Process Optimization and Odor Analysis of Instant Black Tea Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Equipment
2.3. Test Method
2.3.1. Instant Black Tea Powder Preparation Process
2.3.2. Extraction Single-Factor Optimization Test
2.3.3. Sensory Evaluation Method for Instant Black Tea
2.3.4. Design of Process Optimization Experiment Using Response Surface Methodology
2.3.5. Measurement of Instant Black Tea Odor Components Using Electronic Nose
2.3.6. Analysis of Odor Components and Content in Instant Black Tea Using HS-SPME-GC-MS
2.4. Methods of Data Processing and Analysis
3. Results and Discussion
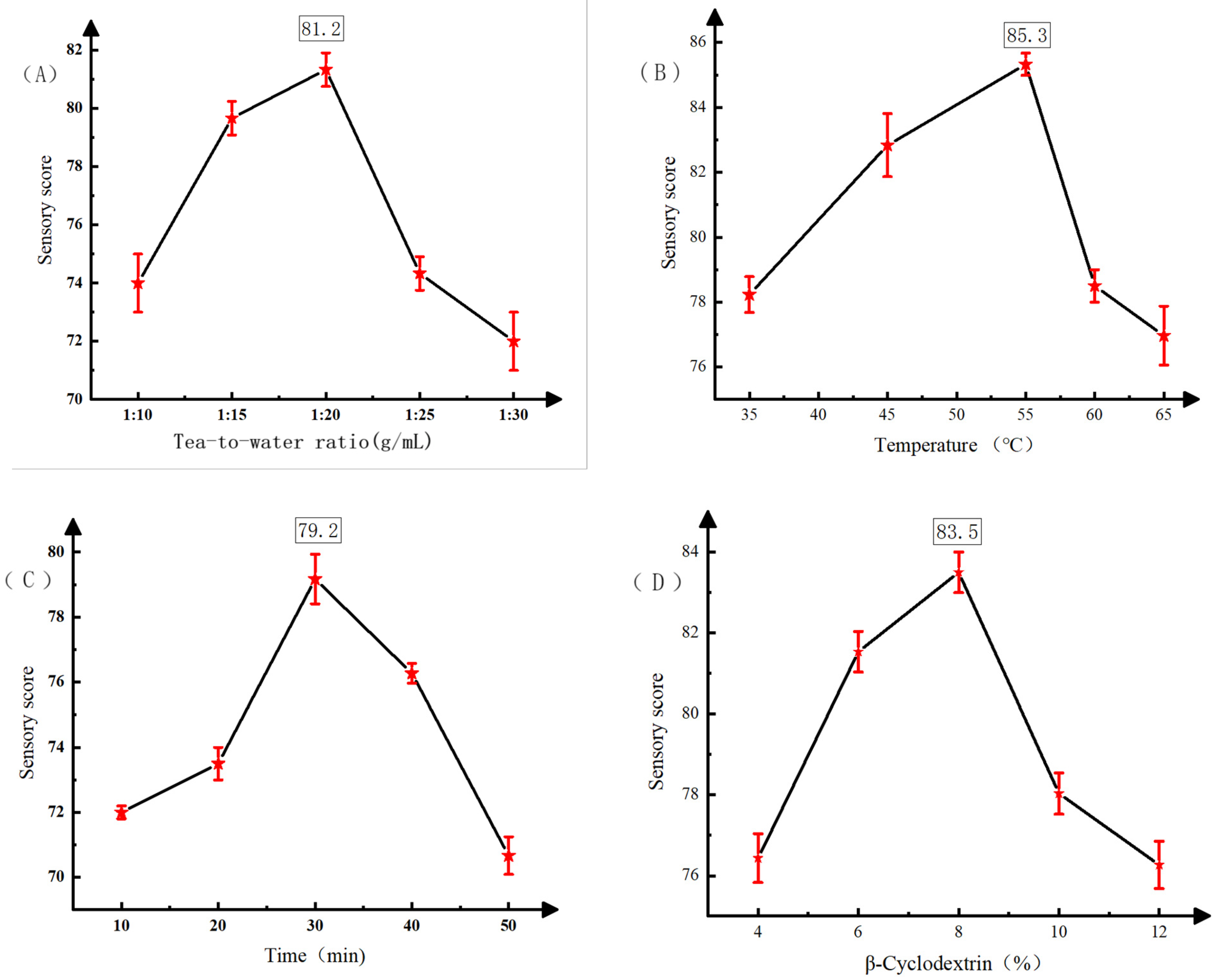
3.1. Analysis of Different Processing Techniques for Instant Black Tea Powder
Single-Factor Test Results
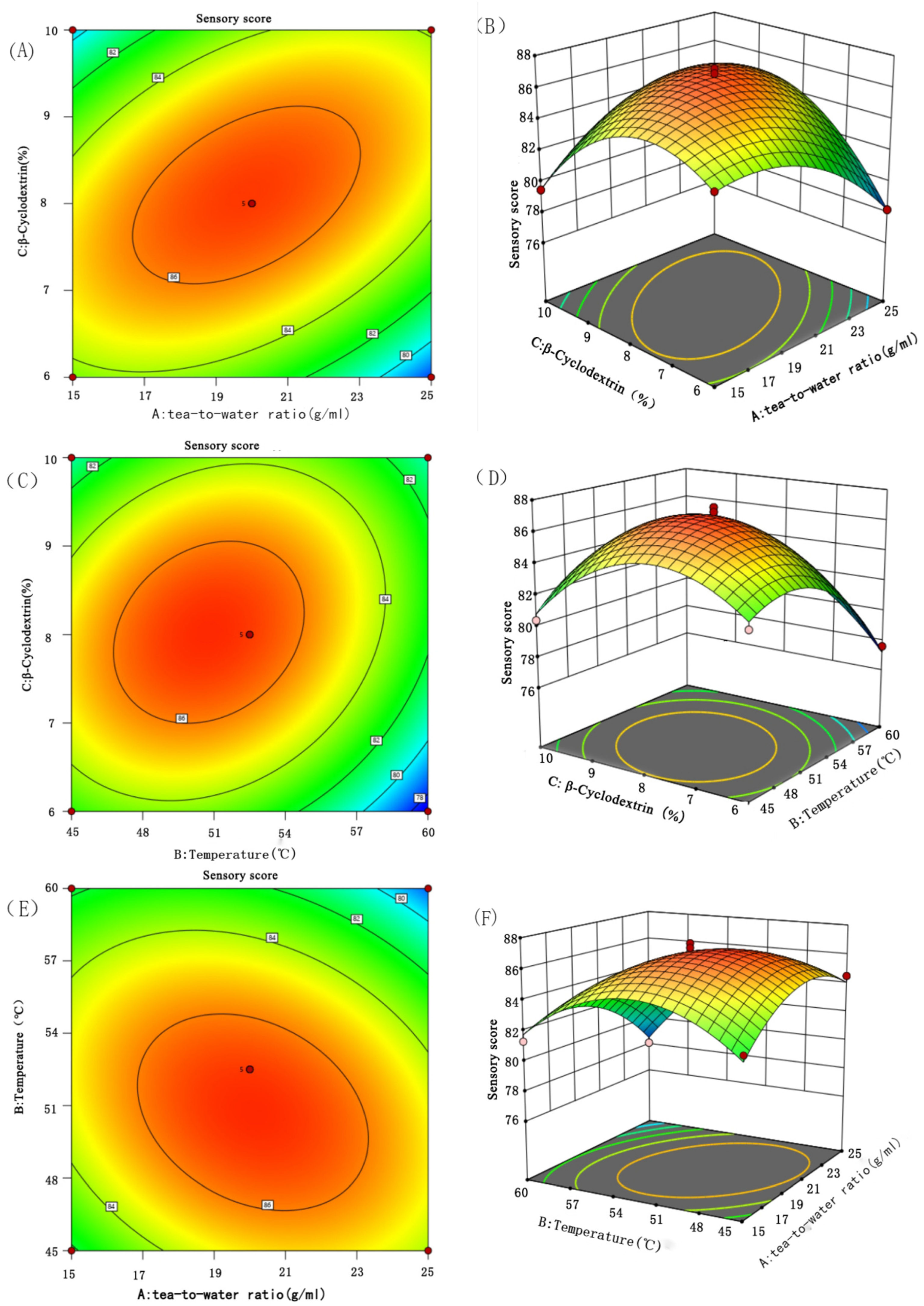
3.2. Response Surface Test Results
3.2.1. Design and Result Analysis of Response Surface Test
3.2.2. Interaction Analysis
3.2.3. Verification of the Response Surface Model
3.3. Results of Analysis of Instant Black Tea with Different Processing Methods by Electronic Nose Processing Techniques
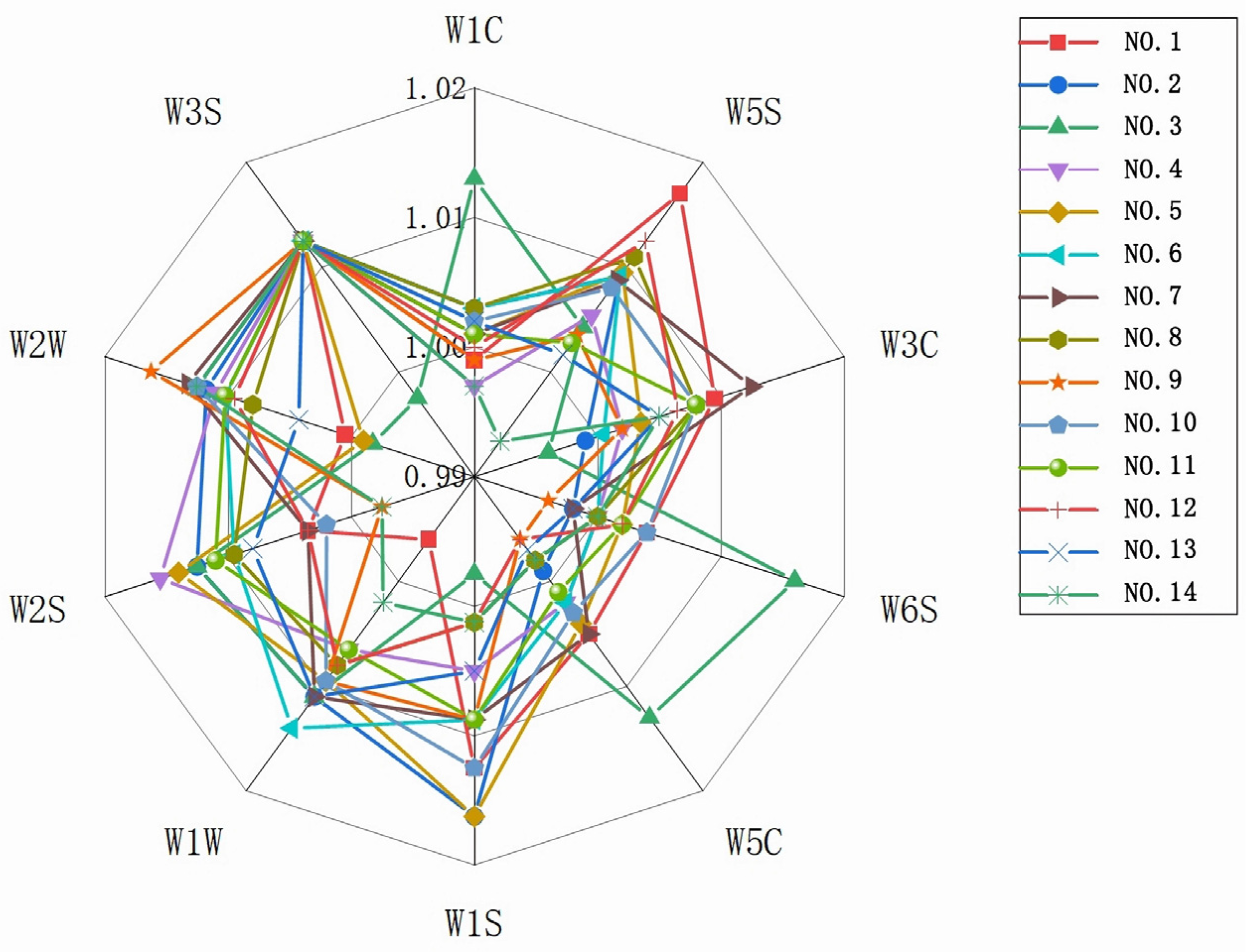
3.3.1. Analysis of the Odor Source of Instant Black Tea by Electronic Nose Sensors
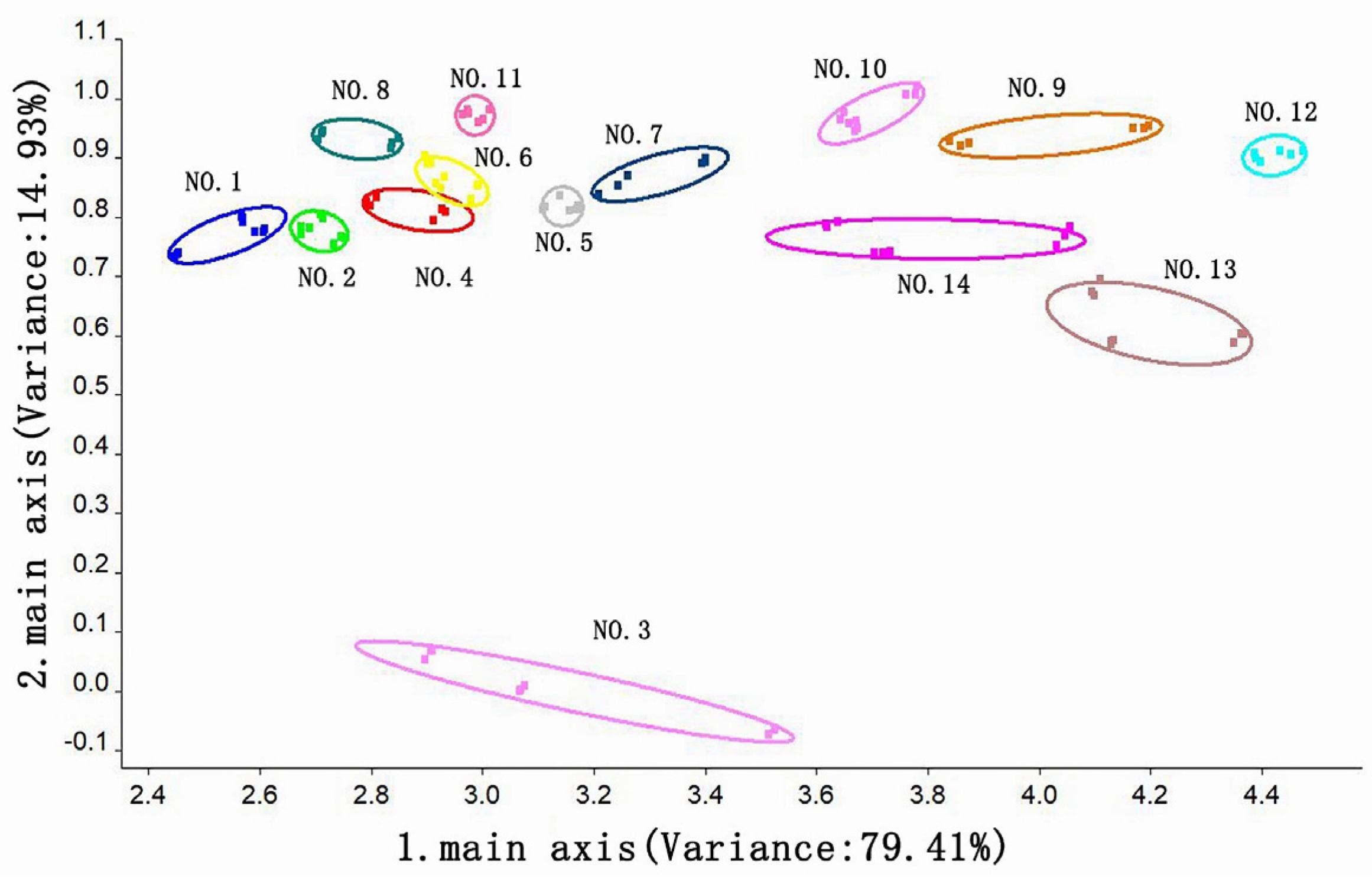
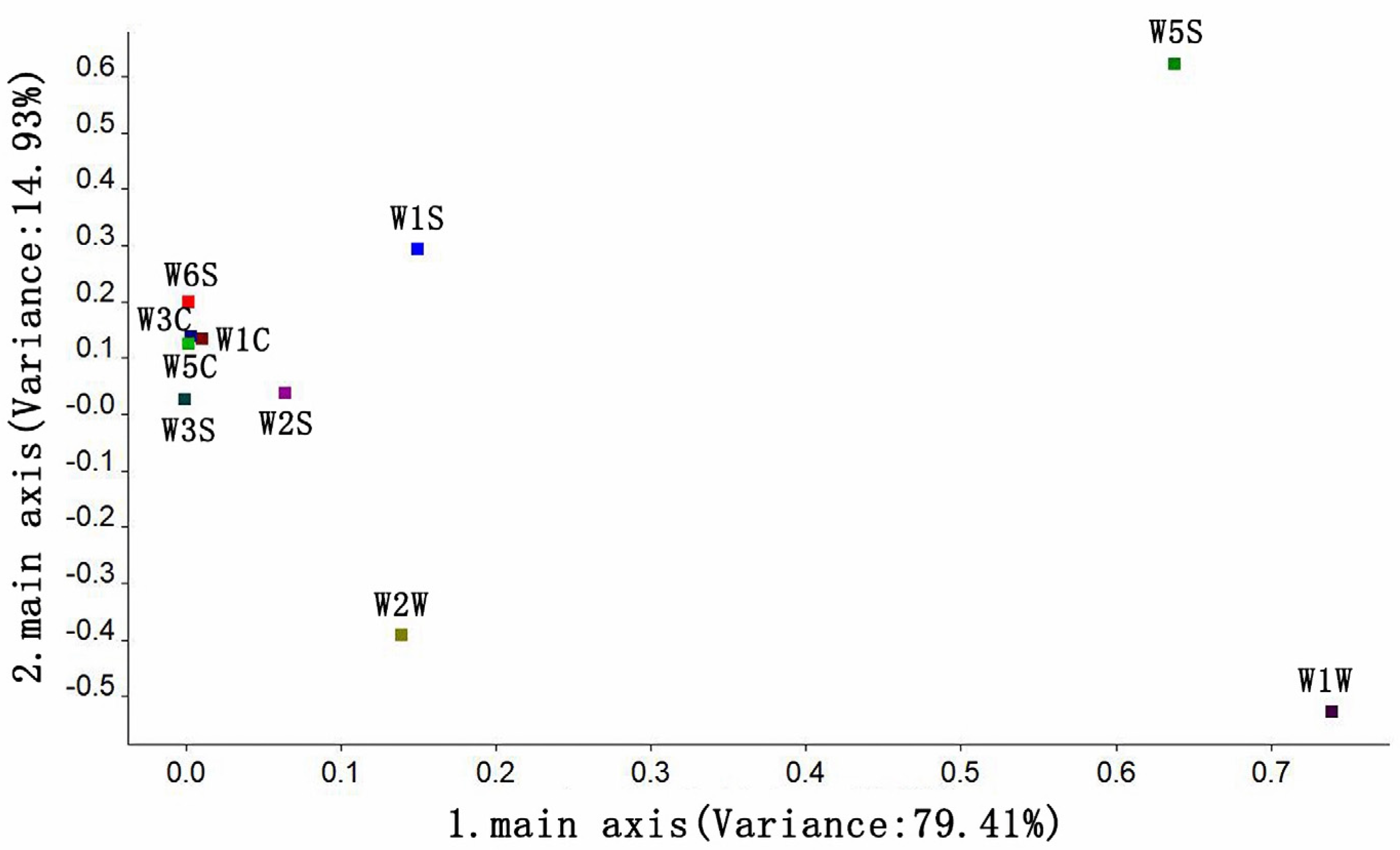
3.3.2. Principal Component Analysis of the Odor of Instant Black Tea Soup
3.3.3. Loading Analysis of the Odor of Instant Black Tea Soup
3.4. HS-SPME-GC-MS Detection and Analysis of the Volatile Components and Contents of Instant Black Tea
Analysis of Volatile Compounds Characteristics of Different Instant Teas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alasalvar, C.; Pelvan, E.; Özdemir, K.S.; Kocadağlı, T.; Mogol, B.A.; Paslı, A.A.; Özcan, N.; Özçelik, B.; Gökmen, V. Compositional, Nutritional, and Functional Characteristics of Instant Teas Produced from Low- and High-Quality Black Teas. J. Agric. Food Chem. 2013, 61, 7529–7536. [Google Scholar] [CrossRef] [PubMed]
- Kraujalytė, V.; Pelvan, E.; Alasalvar, C. Volatile Compounds and Sensory Characteristics of Various Instant Teas Produced from Black Tea. Food Chem. 2016, 194, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent Studies of the Volatile Compounds in Tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, S.; Jin, H.; Jiang, Y.; Xia, T. Effects of Additive β-Cyclodextrin on the Performances of Green Tea Infusion. J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Constable, A.; Varga, N.; Richoz, J.; Stadler, R.H. Antimutagenicity and Catechin Content of Soluble Instant Teas. Mutagenesis 1996, 11, 189–194. [Google Scholar] [CrossRef]
- Qin, Z.; Pang, X.; Chen, D.; Cheng, H.; Hu, X.; Wu, J. Evaluation of Chinese Tea by the Electronic Nose and Gas Chromatography–Mass Spectrometry: Correlation with Sensory Properties and Classification According to Grade Level. Food Res. Int. 2013, 53, 864–874. [Google Scholar] [CrossRef]
