Research Progress on the Mechanism of Action and Screening Methods of Probiotics for Lowering Blood Lipid Levels
Abstract
1. Introduction
2. Mechanisms of Probiotics in Regulating Lipid Metabolism
2.1. Assimilation and Adsorption of Lipids by Microbial Cells
2.2. Regulation of Lipid-Metabolism-Related Transcriptional Factors
2.2.1. Short-Chain Fatty Acids and Their Related Signaling Pathways
2.2.2. Ferulic Acid Esterase
2.2.3. Conjugated Linoleic Acid
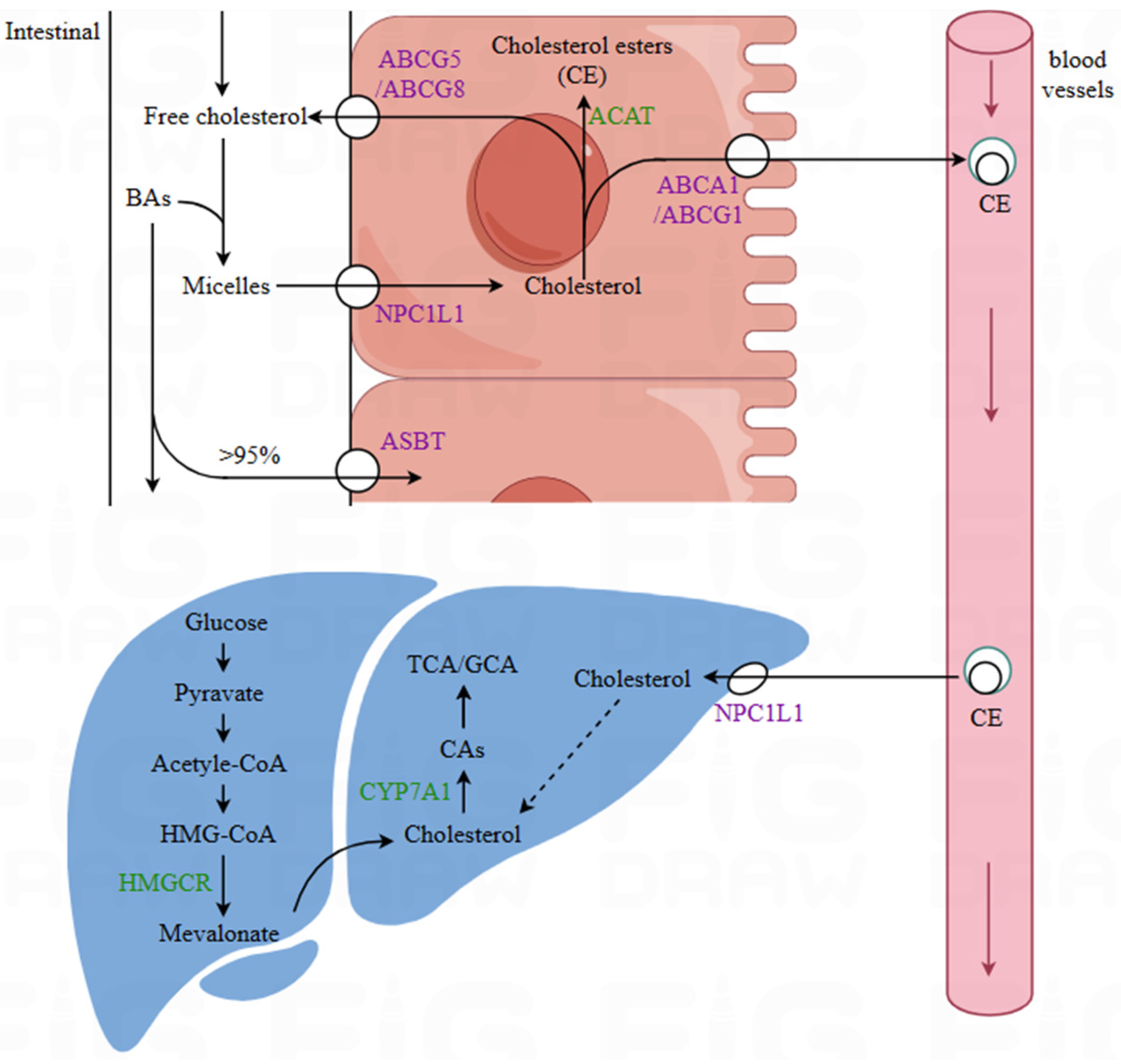
2.3. Influence on Lipid Transport and Absorption
2.3.1. Bile Salt Hydrolase
2.3.2. Exopolysaccharides
2.3.3. Regulation of the Expression of Relevant Transport Proteins
3. Research on Probiotic-Mediated Lipid-Lowering Effects
4. Screening Methods for Lipid-Lowering Probiotics
4.1. Traditional Screening Techniques for Lipid-Lowering Probiotics
4.1.1. In Vitro Cholesterol and Triglyceride Reduction
4.1.2. Qualitative and Quantitative Assays for BSH Activity
4.1.3. Cell Models
4.1.4. High-Fat-Diet Rat and Mouse Models
4.1.5. High-Fat-Diet Zebrafish Model
4.2. Advanced Screening and Prediction Technologies
4.2.1. Digitalized Live Zebrafish-on-a-Chip System
4.2.2. Functional Genomics Analysis of Probiotics
5. Conclusions
6. Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, G. Epidemiology and preventive measures of dyslipidemia. Chin. J. Clin. Healthc. 2021, 24, 733–736. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Gao, R. Chinese guidelines for lipid management (2023). Chin. J. Cardiol. 2023, 51, 221–255. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, H.; Lu, J.; Ding, Q.; Li, X.; Wang, X.; Sun, D.; Tan, L.; Mu, L.; Liu, J.; et al. Prevalence of dyslipidemia and availability of lipid-lowering medications among primary health care settings in China. JAMA Netw. Open 2021, 4, 2127573. [Google Scholar] [CrossRef]
- Bliznakov, E.G. Lipid-lowering drugs (statins), cholesterol, and coenzyme Q10. Biomed. Pharmacother 2002, 56, 56–59. [Google Scholar] [CrossRef]
- Atlee, W.C. Overview of ideal antilipidemic drugs: Past, present and the future. GSC Biol. Pharm. Sci. 2020, 11, 67–74. [Google Scholar] [CrossRef]
- Shattat, G.F. A review article on hyperlipidemia types, treatments and new drug targets. Biomed. Pharmacol. J. 2014, 7, 399–409. [Google Scholar] [CrossRef]
- Mann, G.V. Studies of a surfactant and cholesteremia in the Maasai. Am. J. Clin. Nutr. 1974, 27, 464–469. [Google Scholar] [CrossRef]
- Mann, G.V. A factor in yogurt which lowers cholesteremia in man. Atherosclerosis 1977, 26, 335–340. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Aobchecy, P.; Tateing, S.; Prachansuwan, A.; Sitdhipol, J.; Niwasabutra, K.; Thaveethaptaikul, P.; et al. Safety and effects of Lactobacillus paracasei TISTR 2593 supplementation on improving cholesterol metabolism and atherosclerosis-related parameters in subjects with hypercholesterolemia: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2023, 15, 661. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, C.; Li, Y.; Zhang, L.; A, L.; Yang, C.; Zhao, F.; Han, H.; Shang, D.; Yang, F.; et al. Probio-X relieves symptoms of hyperlipidemia by regulating patients’ gut microbiome, blood lipid metabolism, and lifestyle habits. Microbiol. Spectr. 2023, 11, 0444022. [Google Scholar] [CrossRef]
