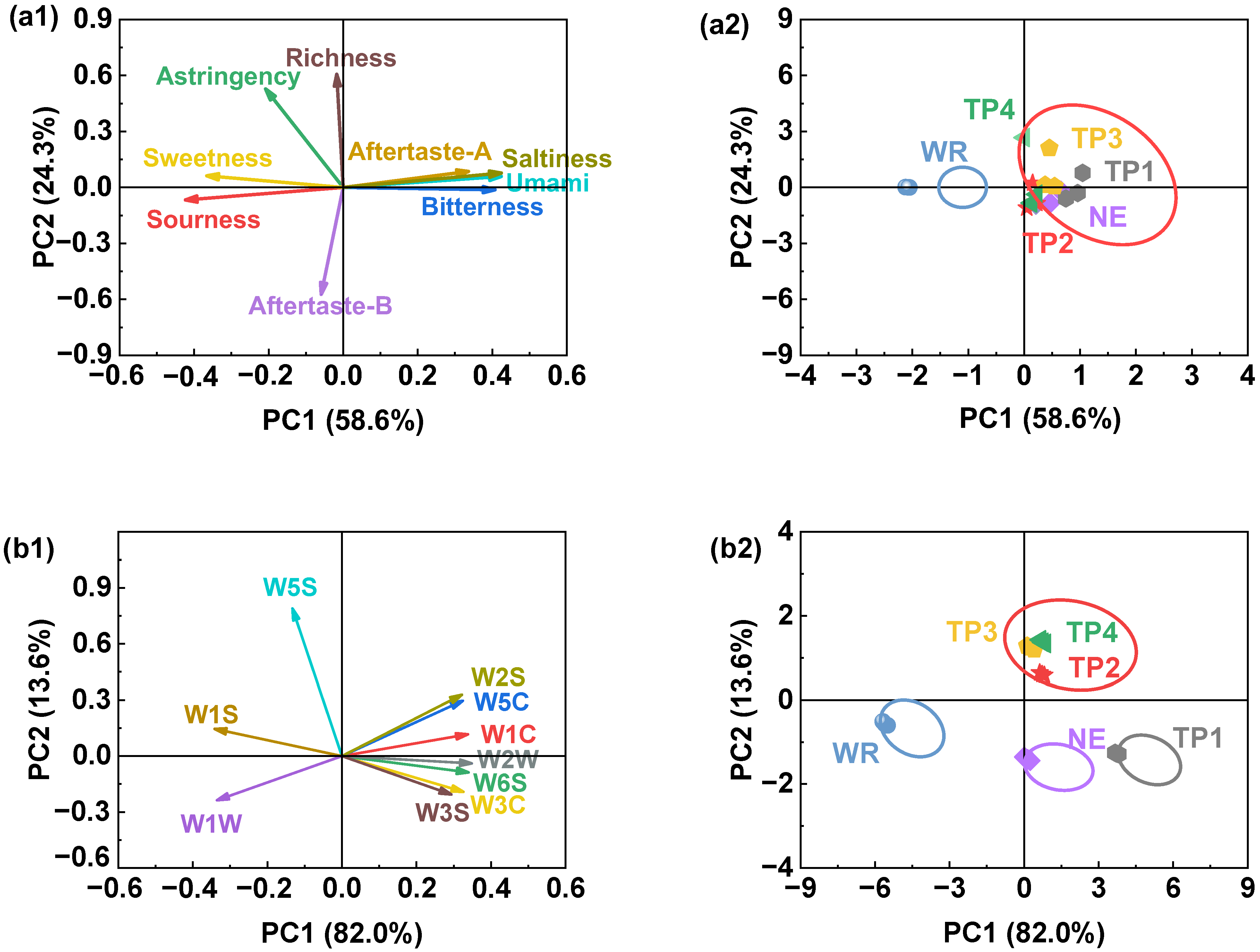
2.5.3. E-Tongue Analysis
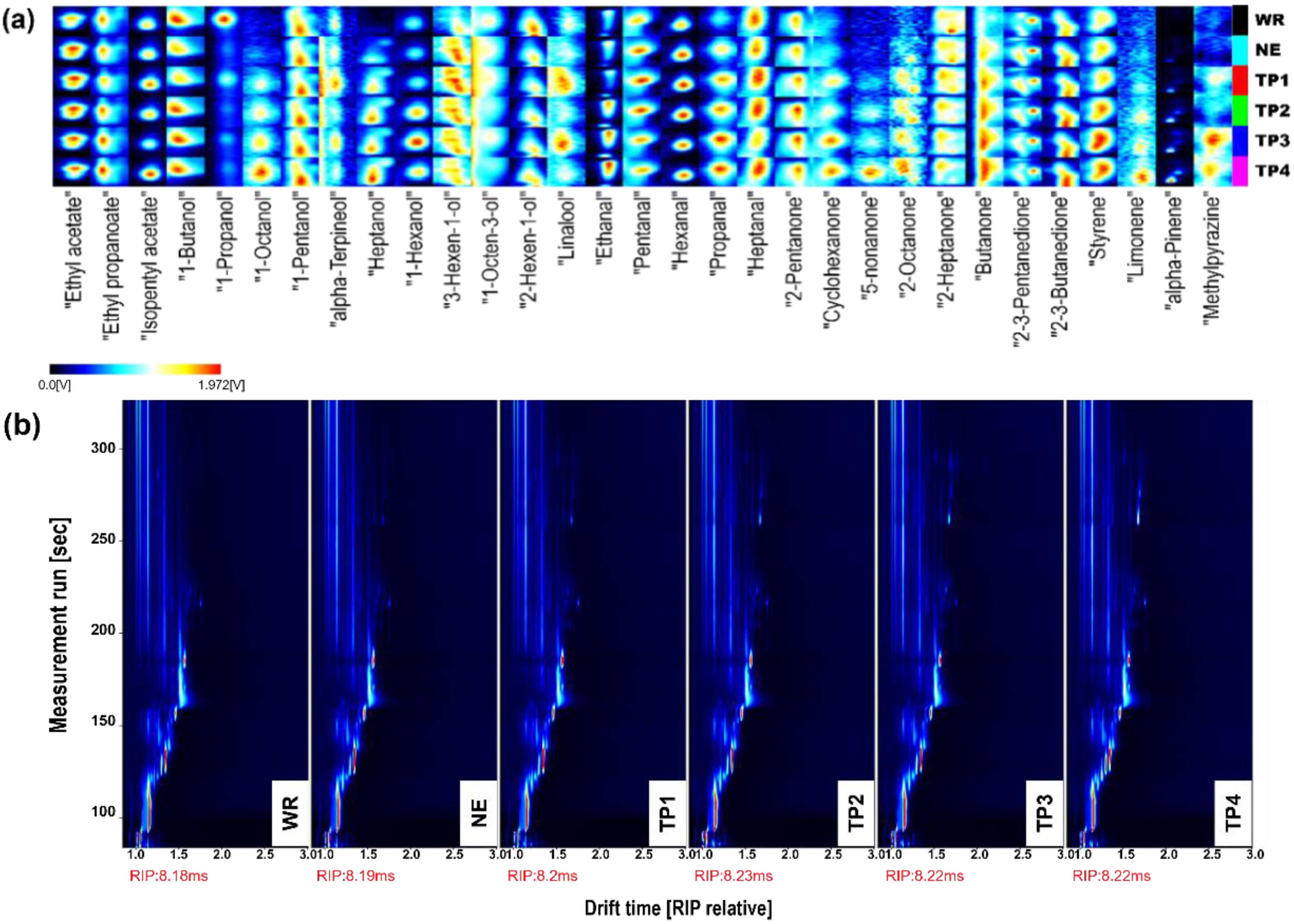
2.5.4. E-Nose Analysis
2.5.5. Determination of Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of RB on Sweet Rice Wine Characteristics
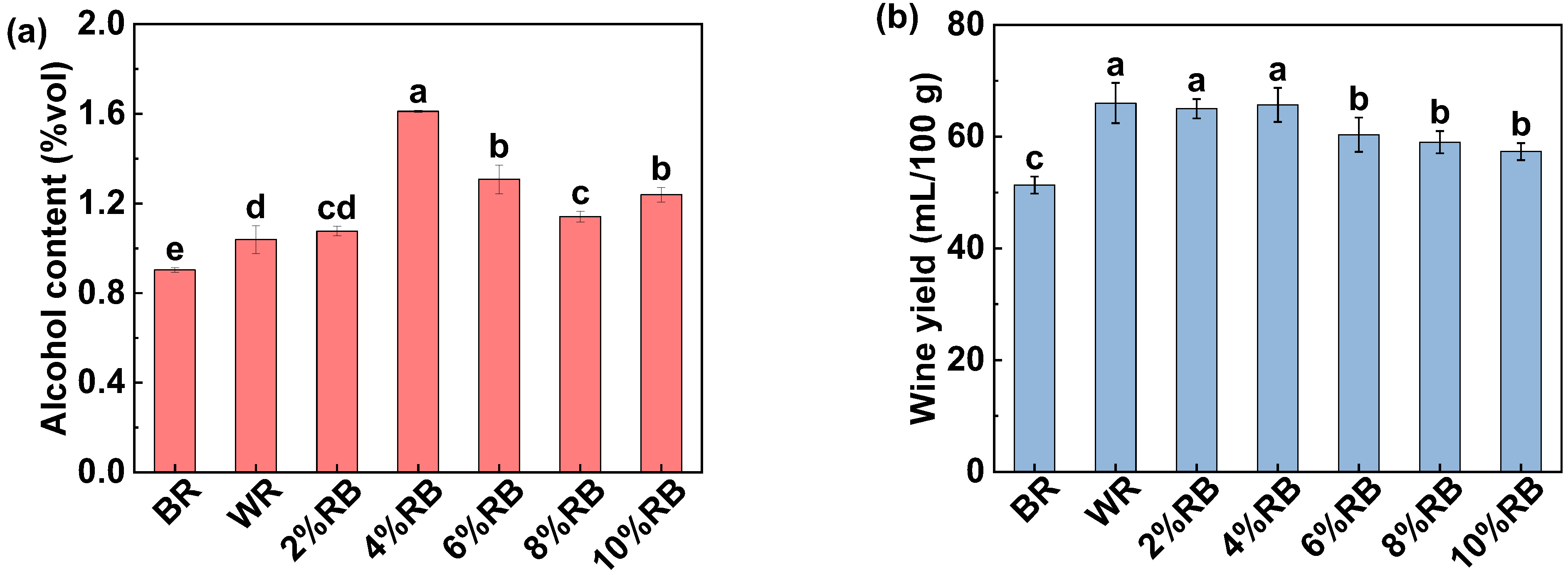
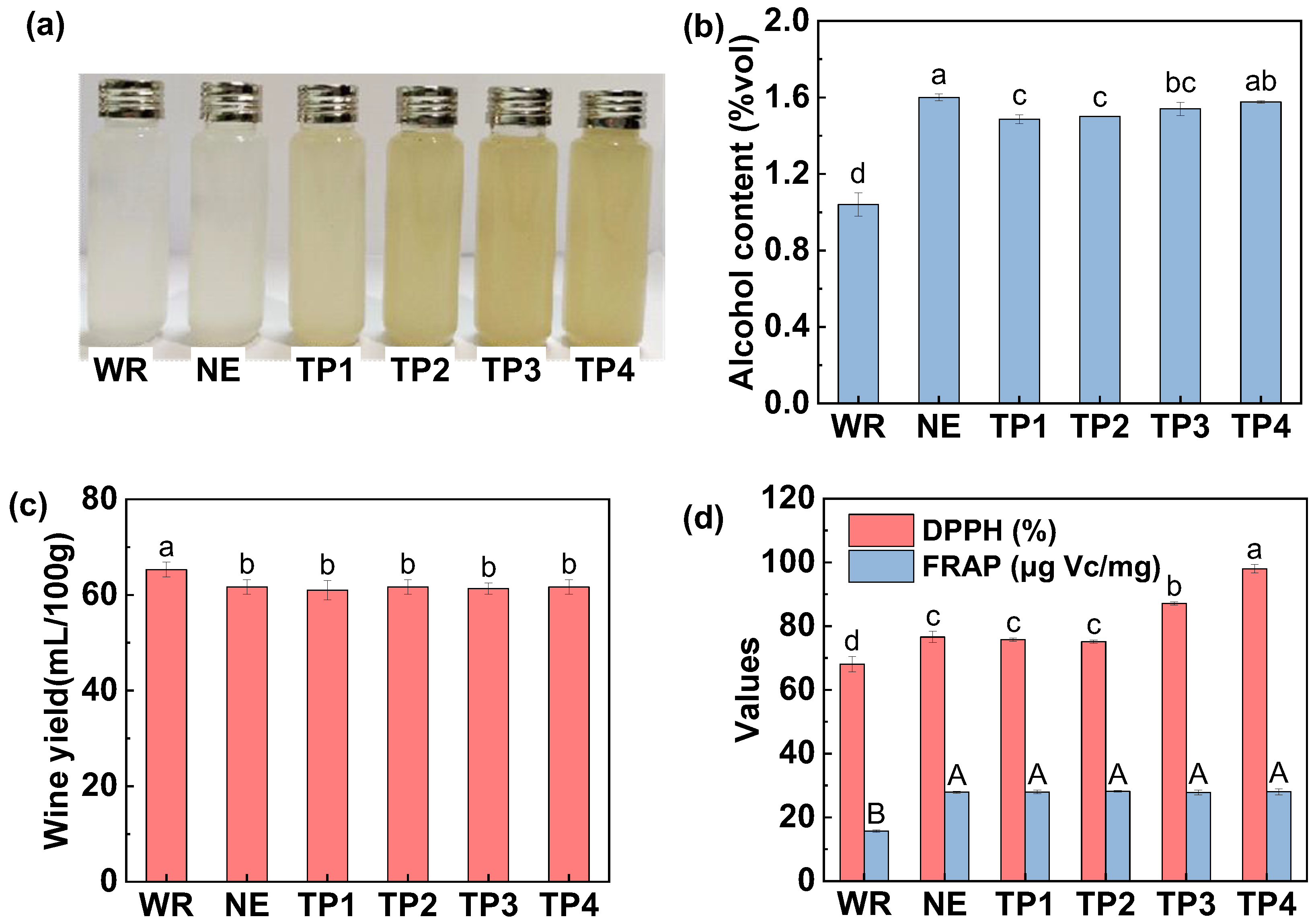
3.1.1. Alcohol Content and Wine Yield
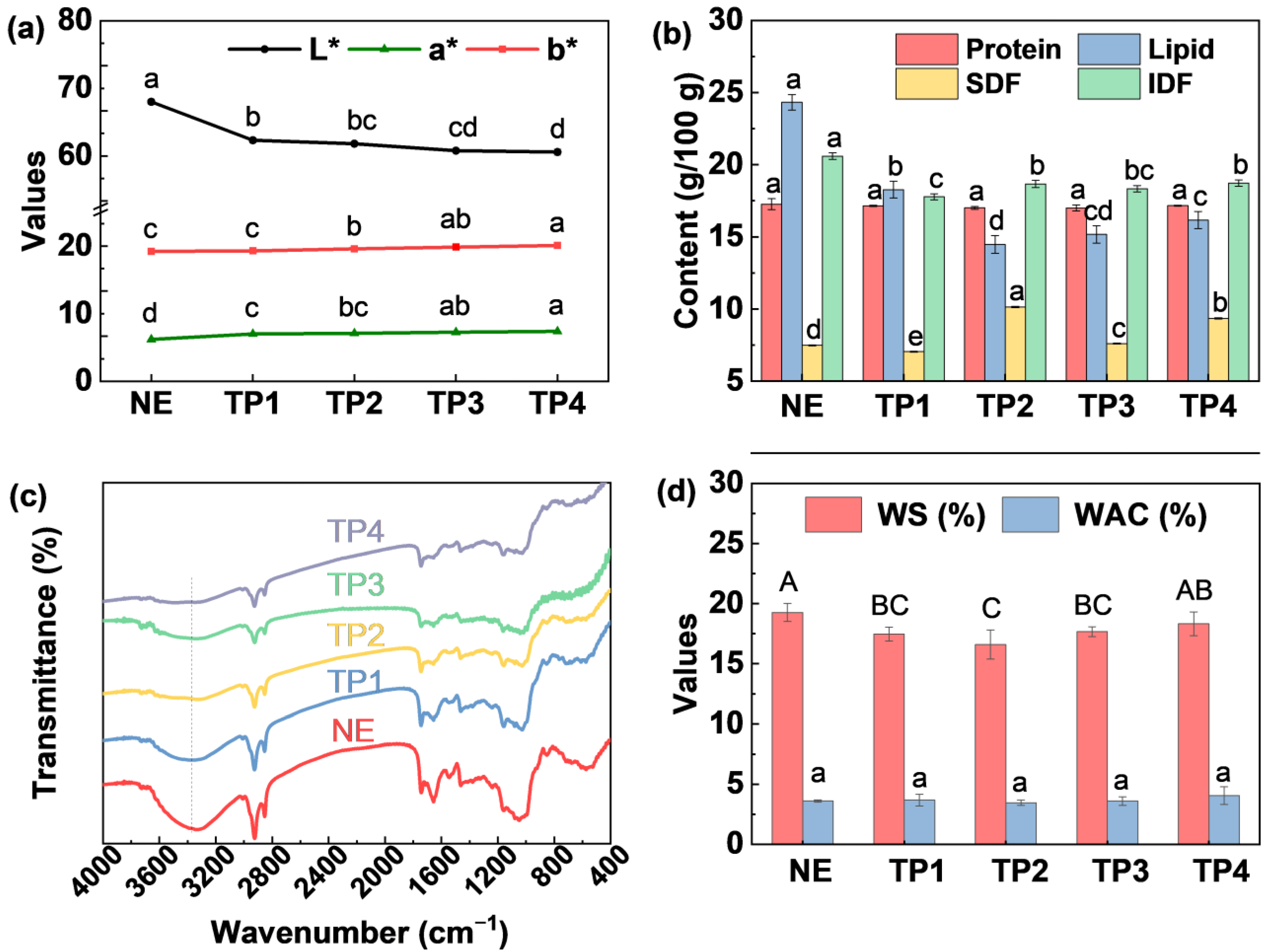
3.1.2. Appearance and Color
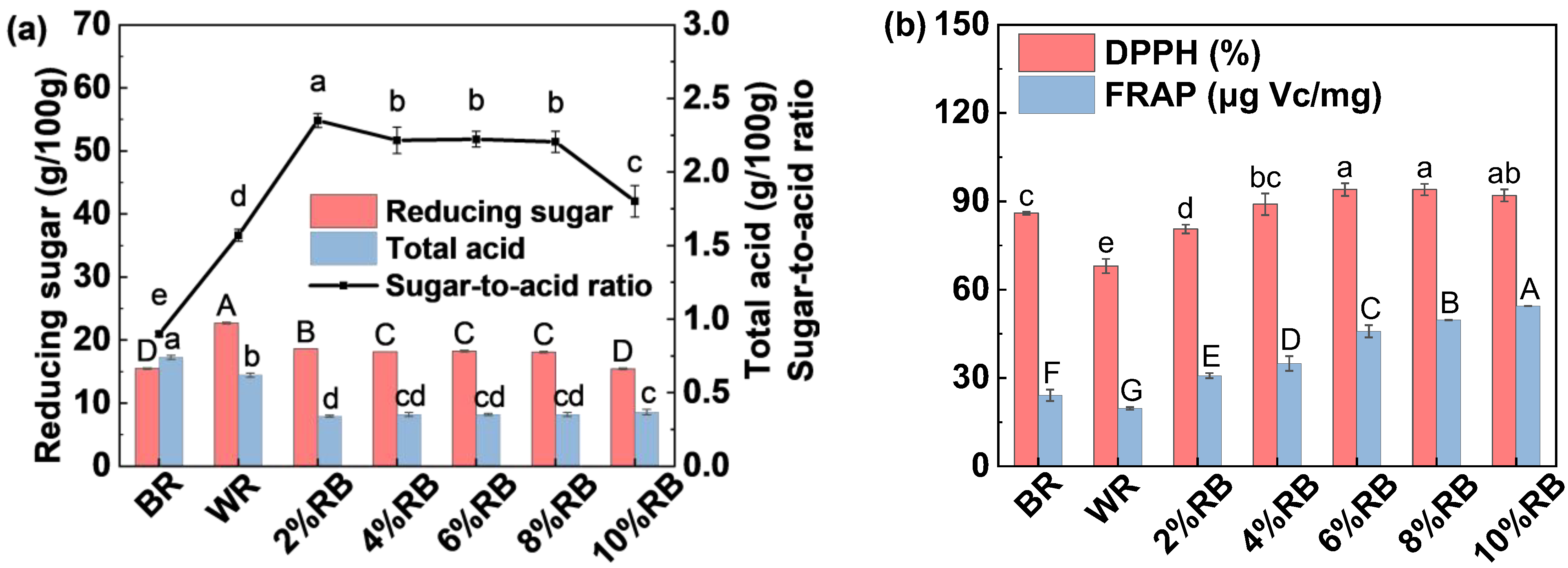
3.1.3. Reducing Sugar Content and Total Acid Content
3.1.4. Antioxidant Capacity
3.2. Effect of Extrusion on the Quality of RB
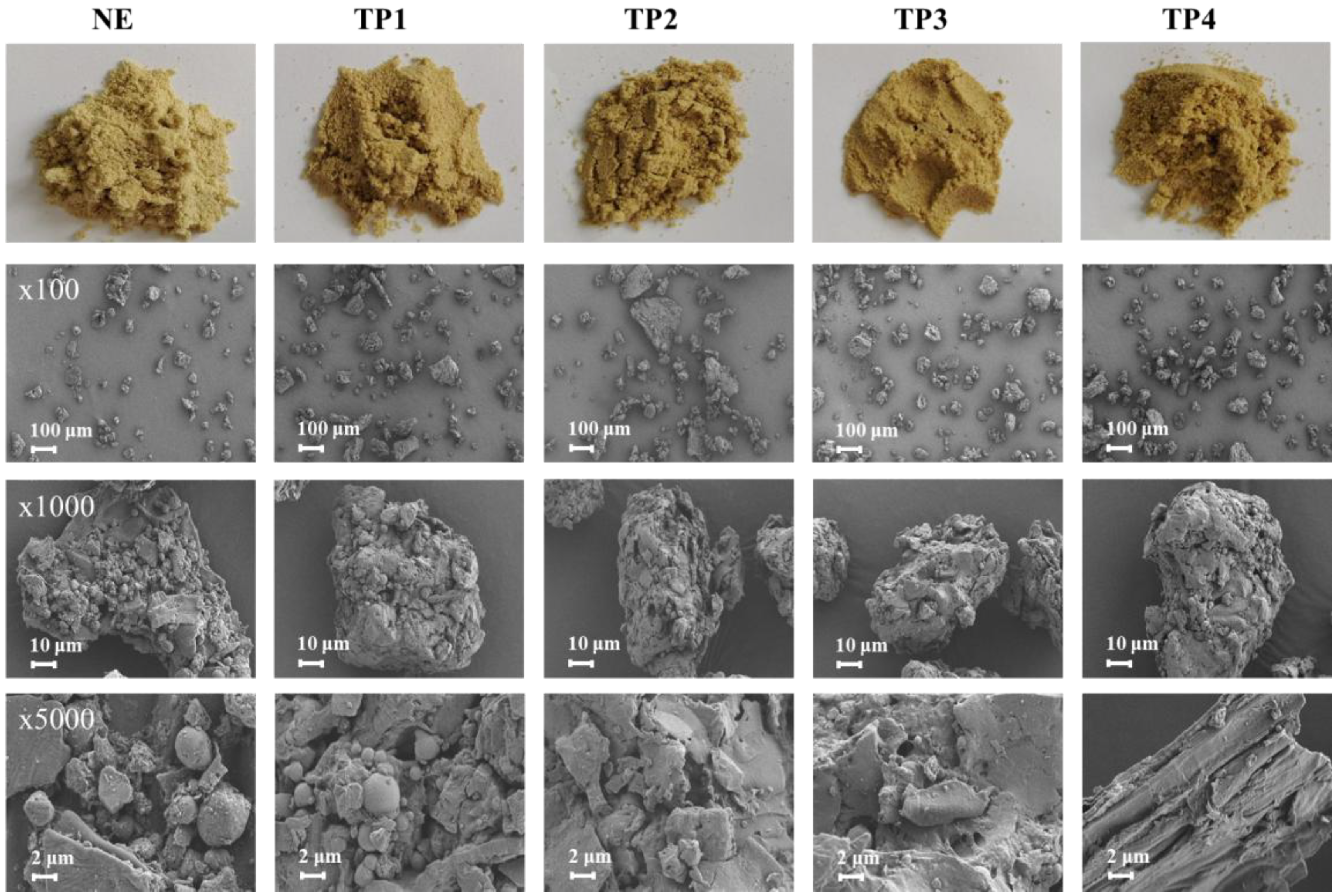
3.2.1. Appearance, Color and Morphology
3.2.2. Basic Component Content
3.2.3. FT-IR Spectra
3.2.4. Water Solubility Index and Water Absorption Index
3.3. Effect of Extruded RB on Sweet Rice Wine Characteristics
3.3.1. Color, Alcohol Content, Wine Yield and In Vitro Antioxidant Capacity
3.3.2. Electronic Tongue and Electronic Nose Results