- Sinija, V.R.; Mishra, H.N. Fuzzy Analysis of Sensory Data for Quality Evaluation and Ranking of Instant Green Tea Powder and Granules. Food Bioprocess Technol. 2011, 4, 408–416. [Google Scholar] [CrossRef]
- Tong, W.; Yu, J.; Wu, Q.; Hu, L.; Tabys, D.; Wang, Y.; Wei, C.; Ling, T.; Ali Inayat, M.; Bennetzen, J.L. Black Tea Quality Is Highly Affected during Processing by Its Leaf Surface Microbiome. J. Agric. Food Chem. 2021, 69, 7115–7126. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.-X.; Wu, D.-T.; Zhao, Y.-X.; Li, H.; Li, H.-B.; Gan, R.-Y. Structural Characteristics of Crude Polysaccharides from 12 Selected Chinese Teas, and Their Antioxidant and Anti-Diabetic Activities. Antioxidants 2021, 10, 1562. [Google Scholar] [CrossRef]
- Ma, Q.; Qin, M.; Song, L.; Sun, H.; Zhang, H.; Wu, H.; Ren, Z.; Liu, H.; Duan, G.; Wang, Y.; et al. Molecular Link in Flavonoid and Amino Acid Biosynthesis Contributes to the Flavor of Changqing Tea in Different Seasons. Foods 2022, 11, 2289. [Google Scholar] [CrossRef]
- Perera, G.A.A.R.; Amarakoon, A.M.T.; Illeperuma, D.C.K.; Muthukumarana, P.K.P. Effects of Raw Material on the Chemical Composition, Organoleptic Properties, Antioxidant Activity, Physical Properties and the Yield of Instant Black Tea. LWT Food Sci. Technol. 2015, 63, 745–750. [Google Scholar] [CrossRef]
- Cao, M.; Cao, Z.; Tian, J.; Lv, W.; Wang, H. Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea. Foods 2024, 13, 821. [Google Scholar] [CrossRef]
- Shuai, D.; Guo, M.; Zhang, W. Summer and fall tea raw material processing of red tea eight process study. J. Zhejiang Agric. Sci. 2022, 63, 804–806. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of Volatile Profile from Ten Different Varieties of Chinese Jujubes by HS-SPME/GC–MS Coupled with E-Nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Wang, L.-F.; Lee, J.-Y.; Chung, J.-O.; Baik, J.-H.; So, S.; Park, S.-K. Discrimination of Teas with Different Degrees of Fermentation by SPME–GC Analysis of the Characteristic Volatile Flavour Compounds. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Zhao, Y.; Zhu, M.; Li, J.; Wang, K. A Meta-Analysis of Dynamic Changes of Key Aroma Compounds during Black Tea Processing. Food Biosci. 2024, 58, 103784. [Google Scholar] [CrossRef]
- Pelvan, E.; Özilgen, M. Assessment of Energy and Exergy Efficiencies and Renewability of Black Tea, Instant Tea and Ice Tea Production and Waste Valorization Processes. Sustain. Prod. Consum. 2017, 12, 59–77. [Google Scholar] [CrossRef]
- Ma, C.; Li, J.; Chen, W.; Wang, W.; Qi, D.; Pang, S.; Miao, A. Study of the Aroma Formation and Transformation during the Manufacturing Process of Oolong Tea by Solid-Phase Micro-Extraction and Gas Chromatography–Mass Spectrometry Combined with Chemometrics. Food Res. Int. 2018, 108, 413–422. [Google Scholar] [CrossRef]
- Parveen, A.; Qin, C.-Y.; Zhou, F.; Lai, G.; Long, P.; Zhu, M.; Ke, J.; Zhang, L. The Chemistry, Sensory Properties and Health Benefits of Aroma Compounds of Black Tea Produced by Camellia sinensis and Camellia assamica. Horticulturae 2023, 9, 1253. [Google Scholar] [CrossRef]
