Evaluation of Polycyclic Aromatic Hydrocarbons Content and Risk Assessment of Tea Products in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. Extraction and Clean-Up
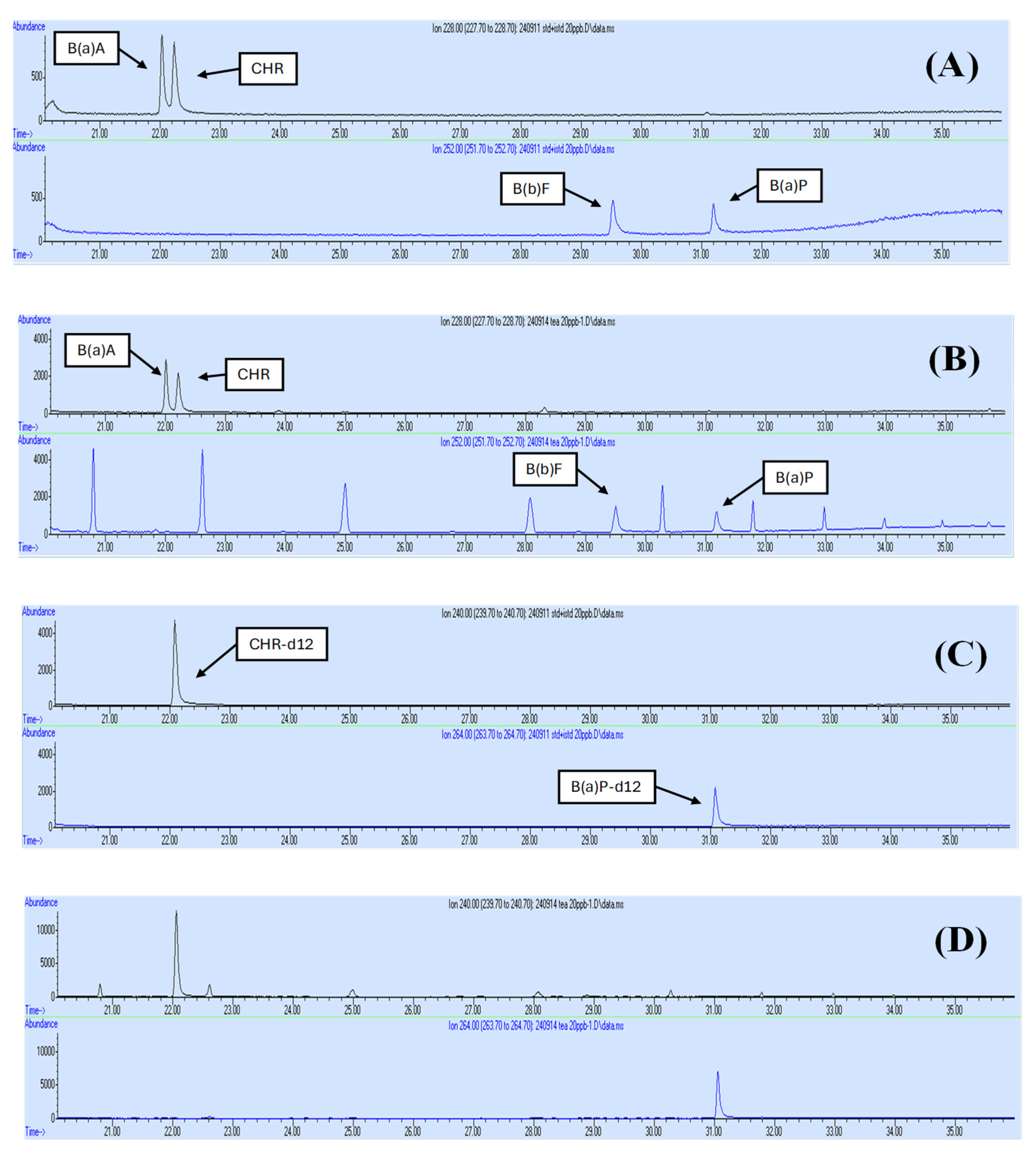
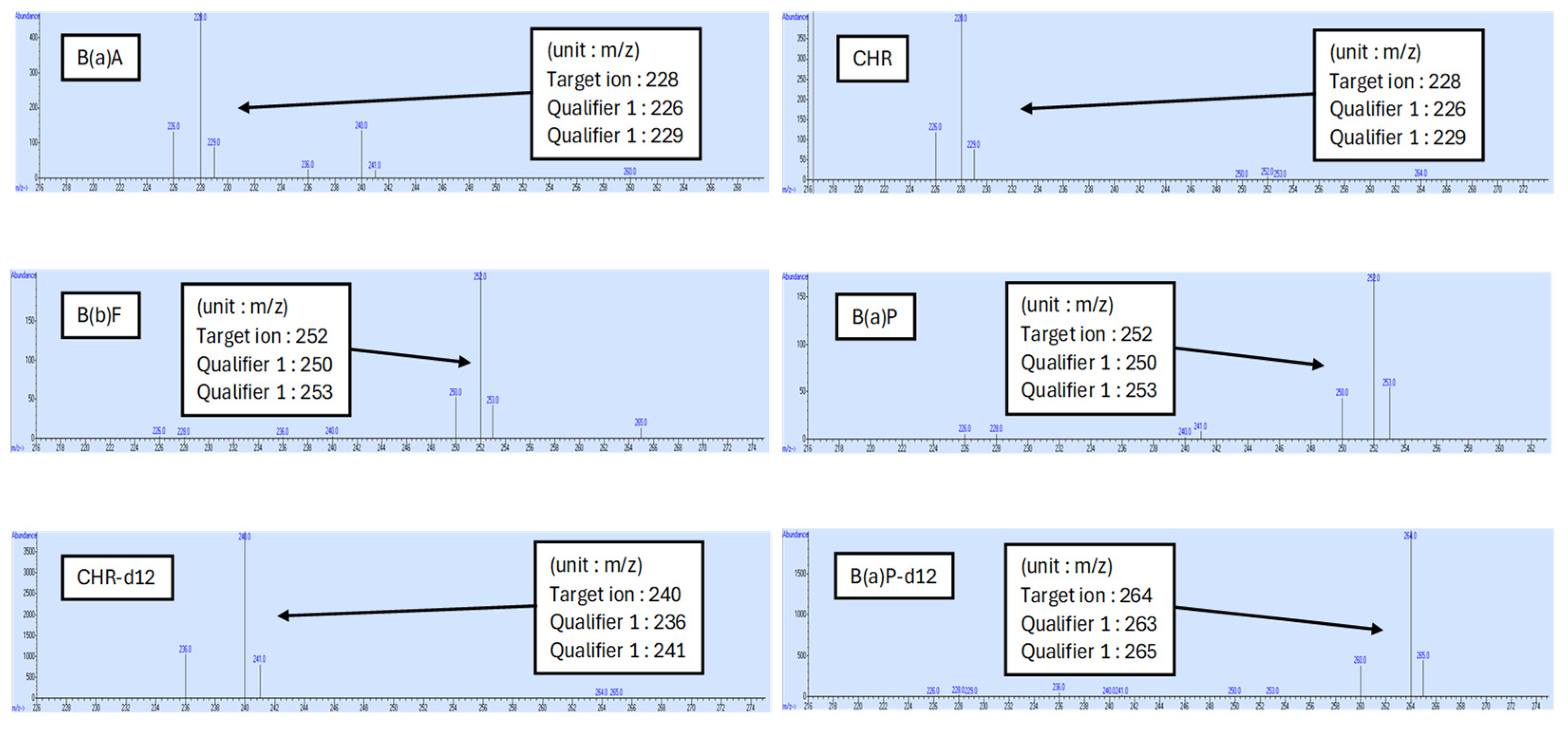
2.4. Determination of PAHs by GC-MS
2.5. Validation of the Analytical Method for PAHs
2.6. Risk Assessment and Toxic Equivalency Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Validation and Analytical Quality Assurance for Four PAHs’ Analysis
3.2. Comparison of PAHs Concentrations Between Tea Leaves and Brewed Tea
3.3. Risk Assessment
3.4. PAH Source Attribution by Diagnostic Ratios
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Zachariadis, G.A. Recent advances in the extraction of polycyclic aromatic hydrocarbons from environmental samples. Molecules 2020, 25, 2182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Wang, Y.; Bai, P.; Hayakawa, K.; Zhang, L.; Tang, N. Characteristics and influencing factors of polycyclic aromatic hydrocarbons emitted from open burning and stove burning of biomass: A brief review. Int. J. Environ. Res. Public Health 2022, 19, 3944. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; Torres, E.A. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- IARC. Chemical Agents and Related Occupations; IARC Monographs on the Identification of Carcinogenic Hazards to Humans, Vol. 100F; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on polycyclic aromatic hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Roudbari, A.; Rafiei Nazari, R.; Shariatifar, N.; Moazzen, M.; Abdolshahi, A.; Mirzamohammadi, S.; Arabameri, M. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ. Sci. Pollut. Res. 2021, 28, 4827–4839. [Google Scholar] [CrossRef]
- Lin, D.; Tu, Y.; Zhu, L. Concentrations and health risk of polycyclic aromatic hydrocarbons in tea. Food Sci. Biotechnol. 2005, 43, 41–48. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Benzo[a]pyrene—General Test Method. In Standards and Specifications for Foods; MFDS Notice No. 2024-29; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2024. [Google Scholar]