- Walker, D.K.; Gilliland, S.E. Relationships among bile tolerance, bile salt deconjugation, and assimilation of cholesterol by Lactobacillus acidophilus. J. Dairy Sci. 1993, 76, 956–961. [Google Scholar] [CrossRef]
- Lye, H.S.; Rahmat-Ali, G.R.; Liong, M.T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175. [Google Scholar] [CrossRef]
- Noh, D.O.; Kim, S.H.; Gilliland, S.E. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 431211. J. Dairy Sci. 1997, 80, 3107–3113. [Google Scholar] [CrossRef]
- Zhu, J.; Mao, Q.; Wang, S.; Liu, H.; Zhou, S.; Zhang, W.; Kong, M.; Zhu, H.; Li, S. Optimization and validation of direct gas chromatography-mass spectrometry method for simultaneous quantification of ten short-chain fatty acids in rat feces. J. Chromatogr. A 2022, 1669, 462958. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Zhou, Q.; Qu, Z.; Wang, N.; Liu, H.; Yang, H.; Wang, H. Miao sour soup influences serum lipid via regulation of high-fat diet-induced intestinal flora in obese rats. Food Nutr. Health 2022, 11, 2232–2242. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Huo, Y.; Zhao, G.; Li, J.; Wang, R.; Ren, F.; Li, Y.; Wang, X. Bifidobacterium animalis subsp. lactis A6 enhances fatty acid β-oxidation of adipose tissue to ameliorate the development of obesity in mice. Nutrients 2022, 14, 598. [Google Scholar] [CrossRef]
- Hara, H.; Haga, S.; Kasai, T.; Kiriyama, S. Fermentation products of sugar-beet fiber by cecal bacteria lower plasma cholesterol concentration in rats. J. Nutr. 1998, 128, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Song, L.; Xiao, Y.; Lu, S.; Xu, J.; Li, J.; Ren, Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods 2020, 73, 104103. [Google Scholar] [CrossRef]
- Melhem, H.; Kaya, B.; Ayata, C.K.; Hruz, P.; Niess, J.H. Metabolite-sensing G protein-coupled receptors connect the diet-microbiota-metabolites axis to inflammatory bowel disease. Cells 2019, 8, 450. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Tian, Y.; Chen, Y.; Guan, W.; Piao, C.; Wang, Y. Lactobacillus plantarum LP104 ameliorates hyperlipidemia induced by AMPK pathways in C57BL/6N mice fed high-fat diet. J. Funct. Foods 2020, 64, 103665. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Modulation of PPAR expression and activity in response to polyphenolic compounds in high fat diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef]
- Frappier, M.; Auclair, J.; Bouasker, S.; Gunaratnam, S.; Diarra, C.; Millette, M. Screening and characterization of some Lactobacillaceae for detection of cholesterol-lowering activities. Probiotics Antimicrob. Proteins 2022, 14, 873–883. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ma, K.Y.; Liang, Y.; Peng, C.; Zuo, Y. Role and classification of cholesterol-lowering functional foods. J. Funct. Foods 2011, 3, 61–69. [Google Scholar] [CrossRef]
- Mougios, V.; Matsakas, A.; Petridou, A.; Ring, S.; Sagredos, A.; Melissopoulou, A.; Tsigilis, N.; Nikolaidis, M. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J. Nutr. Biochem. 2001, 12, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, W. Review of the roles of conjugated linoleic acid in health and disease. J. Funct. Foods 2015, 15, 314–325. [Google Scholar] [CrossRef]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef]
