Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage
Abstract
1. Introduction
History of Kombucha
2. Microbiology
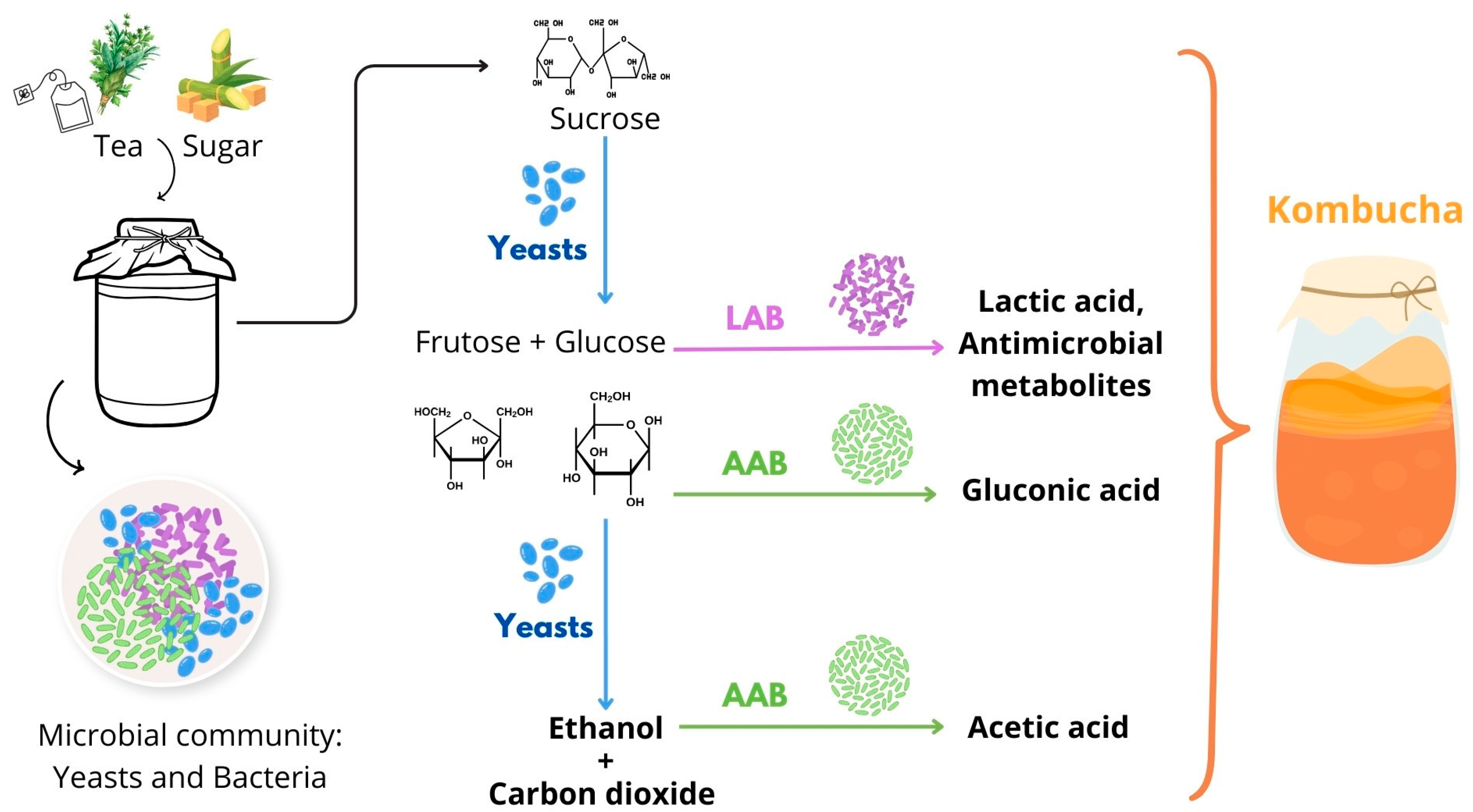
2.1. Fermentation Process and Metabolic Pathways
2.2. Main Microorganisms Present in Kombucha
2.2.1. Yeast
2.2.2. Acid Acetic Bacteria (AAB)
2.2.3. Lactic Acid Bacteria (LAB)
2.3. Microbial Interactions in Kombucha
3. Health Benefits
4. Safety
5. Health Benefits Versus Risks
6. Clinical Studies
7. Other Benefits
8. Kombucha, a Potential Health-Promoting Beverage (But Not Probiotic)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Waisundara, V.Y. Kombucha as a Functional Beverage. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–446. [Google Scholar] [CrossRef]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, Chemical and Antioxidant Activities of Different Types Kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and Microbiological Impacts on the Chemical and Flavor Profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- Haghmorad, D.; Yazdanpanah, E.; Sadighimoghaddam, B.; Yousefi, B.; Sahafi, P.; Ghorbani, N.; Rashidy-Pour, A.; Kokhaei, P. Kombucha Ameliorates Experimental Autoimmune Encephalomyelitis through Activation of Treg and Th2 Cells. Acta Neurol. Belg. 2020, 121, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Smolinske, S.; Greenbaum, D. Probable Gastrointestinal Toxicity of Kombucha Tea: Is This Beverage Healthy or Harmful? J. Gen. Intern. Med. 1997, 12, 643–644. [Google Scholar] [CrossRef]
- Prajapati, K.; Prajapati, J.; Patel, D.; Patel, R.; Varshnei, A.; Saraf, M.; Goswami, D. Multidisciplinary Advances in Kombucha Fermentation, Health Efficacy, and Market Evolution. Arch. Microbiol. 2024, 206, 366. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Isolation and Characterisation of Dominant AAB and Yeast Isolated from Kombucha Samples at Point of Sale in New Zealand. Curr. Res. Food Sci. 2022, 11, 3456. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods 2023, 12, 1818. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X.; Li, Y.; Chen, J.; Chen, X. Microbial Interactions and Dynamic Changes of Volatile Flavor Compounds During the Fermentation of Traditional Kombucha. Food Chem. 2024, 430, 137060. [Google Scholar] [CrossRef]
- Kaashyap, M.; Cohen, M.; Mantri, N. Microbial Diversity and Characteristics of Kombucha as Revealed by Metagenomic and Physicochemical Analysis. Nutrients 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Marechal, R. Microbial Dynamics between Yeasts and AAB in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial Diversity and Interaction Specificity in Kombucha Tea Fermentations. mSystems 2022, 7, e0015722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Todorov, S.D.; Baretto Penna, A.L.; Venema, K.; Holzapfel, W.H.; Chikindas, M.L. Recommendations for the use of Standardized Abbreviations for the Former Lactobacillus Genera, Reclassified in the Year 2020. Benef. Microbes 2023, 15, 1–4. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Liu, N.; Zhang, X.-X.; Mutukumira, A.N. Evaluation of the Probiotic Potential of Yeast Isolated from Kombucha in New Zealand. Curr. Res. Food Sci. 2024, 8, 100711. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Liu, N.; Zhang, X.-X.; Mutukumira, A.N. Probiotic Potential of AAB Isolated from Kombucha in New Zealand In Vitro. Microbe 2024, 4, 100130. [Google Scholar] [CrossRef]