3.3.3. Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhong, H.; Yang, N.; Xu, S.; Yang, T. Quality improvement of sweet rice wine fermented with Rhizopus delemar on key aroma compounds content, phenolic composition, and antioxidant capacity compared to Rhizopus oryzae. J. Food Sci. Technol. 2022, 59, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Sakandar, H.A.; Hussain, R.; Farid Khan, Q.; Zhang, H. Functional microbiota in Chinese traditional Baijiu and Mijiu Qu (starters): A review. Food Res. Int. 2020, 138, 109830. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, L.; Xiao, M.; Zhang, C.; Zhao, J.; Narbad, A.; Chen, W.; Zhai, Q.; Tian, F. Exploring core fermentation microorganisms, flavor compounds, and metabolic pathways in fermented rice and wheat foods. Food Chem. 2025, 463, 141019. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mithul Aravind, S.; Charpe, P.; Ajlouni, S.; Ranadheera, C.S.; Chakkaravarthi, S. Traditional rice-based fermented products: Insight into their probiotic diversity and probable health benefits. Food Biosci. 2022, 50, 102082. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, Y.; Su, J.; Yang, N.; Xu, X.; Jin, Z.; Cui, B.; Wu, F. Changes in the nutritional value, flavor, and antioxidant activity of brown glutinous rice during fermentation. Food Biosci. 2021, 43, 101273. [Google Scholar] [CrossRef]
- Li, C.; Gong, B. Insights into chain-length distributions of amylopectin and amylose molecules on the gelatinization property of rice starches. Int. J. Biol. Macromol. 2020, 155, 721–729. [Google Scholar] [CrossRef]
- Mao, X.; Yue, S.J.; Xu, D.Q.; Fu, R.J.; Han, J.Z.; Zhou, H.M.; Tang, Y.P. Research progress on flavor and quality of Chinese rice wine in the brewing process. ACS Omega 2023, 8, 32311–32330. [Google Scholar] [CrossRef]
- Shen, C.; Yu, Y.; Zhang, X.; Zhang, H.; Chu, M.; Yuan, B.; Guo, Y.; Li, Y.; Zhou, J.; Mao, J.; et al. The dynamic of physicochemical properties, volatile compounds and microbial community during the fermentation of Chinese rice wine with diverse cereals. Food Res. Int. 2023, 198, 115319. [Google Scholar] [CrossRef]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Boue, S.M.; Goufo, P. Health-promoting germinated rice and