- Lv, H.-P.; Zhong, Q.-S.; Lin, Z.; Wang, L.; Tan, J.-F.; Guo, L. Aroma Characterisation of Pu-Erh Tea Using Headspace-Solid Phase Microextraction Combined with GC/MS and GC–Olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Ma, C.; Qu, Y.; Zhang, Y.; Qiu, B.; Wang, Y.; Chen, X. Determination of Nerolidol in Teas Using Headspace Solid Phase Microextraction–Gas Chromatography. Food Chem. 2014, 152, 285–290. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Chang, M.; He, W.; Lu, X.; Fei, S.; Lu, G. Optimization of Electronic Nose Sensor Array for Tea Aroma Detecting Based on Correlation Coefficient and Cluster Analysis. Chemosensors 2021, 9, 266. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC–Olfactometry, GC–FPD, and GC–MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, X. Mulberry leaf instant tea research. J. Compr. Util. Resour. China 2018, 36, 2. [Google Scholar] [CrossRef]
- Xia, T.; Shi, S.Q.; Wan, X.C. Impact of Ultrasonic-Assisted Extraction on the Chemical and Sensory Quality of Tea Infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- He, W.; Hu, X.; Zhao, L.; Liao, X.; Zhang, Y.; Zhang, M.; Wu, J. Evaluation of Chinese Tea by the Electronic Tongue: Correlation with Sensory Properties and Classification According to Geographical Origin and Grade Level. Food Res. Int. 2009, 42, 1462–1467. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Xu, K.; Tian, C.; Zhang, M.; Lu, L.; Zhu, C.; Lai, Z.; Guo, Y. A Comprehensive Investigation of Macro-Composition and Volatile Compounds in Spring-Picked and Autumn-Picked White Tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef]
- Bleibaum, R.N.; Stone, H.; Tan, T.; Labreche, S.; Saint-Martin, E.; Isz, S. Comparison of Sensory and Consumer Results with Electronic Nose and Tongue Sensors for Apple Juices. Food Qual. Prefer. 2002, 13, 409–422. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Wu, Q.-Y.; Wu, Y.; Deng, T.-T.; Chen, X.-H.; Xiao, S.-T.; Zhang, C.-X.; Sun, Y.; Zheng, S.-Z. The Dynamic Changes in Volatile Compounds During Wuyi Rock Tea (WRT) Processing: More than a Contribution to Aroma Quality. Horticulturae 2025, 11, 120. [Google Scholar] [CrossRef]
- Xiao, H.; Tian, Y.; Yang, H.; Zeng, Y.; Yang, Y.; Yuan, Z.; Zhou, H. Are There Any Differences in the Quality of High-Mountain Green Tea before and after the First New Leaves Unfold? A Comprehensive Study Based on E-Sensors, Whole Metabolomics and Sensory Evaluation. Food Chem. 2024, 457, 140119. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Zhang, C.; Zhang, M.; Guo, Y. Volatilomics and Macro-Composition Analyses of Primary Wuyi Rock Teas of Rougui and Shuixian Cultivars from Different Production Areas. Plants 2024, 13, 2206. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jiang, Q.-X.; Zhang, T.; Huang, G.-L.; Li, L.-J.; Chen, F. Characterization of the Aroma of an Instant White Tea Dried by Freeze Drying. Molecules 2020, 25, 3628. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Yin, H.; Deng, Y.; Jiang, Y.; Yuan, H.; Dong, C.; Li, J.; Hua, J.; Wang, J. Rapid Profiling of Volatile Compounds in Green Teas Using Micro-Chamber/Thermal Extractor Combined with Thermal Desorption Coupled to Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. LWT 2018, 96, 42–50. [Google Scholar] [CrossRef]