- Ma, L.; Lengi, A.J.; McGilliard, M.L.; Bauman, D.E.; Corl, B.A. Short communication: Effect of trans-10,cis-12 conjugated linoleic acid on activation of lipogenic transcription factors in bovine mammary epithelial cells. J. Dairy Sci. 2014, 97, 5001–5006. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; Han, C.Y.; Wang, S.; Omer, M.; Chait, A. 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species. J. Lipid Res. 2013, 54, 2964–2978. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.M. Bile salt hydrolase and lipase inhibitory activity in reconstituted skim milk fermented with lactic acid bacteria. J. Funct. Foods 2021, 77, 104342. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Shehata, M.G. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 61–75. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J. Effect of bile salt hydrolase-active Lactobacillus plantarum Y15 on high cholesterol diet induced hypercholesterolemic mice. CyTA-J. Food 2021, 19, 408–417. [Google Scholar] [CrossRef]
- Keleszade, E.; Kolida, S.; Costabile, A. The cholesterol lowering efficacy of Lactobacillus plantarum ECGC 13110402 in hypercholesterolemic adults: A double-blind, randomized, placebo controlled, pilot human intervention study. J. Funct. Foods 2022, 89, 104939. [Google Scholar] [CrossRef]
- Nakajima, H. Cholesterol lowering activity of ropy fermented milk. J. Food Sci. 1992, 57, 75–80. [Google Scholar] [CrossRef]
- Shiomi, M. Antitumor activity in mice of orally administered. Jpn. J. Med. Sci. Biol. 1982, 35, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.Y.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Davis, H.R.; Altmann, S.W. Niemann–Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. BBA-Mol. Cell Biol. Lipids 2009, 1791, 679–683. [Google Scholar] [CrossRef]
- Zhao, K.; Qiu, L.; He, Y.; Tao, X.; Zhang, Z.; Wei, H. Alleviation syndrome of high-cholesterol-diet-induced hypercholesterolemia in mice by intervention with Lactiplantibacillus plantarum WLPL21 via regulation of cholesterol metabolism and transportation as well as gut microbiota. Nutrients 2023, 15, 2600. [Google Scholar] [CrossRef]
- Markowiak-Kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, Y.; Hou, X.; Wang, H.; Huang, L.; Wen, J.; Niu, H.; Zeng, W.; Bai, Y. Novel Lactobacillus reuteri HI120 affects lipid metabolism in C57BL/6 obese mice. Front. Vet. Sci. 2020, 7, 560241. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Ren, F.; Li, Y. Lactobacillus paracasei N1115 attenuates obesity in high-fat diet-induced obese mice. Food Sci. Nutr. 2023, 11, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.J.; Kim, J.Y.; Shim, J.J.; Lee, J.H. A mixture of Lactobacillus HY7601 and KY1032 regulates energy metabolism in adipose tissue and improves cholesterol disposal in high-fat-diet-fed mice. Nutrients 2024, 16, 2570. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Huang, Y.; Song, L.; Li, M.; Ren, Z. Lactobacillus gasseri RW2014 ameliorates hyperlipidemia by modulating bile acid metabolism and gut microbiota composition in rats. Nutrients 2022, 14, 4945. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, F.; Xu, D.; Zhang, Z.; Xu, F.; Tao, X.; Qiu, L.; Wei, H. Enterococcus faecium WEFA23 from infants lessens high-fat-diet-induced hyperlipidemia via cholesterol 7-alpha-hydroxylase gene by altering the composition of gut microbiota in rats. J. Dairy Sci. 2018, 101, 7757–7767. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.L.; Chen, G.M.; Qian, M.; Han, J.Z.; Lv, X.C.; Chen, L.J.; Rao, P.F.; Ai, L.Z.; Ni, L. Pediococcus acidilactici FZU106 alleviates high-fat diet-induced lipid metabolism disorder in association with the modulation of intestinal microbiota in hyperlipidemic rats. Curr. Res. Food Sci. 2022, 5, 775–788. [Google Scholar] [CrossRef]
- Chu, C.; Jiang, J.; Yu, L.; Li, Y.; Zhang, S.; Zhou, W.; Wang, Q.; Zhao, J.; Zhai, Q.; Tian, F.; et al. Bifidobacterium longum CCFM1077 attenuates hyperlipidemia by modulating the gut microbiota composition and fecal metabolites: A randomized, double-blind, placebo-controlled clinical trial. Engineering 2023, 28, 193–205. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.I.; Chu, J.; Kim, B.K.; Choi, I.S.; Kim, J.Y. Effects of Lactobacillus plantarum Q180 on postprandial lipid levels and intestinal environment: A double-blind, randomized, placebo-controlled, parallel trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef]
- Guerrero-Bonmatty, R.; Gil-Fernandez, G.; Rodriguez-Velasco, F.J.; Espadaler-Mazo, J. A combination of Lactoplantibacillus plantarum strains CECT7527, CECT7528, and CECT7529 plus Monacolin K reduces blood cholesterol: Results from a randomized, double-blind, placebo-controlled study. Nutrients 2021, 13, 1206. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, G.; Zhao, X.; Zhang, H.; Ren, M.; Song, X.; Chang, H.; Jing, Z. Probiotics combined with atorvastatin administration in the treatment of hyperlipidemia: A randomized, double-blind, placebo-controlled clinical trial. Medicine 2024, 103, e37883. [Google Scholar] [CrossRef] [PubMed]
- He, S. Isolation and Identification of Intestinal Bacteria Degrading Cholesterol in Vitro by Culturomics and Evaluation of Their Functions. Master’s Thesis, Academy of Military Science, Beijing, China, December 2021. [Google Scholar] [CrossRef]
- Kimoto, H.; Ohmomo, S.; Okamoto, T. Cholesterol removal from media by lactococci. J. Dairy Sci. 2002, 85, 3182–3188. [Google Scholar] [CrossRef]
- Guo, L.D.; Yang, L.J.; Huo, G.C. Cholesterol removal by Lactobacillus plantarum isolated from homemade fermented cream in Inner Mongolia of China. Czech J. Food Sci. 2011, 29, 219–225. [Google Scholar] [CrossRef]
- Pereira, D.I.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, X.; Hang, X.; Li, K.; Yang, H. In vitro selection of probiltic Lactobacillus from the human feces and their mechanism on cholesterol-lowering. Microbiol. China 2006, 33, 43–47. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Font De Valdez, G.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Zhou, X.; Sun, J.; Liang, X.; Lv, Y.; Bai, L.; Zhang, J.; Gong, P.; Liu, T.; et al. Lactobacillus casei YRL577 ameliorates markers of non-alcoholic fatty liver and alters expression of genes within the intestinal bile acid pathway. Brit. J. Nutr. 2021, 125, 521–529. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci. World J. 2014, 2014, 690752. [Google Scholar] [CrossRef]