value-added foods: A comprehensive and systematic review of germination effects on brown rice. Crit. Rev. Food Sci. Nutr. 2023, 63, 11570–11603. [Google Scholar] [CrossRef]
- Manzoor, A.; Pandey, V.K.; Dar, A.H.; Fayaz, U.; Dash, K.K.; Shams, R.; Ahmad, S.; Bashir, I.; Fayaz, J.; Singh, P.; et al. Rice bran: Nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem. Adv. 2023, 2, 100296. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Chan, L.C. Rice Bran: From Waste to Nutritious Food Ingredients. Nutrients 2023, 15, 2503. [Google Scholar] [CrossRef] [PubMed]
- Offiah, V.; Kontogiorgos, V.; Falade, K.O. Extrusion processing of raw food materials and by-products: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2979–2998. [Google Scholar] [CrossRef]
- Yi, C.; Qiang, N.; Zhu, H.; Xiao, Q.; Li, Z. Extrusion processing: A strategy for improving the functional components, physicochemical properties, and health benefits of whole grains. Food Res. Int. 2022, 160, 111681. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Suzuki, K.; Yasui, Y.; Kasumi, T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J. Food Compos. Anal. 2005, 18, 303–316. [Google Scholar] [CrossRef]
- Kuo, C.H.; Shieh, C.J.; Huang, S.M.; Wang, H.M.; Huang, C.Y. The effect of extrusion puffing on the physicochemical properties of brown rice used for saccharification and Chinese rice wine fermentation. Food Hydrocoll. 2019, 94, 363–370. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, J.; Jin, Z.; Tian, Y.; Zhou, X.; Long, J. Improvement of rice bran modified by extrusion combined with ball milling on the quality of steamed brown rice cake. J. Cereal Sci. 2021, 99, 103229. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Mir, M.M. Effect of puffing on physical and antioxidant properties of brown rice. Food Chem. 2016, 191, 139–146. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; American Association of Cereal Chemists: Saint Paul, MN, USA, 2000. [Google Scholar]
- Tian, X.Y.; Liu, J.F.; Cheng, Z.; Wu, N.N.; Tan, B. Structure, thermal stability, physicochemical and functional characteristics of insoluble dietary fiber obtained from rice