- U.S. Environmental Protection Agency. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAH); EPA/600/R-93/089; Office of Research and Development, Office of Health and Environmental Assessment: Washington, DC, USA, 1993. [Google Scholar]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on Polycyclic Aromatic Hydrocarbons: Occurrence, Legislation and Innovative Determination in Foods. Talanta 2007, 73, 1161–1171. [Google Scholar] [CrossRef]
- Wenzl, T.; Simon, R.; Anklam, E.; Kleiner, J. Analytical Methods for Polycyclic Aromatic Hydrocarbons (PAHs) in Food and the Environment. TrAC Trends Anal. Chem. 2006, 25, 716–725. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Santonicola, S.; Albrizio, S.; Murru, N.; Ferrante, M.C.; Mercogliano, R. Polycyclic Aromatic Hydrocarbons (PAHs) in Food: Recent Advances and Future Perspectives. Ital. J. Food Saf. 2017, 6, 7095. [Google Scholar]
- Heck, C.I.; de Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- Vieira, M.A.; Maraschin, M.; Amboni, R.D.D.M.C.; Prudêncio, E.S.; Pagliosa, C.M.; Mantelli, M.B.H.; Amante, E.R. Thermal condition of mate (Ilex paraguariensis) processing. Cienc. Rural. 2023, 53, e20220178. [Google Scholar] [CrossRef]
- Okaru, A.O.; Rullmann, A.; Farah, A.; Gonzalez de Mejia, E.; Stern, M.C.; Lachenmeier, D.W. Comparative oesophageal cancer risk assessment of hot beverage consumption (coffee, mate and tea): The margin of exposure of PAH vs very hot temperatures. BMC Cancer 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oranuba, E.; Deng, H.; Peng, J.; Dawsey, S.M.; Kamangar, F. Polycyclic aromatic hydrocarbons as a potential source of carcinogenicity of mate. J. Environ. Sci. Health C 2019, 37, 26–41. [Google Scholar] [CrossRef]
- Kamangar, F.; Schantz, M.M.; Abnet, C.C.; Fagundes, R.B.; Dawsey, S.M. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.K.; Georgieva, A.; Peteva, Z.V.; Trifonova, T.P. Polycyclic aromatic hydrocarbons contamination levels of dried herbal teas and their infusions. Eur. Food Res. Technol. 2023, 249, 3001–3009. [Google Scholar] [CrossRef]
- Aziz, A.; Abd Karim, K.; Ahmad, M.A.; Ridhwan, M.J.M. Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Moringa oleifera Leaves and Infusion. Sains Malays. 2023, 52, 2889–2906. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Bratu, M.M.; Birghila, S.; Coatu, V.; Danilov, D.A.; Radu, M.D.; Birghila, C. Polycyclic aromatic hydrocarbons (PAHs) in some commercial herbal teas. Polycycl. Aromat. Compd. 2023, 43, 2269–2282. [Google Scholar] [CrossRef]