- Bogdan, M.; Justine, S.; Filofteia, D.C.; Petruta, C.C.; Gabriela, L.U.Ț.Ă.; Roxana, U.E.; Florentina, M.; Camelia Filofteia, D.; Călina Petruța, C.; Gabriela, L. Lactic Acid Bacteria Strains Isolated from Kombucha with Potential Probiotic Effect. Rom. Biotechnol. Lett. 2018, 23, 13592–13598. [Google Scholar]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnostics, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.K.; Fabricio, M.F.; Ayub, M.A.Z. Health Effects and Probiotic and Prebiotic Potential of Kombucha: A Bibliometric and Systematic Review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Almeida, B.M.; Lima, E.M.F.; Fabi, J.P.; Lajolo, F.M.; Hassimotto, N.M.A. Phenolic Compounds and Bacteriocins: Mechanisms, Interactions, and Applications in Food Preservation and Safety. Mol. Nutr. Food Res. 2025, 69, e202400723. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha Tea Fermentation: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Marechal, R. Microbiological and Technological Parameters Impacting the Chemical Composition and Sensory Quality of Kombucha. Compreh. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Gupte, Y.; Kulkarni, A.; Raut, B.; Sarkar, P.; Choudhury, R.; Chawande, A.; Kumar, G.R.K.; Bhadra, B.; Satapathy, A.; Das, G.; et al. Characterization of Nanocellulose Production by Strains of Komagataeibacter sp. Isolated from Organic Waste and Kombucha. Carbohydr. Polym. 2021, 266, 118176. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Winckler, P.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Shedding Light on the Formation and Structure of Kombucha Biofilm Using Two-Photon Fluorescence Microscopy. Front. Microbiol. 2021, 12, 725379. [Google Scholar] [CrossRef]
- Doğan, N. Native Bacterial Cellulose Films Based on Kombucha Pellicle as a Potential Active Food Packaging. J. Food Sci. Technol. 2023, 60, 2893–2904. [Google Scholar] [CrossRef]
- Han, D.; Yang, Y.; Guo, Z.; Dai, S.; Jiang, M.; Zhu, Y.; Wang, Y.; Yu, Z.; Wang, K.; Rong, C.; et al. A Review on the Interaction of AAB and Microbes in Food Fermentation: A Microbial Ecology Perspective. Foods 2024, 13, 2534. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Kombucha: A Systematic Review of the Clinical Evidence. Forsch. Komplementarmed. Klass. Naturheilkd. 2003, 10, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A Systematic Review of the Empirical Evidence of Human Health Benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Microbial Composition, Bioactive Compounds, Potential Benefits and Risks Associated with Kombucha: A Concise Review. Fermentation 2023, 9, 472. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E. Chapter 12—Kombucha as a Therapeutic Soft Drink Targeting Gut Health. In Kombucha—Technology, Traceability, and Health-Promoting Effects; Academic Press: Cambridge, MA, USA, 2025; pp. 223–238. [Google Scholar] [CrossRef]