bran with steam explosion treatment: Effect of different steam pressure and particle size of rice bran. Food Res. Int. 2024, 187, 114310. [Google Scholar] [CrossRef]
- Qiao, C.C.; Zeng, F.K.; Wu, N.N.; Tan, B. Functional, physicochemical and structural properties of soluble dietary fiber from rice bran with extrusion cooking treatment. Food Hydrocoll. 2021, 121, 107057. [Google Scholar] [CrossRef]
- Tang, A.; Peng, B. Uncovering the flavor differences between black rice wine and glutinous rice wine by GC-MS, GC-IMS, HPLC, and electronic sensory analysis. Food Biosci. 2024, 60, 104235. [Google Scholar] [CrossRef]
- Yu, H.; Li, Q.; Guo, W.; Chen, C.; Ai, L.; Tian, H. Dynamic analysis of volatile metabolites and microbial community and their correlations during the fermentation process of traditional Huangjiu (Chinese rice wine) produced around Winter Solstice. Food Chem. X 2023, 18, 100620. [Google Scholar] [CrossRef] [PubMed]
- Sirisoontaralak, P.; Nakornpanom, N.N.; Koakietdumrongkul, K.; Panumaswiwath, C. Development of quick cooking germinated brown rice with convenient preparation and containing health benefits. LWT-Food Sci. Technol. 2015, 61, 138–144. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae—Ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef]
- Chen, C.; Tao, Y.; Han, Y.; Ding, Y.; Jian, X.; Li, D. Preparation of germinated brown rice with high γ-aminobutyric acid content and short root by magnetic field treatment. J. Cereal Sci. 2023, 112, 103720. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Q.; Shen, L.; Luo, J.; Zhu, R.; Mao, J.; Zhao, M.; Cai, C. Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters. LWT-Food Sci. Technol. 2019, 111, 226–234. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef]
- Kc, Y.; Mitchell, J.; Bhandari, B.; Prakash, S. Unlocking the potential of rice bran through extrusion: A systematic review. Sustain. Food Technol. 2024, 2, 594–614. [Google Scholar] [CrossRef]
- Rashid, M.T.; Liu, K.; Han, S.; Jatoi, M.A.; Sarpong, F. Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran. Foods 2023, 12, 1236. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Dong, L.; Jia, X.; Liu, L.; Huang, F.; Chi, J.; Xiao, J.; Zhang, M.; Zhang, R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT-Food Sci. Technol. 2019, 115, 108461. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Han, T.; Wen, Y.; Zhang, H.; Li, D. Preparation of Rice Bran-Enriched Sweet Rice Wine and Its Quality Improvement Through Extrusion. Foods 2025, 14, 1582. https://doi.org/10.3390/foods14091582
Li W, Han T, Wen Y, Zhang H, Li D. Preparation of Rice Bran-Enriched Sweet Rice Wine and Its Quality Improvement Through Extrusion. Foods. 2025; 14(9):1582. https://doi.org/10.3390/foods14091582
Chicago/Turabian StyleLi, Wantian, Teng Han, Yuxin Wen, Hao Zhang, and Dandan Li. 2025. "Preparation of Rice Bran-Enriched Sweet Rice Wine and Its Quality Improvement Through Extrusion" Foods 14, no. 9: 1582. https://doi.org/10.3390/foods14091582
APA StyleLi, W., Han, T., Wen, Y., Zhang, H., & Li, D. (2025). Preparation of Rice Bran-Enriched Sweet Rice Wine and Its Quality Improvement Through Extrusion. Foods, 14(9), 1582. https://doi.org/10.3390/foods14091582