- Lai, J.; Tang, X.; Li, M.; Chen, H. Optimization of tea extraction technology and tea powder processing by response surface method. Food Sci. Technol. 2022, 47, 72–78. (In Chinese) [Google Scholar]
- Zeng, L.; Liu, Y.-Y.; Wang, F.; Jin, S.; Chen, L.-S.; Fu, Y.-Q.; Feng, Z.-H.; Yin, J.-F.; Sun, W.-J.; Yu, X.-M.; et al. Effect of Tea Green Leafhopper (Empoasca onukii Matsuda) Sucking on the Quality of Oriental Beauty. Food Front. 2024, 5, 1571–1582. [Google Scholar] [CrossRef]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterization of the Key Odorants in a High-Grade Chinese Green Tea Beverage (Camellia sinensis; Jingshan Cha) by Means of the Sensomics Approach and Elucidation of Odorant Changes in Tea Leaves Caused by the Tea Manufacturing Process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef]
- Mei, S.; Cao, Y.; Zhang, G.; Zhou, S.; Wang, Y.; Gong, S.; Chu, Q.; Chen, P. Construction of Sensory/Mass Spectrometry Feedback Platform for Seeking Aroma Contributors during the Aroma Enhancement of Congou Black Tea. Plants 2022, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Gulati, A.; Kiranbabu, G.; Acharya, R.; Kaul, V.; Singh, B. Characterization of Volatile Components of Kangra Orthodox Black Tea by Gas Chromatography-Mass Spectrometry. Food Chem. 2007, 105, 229–235. [Google Scholar] [CrossRef]
- Sheibani, E.; Duncan, S.E.; Kuhn, D.D.; Dietrich, A.M.; O’Keefe, S.F. SDE and SPME Analysis of Flavor Compounds in Jin Xuan Oolong Tea. J. Food Sci. 2016, 81, C348–C358. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, H.; Niu, Y.; Liu, Q.; Zhu, J.; Chen, H.; Ma, N. Characterization of Aroma Compositions in Different Chinese Congou Black Teas Using GC–MS and GC–O Combined with Partial Least Squares Regression. Flavour Fragr. J. 2017, 32, 265–276. [Google Scholar] [CrossRef]

| Evaluation Item | Scoring Criteria | Score Range |
|---|---|---|
| Odor (30 points) | Dull, impure odor Pure odor Sweet, floral, or fruity odor | 1~10 11~20 21~30 |
| Taste (30 points) | Bland, slightly astringent taste Fairly mellow, but lacking smoothness Mellow, sweet, refreshing, with lingering aftertaste | 1~10 11~20 21~30 |
| Liquid Color (20 points) | Dark red, slightly cloudy Red, fairly bright Brownish red or reddish brown, clear and bright | 1~6 7~13 14~20 |
| Solubility (10 points) | Needs stirring to dissolve, sediment at the bottom Instantly dissolves, no sediment | 1~5 6~10 |
| Appearance (10 points) | Solid granules, dull, not easily dispersible Loose flakes or powder, glossy, no clumps or impurities | 1~5 6~10 |
| Level | Factor | ||