- Teixeira Oliveira, J.; Machado da Costa, F.; Gonçalvez da Silva, T.; Dotto Simões, G.; dos Santos Pereira, E.; Quevedo da Costa, P.; Andreazza, R.; Cavalheiro Schenkel, P.; Pieniz, S. Green Tea and Kombucha Characterization: Phenolic Composition, Antioxidant Capacity and Enzymatic Inhibition Potential. Food Chem. 2023, 408, 135206. [Google Scholar] [CrossRef]
- Vastrad, J.V.; Badanayak, P.; Goudar, G. Phenolic Compounds in Tea: Phytochemical, Biological, and Therapeutic Applications. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive Compounds in Kombucha: A Review of Substrate Effect and Fermentation Conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Pastore, R.L.; Fratellone, P. Potential Health Benefits of Green Tea (Camellia sinensis): A Narrative Review. Explore 2006, 2, 531–539. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The Chemistry and Biotransformation of Tea Constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT 2022, 168, 113931. [Google Scholar] [CrossRef]
- Martinez-Leal, J.; Ponce-Garcia, N.; Escalante-Aburto, A. Recent Evidence of the Beneficial Effects Associated with Glucuronic Acid Contained in Kombucha Beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wei, Y.; Lin, X.; Liang, H.; Zhang, S.; Chen, Y.; Dong, L.; Ji, C. Microbial Metabolic Transformation and Antioxidant Activity Evaluation of Polyphenols in Kombucha. Food Biosci. 2023, 51, 102287. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Yang, T.; Mac Regenstein, J.; Zhou, P. Functional Properties and Sensory Characteristics of Kombucha Analogs Prepared with Alternative Materials. Trends Food Sci. Technol. 2022, 129, 608–616. [Google Scholar] [CrossRef]
- Chandrakala, S.K.; Lobo, R.O.; Dias, F.O. Kombucha (Bio-Tea): An Elixir for Life? Nutr. Bever. 2019, 12, 591–616. [Google Scholar] [CrossRef]
- Dias, F.O.; Shenoy, C.K. Protective Effect of Kombucha on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. Int. J. Sci. Res. 2016, 5, 945–948. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ghrab, F.; Makni-Ayadi, F.; Pelt, J.V.; Elfeki, A.; Ammar, E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm. Biol. 2015, 53, 1699–1709. [Google Scholar] [CrossRef]
- Gharib, O.A. Effects of Kombucha on oxidative stress induced nephrotoxicity in rats. Chin. Med. 2009, 4, 23. [Google Scholar] [CrossRef]
- Nyiew, K.Y.; Kwong, P.J.; Yow, Y.Y. An Overview of Antimicrobial Properties of Kombucha. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1024–1053. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.-R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Abou Zeid, A.; Enan, G. Chemical Constitution and Antimicrobial Activity of Kombucha Fermented Beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Ferreira, T.V.; Castro, J.C.; da Silva, C.A.J.; Pilau, E.J.; Machinski Junior, M.; Abreu Filho, B.A.d. Evaluation of Antimicrobial Activity of Green Tea Kombucha at Two Fermentation Time Points Against Alicyclobacillus spp. LWT 2020, 130, 109641. [Google Scholar] [CrossRef]
- Selvaraj, S.; Gurumurthy, K. An Overview of Probiotic Health Booster-Kombucha Tea. Chin. Herb. Med. 2023, 15, 27–32. [Google Scholar] [CrossRef]