- Benson, N.U.; Fred-Ahmadu, O.H.; Olugbuyiro, J.A.; Anake, W.U.; Adedapo, A.E.; Olajire, A.A. Concentrations, sources, and risk characterisation of polycyclic aromatic hydrocarbons (PAHs) in green, herbal, and black tea products in Nigeria. J. Food Compos. Anal. 2018, 66, 13–22. [Google Scholar] [CrossRef]
- Ciemniak, A.; Kuźmicz, K.; Rajkowska-Myśliwiec, M.; Cadena, M.F. Assessing the contamination levels of dried teas and their infusions by polycyclic aromatic hydrocarbons (PAHs). J. Consum. Prot. Food Saf. 2019, 14, 263–274. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, Z.; Du, S.; Zhang, D.; Li, X.; Zheng, Q.; Lu, S. Polycyclic aromatic hydrocarbons in commercial tea from China and implications for human exposure. J. Food Compos. Anal. 2023, 116, 105075. [Google Scholar] [CrossRef]
- Adisa, A.; Jimenez, A.; Woodham, C.; Anthony, K.; Nguyen, T.; Saleh, M.A. Determination of polycyclic aromatic hydrocarbons in dry tea. J. Environ. Sci. Health Part B 2015, 50, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Phan Thi, L.A.; Ngoc, N.T.; Quynh, N.T.; Thanh, N.V.; Kim, T.T.; Anh, D.H.; Viet, P.H. Polycyclic aromatic hydrocarbons (PAHs) in dry tea leaves and tea infusions in Vietnam: Contamination levels and dietary risk assessment. Environ. Geochem. Health 2020, 42, 2853–2863. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Navaratnam, M.A.; Jewula, J.; Jensen, A.H. PAH in some brands of tea and coffee. Polycycl. Aromat. Compd. 2015, 35, 74–90. [Google Scholar] [CrossRef]
- Gao, G.; Chen, H.; Liu, P.; Hao, Z.; Ma, G.; Chai, Y.; Lu, C. Residue Pattern of Polycyclic Aromatic Hydrocarbons during Green Tea Manufacturing and Their Transfer Rates during Tea Brewing. Food Addit. Contam. Part A 2017, 34, 990–999. [Google Scholar] [CrossRef]
- Harrison, D.M.; Chang, W.C.; Lin, H.T. Using QuEChERS and HPLC method to monitor the background concentration of polycyclic aromatic hydrocarbons in commercial black tea leaves and infusions in Taiwan. Toxics 2024, 12, 148. [Google Scholar] [CrossRef]