|---|---|---|---|
| A Tea-To-Water Ratio | B Temperature | C β-Cyclodextrin (%) | |
| −1 | 1:15 | 45 | 6 |
| 0 | 1:20 | 55 | 8 |
| 1 | 1:25 | 60 | 10 |
| Test Group | A | B | C | Sensory Score Y (Points) |
|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 82.3 |
| 2 | 0 | −1 | 1 | 80.4 |
| 3 | −1 | −1 | 0 | 82.2 |
| 4 | 1 | −1 | 0 | 84.5 |
| 5 | −1 | 0 | −1 | 83.6 |
| 6 | 1 | 0 | −1 | 78.2 |
| 7 | 0 | 0 | 0 | 86.4 |
| 8 | −1 | 0 | 1 | 79.5 |
| 9 | 1 | 0 | 1 | 83.8 |
| 10 | 0 | 1 | −1 | 77.6 |
| 11 | −1 | 1 | 0 | 81.3 |
| 12 | 1 | 1 | 0 | 78.1 |
| 13 | 0 | 1 | 1 | 81.2 |
| 14 | 0 | 0 | 0 | 86.2 |
| 15 | 0 | 0 | 0 | 87.2 |
| 16 | 0 | 0 | 0 | 86.9 |
| 17 | 0 | 0 | 0 | 86.6 |
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 169.78 | 9 | 18.86 | 63.27 | <0.0001 | ** |
| A—tea–water ratio | 0.5000 | 1 | 0.5000 | 1.68 | 0.2364 | |
| B—temperature | 15.68 | 1 | 15.68 | 52.59 | 0.0002 | ** |
| C—β-cyclodextrin | 1.28 | 1 | 1.28 | 4.29 | 0.0770 | |
| AB | 7.56 | 1 | 7.56 | 25.37 | 0.0015 | ** |
| AC | 23.52 | 1 | 23.52 | 78.90 | <0.0001 | ** |
| BC | 7.56 | 1 | 7.56 | 25.37 | 0.0015 | ** |
| A2 | 18.88 | 1 | 18.88 | 63.32 | <0.0001 | ** |
| B2 | 38.34 | 1 | 38.34 | 128.59 | <0.0001 | ** |
| C2 | 44.95 | 1 | 44.95 | 150.78 | <0.0001 | ** |
| Residuals | 2.09 | 7 | 0.2981 | |||
| Lack of fit | 1.45 | 3 | 0.4850 | 3.07 | 0.1535 | Not significant |
| Pure error | 0.6320 | 4 | 0.1580 | |||
| Total | 171.87 | 16 | ||||
| R2 | 0.9879 | |||||
| Adjusted R2 | 0.9722 | |||||
| Predicted R2 | 0.8588 | |||||
| Adeq precision | 22.5893 |
| Sensor | Determination of the Substance |
|---|---|
| W1C | Aliphatic aromatic compounds, benzene series |
| W5S | Nitrogen oxides |
| W3C | Amines, aromatic substances |
| W6S | Hydrogenated compounds |
| W5C | Short-chain alkanes, aromatic substances |
| W1S | Alkanes, methane, methyl compounds |
| W1W | Sulfides, terpenes |
| W2S | Alcohols, aldehydes, ketones, aromatic compounds |
| W2W | Organic sulfides, aromatic substances |
| W3S | Long-chain alkanes |
| Number | Compounds | No.1 | No.2 | No.3 | No.4 | No.5 | No.6 | No.7 | No.8 | No.9 | No.10 | No.11 | No.12 | No.13 | No.14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||||||||
| 1 | Benzaldehyde | 210.0 | 319.3 | 254.4 | 370.7 | 440.7 | 310.6 | 138.4 | 541.7 | 569.2 | 659.9 | 885.7 | 646.9 | 289.6 | 591.0 |
| 2 | (E,E)-2,4-Heptadi-enal | 161.0 | 566.3 | 356.9 | 366.2 | 94.4 | 300.3 | 288.0 | 400.3 | 293.8 | 614.0 | 522.0 | 524.8 | 260.9 | 354.8 |
| 3 | Benzeneacetaldehyde | 165.7 | 926.7 | 815.5 | 1266.9 | 870.5 | 976.5 | 1065.1 | 1021.8 | 1310.0 | 1128.5 | 1307.7 | 1327.0 | 994.2 | 1586.7 |
| 4 | 2-Undecenal | 368.7 | 404.5 | 215.6 | 500.0 | 122.7 | 168.4 | 439.7 | 570.7 | 395.4 | 156.0 | 362.6 | 174.4 | 562.9 | 436.9 |
| 5 | Dodecanal | 16,462.8 | 437.5 | 346.4 | 540.7 | 403.0 | 419.6 | 413.7 | 387.4 | 474.0 | 517.8 | 513.7 | 438.1 | 359.4 | 474.2 |
| 6 | 2,4-Nonadienal, (E,E)- | 108.9 | 108.9 | 56.7 | 109.6 | 57.3 | 92.9 | 122.7 | 132.2 | 46.8 | 865.1 | 97.2 | 71.9 | 28.8 | 34.7 |