- Kaur, P.; Robin; Mehta, R.G.; Arora, S.; Singh, B. Progression of conventional hepatic cell culture models to bioengineered HepG2 cells for evaluation of herbal bioactivities. Biotechnol. Lett. 2018, 40, 881–893. [Google Scholar] [CrossRef]
- Tang, C.; Meng, F.; Pang, X.; Chen, M.; Zhou, L.; Lu, Z.; Lu, Y. Protective effects of Lactobacillus acidophilus NX2-6 against oleic acid-induced steatosis, mitochondrial dysfunction, endoplasmic reticulum stress and inflammatory responses. J. Funct. Foods 2020, 74, 104206. [Google Scholar] [CrossRef]
- Hengpratom, T.; Lowe, G.M.; Thumanu, K.; Suknasang, S.; Tiamyom, K.; Eumkeb, G. Oroxylum indicum (L.) Kurz extract inhibits adipogenesis and lipase activity in vitro. BMC Complement. Altern. Med. 2018, 18, 177. [Google Scholar] [CrossRef]
- Won, S.M.; Chen, S.Y.; Park, K.W.; Yoon, J.H. Isolation of lactic acid bacteria from kimchi and screening of Lactobacillus sakei ADM14 with anti-adipogenic effect and potential probiotic properties. LWT-Food Sci. Technol. 2020, 126, 109296. [Google Scholar] [CrossRef]
- Hsieh, F.C.; Lan, C.C.E.; Huang, T.Y.; Chen, K.W.; Chai, C.Y.; Chen, W.T.; Fang, A.H.; Chen, Y.H.; Wu, C.S. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; Dong, Y.; Zhou, X. Anti-obesity action of fermented barley extracts with Lactobacillus plantarum dy-1 and associated microRNA expression in high-fat diet-induced obese rats. Biomed. Environ. Sci. 2019, 32, 755–768. [Google Scholar] [CrossRef]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Maria Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Lu, F.; Zhang, J.; Guo, L. Application of zebrafish in the study of the gut microbiome. Anim. Models Exp. Med. 2022, 5, 323–336. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef]
- Chen, S.; Ye, W.; Clements, K.D.; Zan, Z.; Zhao, W.; Zou, H.; Wang, G.; Wu, S. Bacillus licheniformis FA6 affects zebrafish lipid metabolism through promoting acetyl-CoA synthesis and inhibiting β-oxidation. Int. J. Mol. Sci. 2023, 24, 673. [Google Scholar] [CrossRef]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.L.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 9336. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Weitekamp, C.A.; Kvasnicka, A.; Keely, S.P.; Brinkman, N.E.; Howey, X.M.; Gaballah, S.; Phelps, D.; Catron, T.; Zurlinden, T.; Wheaton, E.; et al. Monoassociation with bacterial isolates reveals the role of colonization, community complexity and abundance on locomotor behavior in larval zebrafish. Anim. Microbiome 2021, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci. Rep. 2016, 6, 30046. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Avella, M.A.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocr. 2013, 188, 297–302. [Google Scholar] [CrossRef]
- Lin, X.; Duan, X.; Jacobs, C.; Ullmann, J.; Chan, C.; Chen, S.; Cheng, S.; Zhao, W.; Poduri, A.; Wang, X.; et al. High-throughput brain activity mapping and machine learning as a foundation for systems neuropharmacology. Nat. Commun. 2018, 9, 5142. [Google Scholar] [CrossRef]