- Batista, P.; Penas, M.R.; Pintado, M.; Oliveira-Silva, P. Kombucha: Perceptions and Future Prospects. Foods 2022, 11, 1977. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Nummer, B.A. Kombucha Brewing Under the Food and Drug Administration Model Food Code: Risk Analysis and Processing Guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar] [PubMed]
- Kole, A.S.; Jones, H.D.; Christensen, R.; Gladstein, J. A Case of Kombucha Tea Toxicity. J. Intensive Care Med. 2009, 24, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- de Oliveira Duarte, F.A.; Ramos, K.K.; Gini, C.; Morasi, R.M.; Silva, N.C.C.; Efraim, P. Microbiological Characterization of Kombucha and Biocellulose Film Produced with Black Tea and Cocoa Bean Shell Infusion. Food Res. Int. 2024, 190, 114568. [Google Scholar] [CrossRef]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-Lactate: Implications for Gastrointestinal Diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef]
- Maccari, C.; Kamel, K.S.; Davids, M.R.; Halperin, M.L. The patient with a severe degree of metabolic acidosis: A deductive analysis. QJM Int. J. Med. 2006, 99, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Armanpour, V.; Ghorbani, M.; Tabibiazar, M.; Soofi, M.; Roufegarinejad, L. Determination of Phenolic Composition, Antioxidant Activity, and Cytotoxicity Characteristics of Kombucha Beverage Containing Echium amoenum. J. Food Meas. Charact. 2023, 17, 3162–3172. [Google Scholar] [CrossRef]
- Murphy, T.E.; Walia, K.; Farber, J.M. Safety Aspects and Guidance for Consumers on the Safe Preparation, Handling, and Storage of Kombucha—A Fermented Tea Beverage. Food Prot. Trends 2018, 38, 329–337. [Google Scholar]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of Substrates, Regulations, Composition, and Biological Properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Gonçalves, T.; Martins, Z.E.; Bäuerl, C.; Cortés-Macías, E.; Collado, M.C.; Ferreira, I.M.P.L.V.O. Mycotoxin Interactions along the Gastrointestinal Tract: In Vitro Semi-Dynamic Digestion and Static Colonic Fermentation of a Contaminated Meal. Toxins 2022, 14, 28. [Google Scholar] [CrossRef]
- Solís-Cruz, B.; Hernández-Patlán, D.; Beyssac, E.; Latorre, J.; Hernandez-Velasco, X.; Merino-Guzman, R.; Tellez, G.; López-Arellano, R. Evaluation of Chitosan and Cellulosic Polymers as Binding Adsorbent Materials to Prevent Aflatoxin B1, Fumonisin B1, Ochratoxin, Trichothecene, Deoxynivalenol, and Zearalenone Mycotoxicoses Through an In vitro Gastrointestinal Model for Poultry. Polymers 2017, 9, 529. [Google Scholar] [CrossRef]
- Zavala-Franco, A.; Hernández-Patlán, D.; Solís-Cruz, B.; López-Arellano, R.; Tellez-Isaias, G.; Vázquez-Durán, A.; Méndez-Albores, A. Assessing the Aflatoxin B1 Adsorption Capacity between Biosorbents Using an In vitro Multicompartmental Model Simulating the Dynamic Conditions in the Gastrointestinal Tract of Poultry. Toxins 2018, 10, 484. [Google Scholar] [CrossRef]
- Tso, K.-H.; Ju, J.-C.; Fan, Y.-K.; Chiang, H.-I. Enzyme Degradation Reagents Effectively Remove Mycotoxins Deoxynivalenol and Zearalenone from Pig and Poultry Artificial Digestive Juices. Toxins 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Mahbub, N.U.; Islam, M.A. Gut Microorganism-Mediated Neutralization of Mycotoxins: A Promising Approach to Combat Fungal Toxicity. Adv. Gut Microb. Res. 2024, 2024, 8448547. [Google Scholar] [CrossRef]