| 7 | Tetradecanal | 20,155.6 | 129.3 | 292.1 | 185.3 | 100.2 | 199.1 | 100.6 | 67.3 | 168.1 | 210.8 | 106.2 | 164.3 | - | 270.8 |
| 8 | Nonanal | 169.7 | 166.8 | 119.7 | 269.3 | 152.5 | 89.2 | 208.0 | 236.3 | 347.5 | 151.0 | 235.4 | 198.5 | 172.1 | 183.5 |
| 9 | Undecanal | 8291.3 | 66.2 | 48.1 | 84.5 | 51.5 | 26.3 | 71.2 | 64.9 | 89.4 | 65.9 | 74.0 | 77.8 | 65.3 | 91.2 |
| 10 | Decanal | 335.3 | 188.2 | 126.9 | 287.4 | 154.0 | 167.3 | 225.1 | 202.6 | 257.3 | 160.2 | 212.4 | 197.8 | 147.5 | 203.6 |
| 11 | 2-Hexenal | 43.8 | 51.2 | 31.1 | 74.3 | - | - | 46.7 | 51.7 | 50.9 | 35.2 | 46.1 | - | - | 182.0 |
| 12 | 2-Octenal, (E)- | 20.5 | 24.4 | 13.3 | 63.6 | 12.7 | 30.5 | 14.6 | 49.5 | 17.7 | 14.1 | 27.8 | - | - | - |
| 13 | 2-Nonenal, (E)- | 30.7 | 34.4 | 39.5 | 83.1 | 43.5 | 51.7 | 79.1 | 85.4 | 89.2 | 51.6 | 78.1 | 59.4 | 26.1 | 66.8 |
| 14 | Tridecanal | 79.3 | 98.3 | 76.9 | 97.4 | 192.1 | 139.1 | 182.6 | 175.9 | 85.0 | 152.5 | 153.9 | 95.4 | 147.5 | 183.4 |
| 15 | (E)-Tetradec-2-enal | 1372.1 | - | - | - | - | - | - | - | - | 9.3 | - | - | - | - |
| 16 | 13-Methyltetradecanal | 500.0 | 91.6 | 31.2 | - | - | - | - | 122.1 | 95.6 | - | 104.7 | - | 213.9 | - |
| 17 | (Z)-9-Hexadecenal | 1722.1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Alcohols | |||||||||||||||
| 18 | Benzyl alcohol | 578.1 | 566.3 | 491.2 | 842.3 | 264.3 | 553.0 | 777.5 | 465.1 | 436.0 | 435.9 | 871.8 | 795.6 | 605.0 | 1200.2 |
| 19 | Phenylethyl alcohol | 1476.7 | 1830.4 | 1543.9 | 2596.6 | 1793.1 | 2080.1 | 2529.9 | 2293.9 | 2749.9 | 2295.8 | 2587.0 | 2489.0 | 1990.2 | 3273.9 |
| 20 | Phytol | 2183.1 | 3038.8 | 3187.5 | 4075.1 | 4536.6 | 4097.1 | 4138.2 | 6252.1 | 4541.8 | 4339.0 | 4462.2 | 4039.7 | 4173.9 | 2710.9 |
| 21 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | 266.7 | 511.5 | 424.1 | 274.4 | 505.1 | 235.0 | 562.9 | 599.8 | 490.3 | 455.0 | 583.8 | 538.4 | 430.0 | 505.6 |
| 22 | Geraniol | 121.1 | 750.2 | 195.4 | 319.2 | 184.9 | 279.1 | 812.7 | 400.8 | 648.4 | 661.8 | 760.7 | 827.5 | 375.3 | 697.4 |
| 23 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-,(E)- | 2554.4 | 4941.5 | 4818.1 | 3931.5 | 4086.0 | 3507.0 | 5721.1 | 5611.4 | 4306.9 | 4277.0 | 4885.6 | 7281.7 | 3803.5 | 4641.2 |
| 24 | Linalool | 20.9 | 232.6 | 55.2 | 38.6 | 39.5 | 26.7 | 95.2 | 38.5 | 76.4 | 210.5 | 253.3 | 268.6 | 31.4 | 132.5 |
| 25 | 2-Decenal, (E)- | - | 424.2 | 118.3 | 193.6 | 128.5 | 251.5 | 348.2 | 395.3 | 280.7 | 135.2 | 190.6 | 170.6 | 198.6 | 212.4 |
| Esters | |||||||||||||||
| 26 | Propyl decanoate | 2177.5 | 544.2 | 713.6 | 634.9 | 718.0 | 489.9 | - | - | - | 522.4 | 479.6 | 506.3 | 829.3 | 504.2 |
| 27 | Undecanoic acid, ethyl ester | 1872.9 | 2846.7 | 2634.8 | 3697.6 | 2713.5 | 2776.2 | 2390.2 | 2848.5 | 2816.9 | 3577.6 | 3323.6 | 3736.8 | 3006.4 | 3257.6 |
| 28 | Dodecanoic acid, ethyl ester | 875.7 | 1398.6 | 1276.3 | 1610.3 | 1369.2 | 1308.4 | 1233.4 | 1440.2 | 1409.2 | 1743.4 | 1543.4 | 1702.5 | 1433.3 | 1399.1 |