- Tang, M.; Duan, X.; Yang, A.; He, S.; Zhou, Y.; Liu, Y.; Zhang, L.; Luo, X.; Shi, P.; Li, H.; et al. Fish Capsules: A System for High-Throughput Screening of Combinatorial Drugs. Adv. Sci. 2022, 9, 2104449. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H. Application of probiogenomics in screening and functional evaluation of lactic acid bacteria. J. Chin. Inst. Food Sci. Technol. 2024, 24, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Zheng, L.; Li, J.; Hong, Y.; Liang, P.; Kwok, L.; Zuo, Y.; Zhang, W.; Zhang, H. iProbiotics: A machine learning platform for rapid identification of probiotic properties from whole-genome primary sequences. Brief. Bioinform. 2022, 23, bbab477. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Li, H.; Zhu, J.; Liu, Z.; Hu, X.; Yi, J. Assessments of probiotic potentials of Lactiplantibacillus plantarum strains isolated from chinese traditional fermented food: Phenotypic and genomic analysis. Front. Microbiol. 2022, 13, 895132. [Google Scholar] [CrossRef]
| Study Type | Probiotic Strain | Subjects | Intervention Duration | Key Outcomes | Reference |
|---|---|---|---|---|---|
| Animal | Lactobacillus plantarum HAC01 | High-fat-diet (HFD)-fed C57BL/6J mice | 8 weeks | ↓ Serum TC and TG levels (TC: ↓ 19.75%; TG: ↓ 16.67% vs. model) | (Park et al., 2017) [49] |
| Lactobacillus reuteri HI120 | HFD-fed C57BL/6J mice | 12 weeks | ↓ Serum TC levels in the HI120 group (↓ 47.82% vs. model) ↓ Serum TC levels in atorvastatin group (↓ 48.26% vs. model) | (Sun et al., 2020) [50] | |
| Lactobacillus plantarum Y15 | HFD-fed C57BL/6J mice | 4 weeks | ↓ Serum TC, TG, and LDL-C levels (TC, TG, and LDL-C decreases were comparable to the pravastatin group) | (Liu et al., 2017) [40] | |
| Lactobacillus paracasei N1115 | HFD-fed C57BL/6J mice | 12 weeks | ↓ Serum TC, TG, and LDL-C levels (TC: ↓ 46.79%; TG: ↓ 17.99%; LDL-C: ↓ 31.59% vs. model) | (Sun et al., 2023) [51] | |
| Lactobacillus curvatus HY7601, Lactobacillus plantarum KY1032 | HFD-fed C57BL/6 mice | 7 weeks | ↓ Serum TC, TG, and LDL-C levels (TC: ↓ 20.1%; TG: ↓ 40.9%; LDL-C: ↓ 29.4% vs. model) | (Lee et al., 2024) [52] | |
| Lactobacillus gasseri RW2014 | HFD-fed SD rats | 6 weeks | ↓ Serum TC, TG, and LDL-C levels (TC and TG decreases were comparable to the simvastatin group) ↑ Serum HDL-C levels | (Li et al., 2022) [53] | |
| Enterococcus faecium WEFA23 | HFD-fed SD rats | 35 days | ↓ Serum TC, TG, and LDL-C levels (TC: ↓ 33.90%; TG: ↓ 35.17%; LDL-C: ↓ 30.02% vs. model) ↑ Serum HDL-C levels (HDL-C: ↑ 30.02% vs. model) | (Huang et al., 2018) [54] | |
| Pediococcus acidilactici FZU106 | HFD-fed SD rats | 8 weeks | ↓ Serum TC, TG, and LDL-C levels (TC and LDL-C decreases were comparable to the simvastatin group) ↑ Serum HDL-C levels (HDL-C increase was comparable to the simvastatin group) | (Zhang et al., 2022) [55] | |
| Clinical | Lactobacillus paracasei TISTR 2593 | 50 hyperlipidemic patients | 90 days | ↓ Serum LDL-C level (↓ 15.56% vs. placebo) | (Khongrum et al., 2023) [11] |
| Bifidobacterium longum CCFM1077 | 62 hyperlipidemic patients | 6 weeks | ↓ Serum TC and LDL-C levels (TC: ↓ 5.7%; LDL-C ↓ 4.7% vs. placebo) | (Chu et al., 2023) [56] | |
| Lactobacillus plantarum ECGC 13110402 | 16 hypercholesterolemic adults, TC ≥ 6 mmol/L | 6 weeks | ↓ Serum TC and LDL-C levels (TC: ↓ 34.6%; LDL-C: ↓ 28.4% vs. before intervention) | (Keleszade et al., 2022) [41] | |