- Abraham, N.; Chan, E.T.S.; Zhou, T.; Seah, S.Y.K. Microbial detoxification of mycotoxins in food. Front. Microbiol. 2022, 13, 957148. [Google Scholar] [CrossRef] [PubMed]
- Gari, J.; Abdella, R. Degradation of zearalenone by microorganisms and enzymes. PeerJ 2023, 11, e15808. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; McIntyre, L.; Chan, M.; Brown, P.N.; Finley, J.; Chen, S.X. Ethanol Concentration of Kombucha Teas in British Columbia, Canada. J. Food Prot. 2021, 84, 1878–1883. [Google Scholar] [CrossRef]
- FSANZ. Labelling of Alcoholic Beverages User Guide; November 2014. Available online: https://www.foodstandards.gov.au/consumer/labelling/Labelling-of-alcoholic-beverages (accessed on 24 April 2025).
- Chan, M.; Sy, H.; Finley, J.; Robertson, J.; Brown, P.N. Determination of Ethanol Content in Kombucha Using Headspace Gas Chromatography with Mass Spectrometry Detection: Single-Laboratory Validation. J. AOAC Int. 2021, 104, 122–128. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological Aspects of Kombucha, Its Applications and the Symbiotic Culture (SCOBY), and Extraction of Compounds of Interest: A Literature Review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Batista, P.; Rodrigues Penas, M.; Vila-Real, C.; Pintado, M.; Oliveira-Silva, P. Kombucha: Challenges for Health and Mental Health. Foods 2023, 12, 3378. [Google Scholar] [CrossRef]
- Liu, M.; Yang, S.; Ye, Z.; Zhang, Y.; Zhang, Y.; He, P.; Zhou, C.; Hou, F.F.; Qin, X. Tea consumption and new-onset acute kidney injury: The effects of milk or sweetners addition and caffeine/coffee. Nutrients 2023, 15, 2201. [Google Scholar] [CrossRef]
- Sannapaneni, S.; Philip, S.; Desai, A.; Mitchell, J.; Feldman, M. Kombucha-induced massive hepatic necrosis: A case report and a review of literature. Gastro Hep. Adv. 2023, 2, 196–198. [Google Scholar] [CrossRef]
- Morales, D. biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Cardenas, D. Let not thy food be Confused with thy Medicince: The Hippocatic misquotation. e-SPEN J. 2013, 8, e260–e262. [Google Scholar] [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha Tea as an Anti-Hyperglycemic Agent in Humans with Diabetes—A Randomized Controlled Pilot Investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef] [PubMed]
- Abuduaibifu, A.; Tamer, C.E. Evaluation of Physicochemical and Bioaccessibility Properties of Goji Berry Kombucha. J. Food Process. Preserv. 2019, 43, e14077. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Miller, R.; Maseng, M.G.; Hawes, V.; Hinz, D.; Kim, C.; Gilbert, J.A. Modulating the Human Gut Microbiome and Health Markers Through Kombucha Consumption: A Controlled Clinical Study. Sci. Rep. 2024, 14, 31647. [Google Scholar] [CrossRef]
- Diepvens, K.; Kovacs, E.M.R.; Vogels, N.; Westerterp-Plantenga, M.S. Metabolic Effects of Green Tea and of Phases of Weight Loss. Physiol. Behav. 2006, 87, 185–191. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Burleson, L.E.; Holmes, A.K.; Geist, C.R. Effects of Chronic Kombucha Ingestion on Open-Field Behaviors, Longevity, Appetitive Behaviors, and Organs in C57-BL/6 Mice: A Pilot Study. Nutrition 2000, 16, 755–761. [Google Scholar] [CrossRef]