| 29 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 64.02 | 44.6 | - | 42.67 | 130.54 | 19.48 | 74.82 | 51.71 | 67.43 | 59.58 | 35.42 | 94.52 | 28.0 | 56.0 |
| 30 | Hexadecanoic acid, methyl ester | 1986.4 | 356.7 | 358.4 | 409.5 | 398.2 | 622.3 | 493.2 | 498.8 | 383.4 | 425.6 | 418.6 | 428.9 | 461.1 | 311.3 |
| 31 | 1,4-Dibutyl benzene-1,4-dicarboxylate | - | - | 270.8 | 452.7 | 414.6 | 534.9 | 526.4 | 561.6 | 2714.1 | 326.9 | 427.8 | 425.3 | 340.6 | 422.4 |
| 32 | Isopropyl palmitate | 1111.3 | 115.7 | - | 186.3 | 41.8 | 135.4 | - | - | 290.0 | 75.4 | 69.0 | - | 100.1 | 33.2 |
| 33 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 199.04 | 469.79 | 310.8 | - | 453.91 | 547.68 | 524.08 | 538.17 | 335.11 | 194.9 | 188.11 | 267.17 | 193.24 | 386.47 |
| 34 | Pentanedioic acid, dimethyl ester | 98.0 | 127.9 | 148.0 | 70.8 | 98.2 | 100.4 | 131.7 | 271.7 | 205.6 | 199.3 | 267.5 | 75.8 | 140.4 | 246.5 |
| 35 | Bis(2-ethylhexyl) phthalate | 19.92 | 37.43 | - | - | 26.27 | 55.2 | 88.89 | 114.01 | 64.89 | 33.74 | 23.5 | 18.27 | 35.62 | 71.23 |
| 36 | Hexanedioic acid, dimethyl ester | 136.6 | 207.7 | 83.0 | 108.5 | 194.9 | 180.1 | 243.8 | 369.2 | 260.9 | 241.0 | 286.7 | 103.7 | 184.0 | 304.0 |
| 37 | .gamma.-Tetradecalactone | 4593.1 | - | - | - | - | 924.2 | - | 1749.3 | 439.0 | - | - | - | - | - |
| 38 | .beta.-Phenylethyl butyrate | - | 245.7 | 42.0 | 87.0 | - | 370.4 | - | - | - | - | - | - | - | - |
| 39 | Nerolidyl acetate | 961.3 | - | - | - | - | - | - | - | - | 5165.7 | 4420.5 | - | - | - |
| Alkanes | |||||||||||||||
| 40 | Tetradecane | 1379.8 | 1157.9 | 1532.0 | 1091.8 | 2027.0 | 1191.5 | 1123.9 | 1103.1 | 1112.6 | 1034.3 | 991.3 | 1171.4 | 1078.6 | 1067.9 |
| 41 | Pentadecane | 815.7 | 717.2 | 554.0 | 852.6 | 711.7 | 961.8 | 730.9 | 842.2 | 1172.1 | 992.4 | 847.5 | 988.8 | 878.6 | 1049.2 |
| 42 | Eicosane | 184.7 | 287.9 | 243.9 | 392.0 | 305.6 | 454.4 | 346.1 | 413.5 | 432.2 | 363.3 | 409.9 | 395.0 | 328.3 | 447.2 |
| 43 | Heptadecane | 146.84 | 93.44 | - | 96.55 | 206.41 | 111.93 | 95.19 | 116.87 | 89.2 | 98.23 | 95.19 | 196.87 | 229.27 | 185.43 |
| 44 | Nonadecane | 103.51 | 103.51 | - | 93.99 | 315.32 | 216.4 | 280.11 | 172.86 | 246.28 | 213.88 | 196.96 | 216.4 | 213.88 | 196.96 |
| 45 | Heptadecane, 3-methyl- | - | - | - | - | 188.27 | 75.51 | 139.22 | 63.27 | 61.47 | 139.22 | 75.51 | 139.22 | 75.51 | - |
| 46 | Hexadecane | - | - | - | 96.39 | 83.91 | - | - | - | - | - | - | - | - | - |
| 47 | Pentadecane, 2,6,10,14-tetramethyl- | - | - | - | - | - | 101.27 | 107.47 | 140.61 | 135.47 | 144.26 | 102.33 | 101.27 | 101.27 | - |
| 48 | 2-Methyltetracosane | 427.3 | 141.2 | 132.6 | 120.3 | 109.8 | 185.1 | 155.8 | 116.6 | 173.8 | 144.8 | 184.4 | 187.0 | 190.6 | 196.3 |
| 49 | 11-Methyltricosane | 804.3 | - | - | - | - | - | - | 23.6 | 112.3 | 107.2 | 114.0 | 219.4 | 97.6 | 98.1 |
| 50 | 2,6,10-Trimethyltridecane | - | - | - | 100.55 | 108.11 | 100.07 | 108.65 | 100.07 | 108.65 | 108.65 | 108.65 | 108.65 | 81.71 | - |