| Lactobacillus plantarum Q180 | 70 participants, TG < 200 mg/dL | 12 weeks | ↓ Serum LDL-C levels (LDL-C: ↓ 9.5% vs. placebo) | (Park et al., 2020) [57] | |
| Lactoplantibacillus plantarum CECT7527, Lactoplantibacillus plantarum CECT7528 and Lactoplantibacillus plantarum CECT7529 | 39 participants, TC ≥ 200 mg/dL | 12 weeks | ↓ Serum TC and LDL-C levels (TC: ↓ 11%; LDL-C: ↓ 13% vs. placebo) | (Guerrero-Bonmatty et al., 2020) [58] | |
| Lactiplantibacillus casei Zhang, Bifidobacterium lactis V9, Bifidobacterium lactis Probio-M8, Lactiplantibacillus rhamnosus Probio-M9, Lactiplantibacillus plantarum P-8 | 56 hyperlipidemic patients | 3 months | ↓ Serum LDL-C levels (LDL-C: ↓ 11.8% vs. placebo) ↑ Serum HDL-C levels (HDL-C: ↑ 9.5% vs. placebo) | (Wang et al., 2023) [12] |
| Reference | Year | Method | Bile Salt |
|---|---|---|---|
| Bile salt hydrolase and lipase inhibitory activity in reconstituted skim milk fermented with lactic acid bacteria [37] | 2021 | Wells were drilled in MRS agar plates containing CaCl2 and 0.3% ox bile. Each well was inoculated with 25 µL of bacterial suspension and incubated under anaerobic conditions at 37 °C for 72 h. | 0.3% ox bile |
| Lactobacillus casei YRL577 ameliorates markers of non-alcoholic fatty liver and alters the expression of genes within the intestinal bile acid pathway [66] | 2021 | MRS agar medium was supplemented with 0.3% taurodeoxycholate, 0.2% sodium thioglycolate, and 0.37 g/L of CaCl2. Sterile filter paper discs were evenly placed on the agar plate, and 10 µL of bacterial suspension was added to each disc; this was followed by incubation at 37 °C for 48 h. | 0.3% taurodeoxycholate (TDCA) |
| Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo [67] | 2014 | MRS agar plates containing 0.5% (w/v) sodium tauro (deoxy) cholate and 0.37 g/L of CaCl2 were prepared. Sterile filter discs (6 mm) were soaked in overnight bacterial cultures and placed on the agar plates; this was followed by anaerobic incubation at 37 °C for 72 h. | 0.5% tauro (deoxy) cholate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, J.; Gao, M.; Ouyang, Z.; Li, Y.; Liu, D.; Zhu, M.; Sun, H. Research Progress on the Mechanism of Action and Screening Methods of Probiotics for Lowering Blood Lipid Levels. Foods 2025, 14, 1583. https://doi.org/10.3390/foods14091583
Wang J, Chen J, Gao M, Ouyang Z, Li Y, Liu D, Zhu M, Sun H. Research Progress on the Mechanism of Action and Screening Methods of Probiotics for Lowering Blood Lipid Levels. Foods. 2025; 14(9):1583. https://doi.org/10.3390/foods14091583
Chicago/Turabian StyleWang, Jingli, Jieyu Chen, Mingkun Gao, Zijun Ouyang, Yanhui Li, Dong Liu, Mingjun Zhu, and Haiyan Sun. 2025. "Research Progress on the Mechanism of Action and Screening Methods of Probiotics for Lowering Blood Lipid Levels" Foods 14, no. 9: 1583. https://doi.org/10.3390/foods14091583
APA StyleWang, J., Chen, J., Gao, M., Ouyang, Z., Li, Y., Liu, D., Zhu, M., & Sun, H. (2025). Research Progress on the Mechanism of Action and Screening Methods of Probiotics for Lowering Blood Lipid Levels. Foods, 14(9), 1583. https://doi.org/10.3390/foods14091583