- Fraiz, G.M.; Bonifácio, D.B.; Lacerda, U.V.; Cardoso, R.R.; Corich, V.; Giacomini, A.; Martino, H.S.D.; Echeverría, S.E.; Barros, F.A.R.; Milagro, F.I.; et al. Green Tea Kombucha Impacts Inflammation and Salivary Microbiota in Individuals with Excess Body Weight: A Randomized Controlled Trial. Nutrients 2024, 16, 3186. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.C.; Vilela, D.L.S.; Fraiz, G.M.; Lopes, I.L.; Coelho, A.I.M.; Castro, L.C.V.; Martin, J.G.P. Effect of Kombucha Intake on the Gut Microbiota and Obesity-Related Comorbidities: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3851–3866. [Google Scholar] [CrossRef]
- Diguta, C.F.; Nitoi, G.D.; Matei, F.; Luta, G.; Cornea, C.P. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods 2020, 9, 1780. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Kangari, A.; Homayouni, A. Evaluation of the Glucuronic Acid Production and Antibacterial Properties of Kombucha Black Tea. Curr. Pharm. Biotechnol. 2019, 20, 985–990. [Google Scholar] [CrossRef]
- Choi, G.H.; Holzapfel, W.H.; Todorov, S.D. Diversity of the Bacteriocins, Their Classification and Potential Applications in Combat of Antibiotic Resistant and Clinically Relevant Pathogens. Crit. Rev. Microbiol. 2023, 49, 578–597. [Google Scholar] [CrossRef]
- Mihai, R.A.; Cubi-Insuaste, N.S.; Catana, R.D. Biological Activity and Phenolic Content of Kombucha Beverages under the Influence of Different Tea Extract Substrates. Fermentation 2024, 10, 338. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaśa, R.V.; Sathishkumar, M. Kombucha Tea: Metabolites. In Fungal Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 965–978. [Google Scholar] [CrossRef]
- Jafari, R.; Naghavi, N.S.; Khosravi-Darani, K.; Doudi, M.; Shahanipour, K. Kombucha Microbial Starter with Enhanced Production of Antioxidant Compounds and Invertase. Biocatal. Agric. Biotechnol. 2020, 29, 101789. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent Progress in Chemical Composition, Production, and Pharmaceutical Effects of Kombucha Beverage: A Complementary and Alternative Medicine. Evid. Based Complement. Alternat. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef] [PubMed]
- de Noronha, M.C.; Cardoso, R.R.; dos Santos D’Almeida, C.T.; Vieira do Carmo, M.A.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black Tea Kombucha: Physicochemical, Microbiological and Comprehensive Phenolic Profile Changes during Fermentation, and Antimalarial Activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and Water Soluble Vitamin Content in the Kombucha Drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan Tea-Based Kombucha: Physicochemical, Sensorial, and Antioxidant Profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kaldunska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M.Y. Exploring Biotechnological and Functional Characteristics of Probiotic Yeasts: A Review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.H.; Babji, A.S.; Lim, S.J.; Sarbini, S.R. A systematic review of edible swiftlet’s nest (ESN): Nutritional bioactive compounds, health benefits as functional food, and recent development as bioactive ESN glycopeptide hydrolysate. Trends Food Sci. Technol. 2021, 115, 117–132. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating what they are and what they are not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canini, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