| 51 | 2-Methylhexacosane | 404.8 | 141.2 | 53.7 | 66.7 | 109.8 | 267.5 | 155.8 | 251.8 | 197.4 | 144.8 | 187.8 | 136.6 | 190.6 | - |
| 52 | .beta.-Phenylethyl butyrate | - | 245.7 | 42.0 | 87.0 | - | 370.4 | - | - | - | - | - | - | - | - |
| Ketones | |||||||||||||||
| 53 | γ-Dodecalactone | 267.5 | - | - | 650.0 | - | 577.6 | - | 717.4 | 663.4 | 530.0 | 339.5 | 318.3 | 327.6 | 308.6 |
| 54 | 2-Pentadecanone, 6,10,14-trimethyl- | 2885.1 | 1533.7 | 444.1 | 1389.2 | 1051.6 | 3595.6 | 4870.5 | 2462.2 | 1017.7 | 490.0 | 520.5 | 893.4 | 879.9 | 597.2 |
| 55 | .alpha.-Ionone | 110.9 | 125.7 | 125.4 | 154.8 | - | 113.0 | - | - | 201.2 | 304.8 | 315.9 | 316.9 | - | 340.6 |
| 56 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- | - | 201.6 | 103.8 | - | 460.8 | 238.5 | 609.0 | 578.6 | 509.2 | 472.0 | 489.2 | 518.4 | 345.8 | 568.3 |
| Acids | |||||||||||||||
| 57 | n-Decanoic acid | - | - | 1166.8 | 1754.6 | 1144.6 | 1451.8 | 1492.5 | 1781.1 | 1506.0 | 873.1 | 1298.5 | 616.6 | 863.1 | 1540.9 |
| 58 | Pentadecanoic acid | - | - | - | - | - | 237.24 | - | - | - | - | - | 181.97 | - | - |
| 59 | Eicosanoic acid | 6035.7 | 75.7 | 71.2 | 64.1 | 59.8 | - | - | - | - | - | 68.2 | 63.4 | 55.3 | 69.9 |
| 60 | Nonanoic acid | 72.0 | 52.1 | - | 63.4 | - | 51.9 | - | - | 151.6 | - | 47.8 | 40.4 | 105.7 | 59.8 |
| Phenols | |||||||||||||||
| 61 | 2,4-Di-tert-butylphenol | 757.0 | 1250.3 | 303.9 | 1488.9 | 1466.6 | 1225.5 | 1561.0 | 1561.2 | 1496.8 | 1514.7 | 1570.9 | 1402.1 | 1285.4 | 2167.8 |
| 62 | Phenol, 2,4,6-tri-tert-butyl- | 88.6 | 59.1 | 86.7 | 81.5 | 172.8 | 173.7 | 231.3 | 212.2 | 146.2 | 165.8 | 179.4 | 213.9 | 125.9 | 235.4 |
| Other categories | |||||||||||||||
| 63 | Indole | 1088.3 | 581.5 | 555.1 | 806.7 | 637.4 | 748.9 | 907.4 | 880.4 | 1086.6 | 791.4 | 985.8 | 873.5 | 691.0 | 957.3 |
| 64 | Caffeine | 701.8 | - | 512.4 | 613.8 | 993.5 | 1227.0 | 1385.1 | 1994.1 | 2442.4 | 865.4 | 1202.6 | 1562.0 | 988.1 | 1140.2 |
| 65 | Neophytadiene | 1664.1 | 1118.4 | 1386.4 | 1871.2 | 1868.7 | 1842.6 | 1786.2 | 2056.8 | 1725.1 | 1740.0 | 2020.0 | 1596.0 | 1468.3 | 1181.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Liao, H.; Liao, H.; Song, X.; Ma, C.; Huang, Y. Process Optimization and Odor Analysis of Instant Black Tea Powder. Foods 2025, 14, 1552. https://doi.org/10.3390/foods14091552
Xiong Y, Liao H, Liao H, Song X, Ma C, Huang Y. Process Optimization and Odor Analysis of Instant Black Tea Powder. Foods. 2025; 14(9):1552. https://doi.org/10.3390/foods14091552
Chicago/Turabian StyleXiong, Yuqin, Haomu Liao, Haiyue Liao, Xiaoyue Song, Chunhua Ma, and Yan Huang. 2025. "Process Optimization and Odor Analysis of Instant Black Tea Powder" Foods 14, no. 9: 1552. https://doi.org/10.3390/foods14091552
APA StyleXiong, Y., Liao, H., Liao, H., Song, X., Ma, C., & Huang, Y. (2025). Process Optimization and Odor Analysis of Instant Black Tea Powder. Foods, 14(9), 1552. https://doi.org/10.3390/foods14091552