| Microbial Group | Genera | Species | Benefits |
|---|---|---|---|
| Yeast | Zygosaccharomyces | Zygosaccharomyces bisporus | Tolerant to high sugar and acidic environments, making it well-suited for kombucha fermentation with contribution in carbohydrate metabolism and production of flavor compounds, CO2, and alcohol |
| Dekkera | |||
| Starmerella | |||
| Galiella | |||
| Hanseniaspora | |||
| Microidium | |||
| Brettanomyces | Brettanomyces bruxellensis | ||
| Candida | Candida stellata | ||
| Candida krusei | |||
| Acetic Acid Bacteria | Acetobacter | Acetobacter aceti | With the ability to produce acetic acid and bacterial cellulose, AAB contributes to the formation of the characteristic biofilm/cellulosic pellicle named SCOBY. The produced acetic acid reduces pH and contributes to safety. AAB are involved in the production of gluconic acid and the oxidation of glucose to gluconic acid, impacting acidity and flavor profile of kombucha. AAB are responsible for the conversion of ethanol into acetic acid and reducing alcohol levels in final product. |
| Gluconobacter | Gluconobacter oxydans | ||
| Swingsia | |||
| Komagataeibacter | Komagataeibacter rhaeticus | ||
| Asaia | |||
| Lactic Acid Bacteria | Lactiplantibacillus | Lactiplantibacillus plantarum | LAB contributes to production of lactic acid, which causes the tangy flavor, reduction in pH, and production of different metabolites with contribution to the flavors and varieties of antimicrobials, including lactic acid, diacetyl, low molecular metabolites, bacteriocins, carbon dioxide, and hydrogen peroxide. Some LAB can have potential health-promoting benefits. |
| Lacticaseibacillus | Lacticaseibacillus casei |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, D.K.A.; Wang, B.; Lima, E.M.F.; Shebeko, S.K.; Ermakov, A.M.; Khramova, V.N.; Ivanova, I.V.; Rocha, R.d.S.; Vaz-Velho, M.; Mutukumira, A.N.; et al. Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Foods 2025, 14, 1547. https://doi.org/10.3390/foods14091547
Andrade DKA, Wang B, Lima EMF, Shebeko SK, Ermakov AM, Khramova VN, Ivanova IV, Rocha RdS, Vaz-Velho M, Mutukumira AN, et al. Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Foods. 2025; 14(9):1547. https://doi.org/10.3390/foods14091547
Chicago/Turabian StyleAndrade, Dhuelly Kelly Almeida, Boying Wang, Emília Maria França Lima, Sergei Konstantinovich Shebeko, Alexey Mikhailovich Ermakov, Valentina Nikolaevna Khramova, Iskra Vitanova Ivanova, Ramon da Silva Rocha, Manuela Vaz-Velho, Anthony Nhamo Mutukumira, and et al. 2025. "Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage" Foods 14, no. 9: 1547. https://doi.org/10.3390/foods14091547
APA StyleAndrade, D. K. A., Wang, B., Lima, E. M. F., Shebeko, S. K., Ermakov, A. M., Khramova, V. N., Ivanova, I. V., Rocha, R. d. S., Vaz-Velho, M., Mutukumira, A. N., & Todorov, S. D. (2025). Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Foods, 14(9), 1547. https://doi.org/10.3390/foods14091547