- Harrison, D.M.; Chang, W.C.; Lin, H.T. Dietary exposure and health risk assessment of polycyclic aromatic hydrocarbons in black tea consumed in Taiwan. Toxics 2024, 12, 134. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, L.; Luo, L. Factors affecting transfer of polycyclic aromatic hydrocarbons from made tea to tea infusion. J. Agric. Food Chem. 2006, 54, 4350–4354. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, Z.; Cao, D.; Yang, Q. Occurrence and exposure evaluation of polycyclic aromatic hydrocarbons in tea samples consumed in Beijing-Tianjin-Hebei regions of China. Food Sci. Biotechnol. 2023, 123, 105606. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M. Levels and risk assessment of polycyclic aromatic hydrocarbons, acrylamide and 5-hydroxymethylfurfural in yerba mate and its infusions. Food Sci. Biotechnol. 2023, 152, 109860. [Google Scholar] [CrossRef]
- Elzein, A.; Dunmore, R.E.; Ward, M.W.; Hamilton, J.F.; Lewis, A.C. Variabil-ity of polycyclic aromatic hydrocarbons and their oxidative derivatives in wintertime Beijing, China. Atmos. Chem. Phys. 2019, 19, 8741–8758. [Google Scholar] [CrossRef]
- Gurkan Ayyildiz, E.; Esen, F. Atmospheric polycyclic aromatic hydrocarbons (PAHs) at two sites, in Bursa, Turkey: Determination of concentrations, gas–particle partitioning, sources, and health risk. Arch. Environ. Contam. Toxicol. 2020, 78, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Kargar, N.; Matin, G.; Matin, A.A.; Buyukisik, H.B. Biomonitoring, status and source risk assessment of polycyclic aromatic hydrocarbons (PAHs) using honeybees, pine tree leaves, and propolis. Chemosphere 2017, 186, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Z.; Guo, Y.; Yu, F.; Zhang, Z.; Zhang, R. Characterization of PM₂.₅-bound polycyclic aromatic hydrocarbons at two Central China cities: Seasonal variation, sources, and health risk assessment. Arch. Environ. Contam. Toxicol. 2020, 78, 20–33. [Google Scholar] [CrossRef]
| Sample Type | PAHs | Equation (y = ax + b) | Linearity (R2) | LOD (μg/kg) (1) | LOQ (μg/kg) (2) |
|---|---|---|---|---|---|
| Tea leaf (solid) | BaA | y = 0.0096x + 0.0023 | 0.9992 | 0.0954 | 0.2891 |
| CHR | y = 0.0081x + 0.0016 | 0.9997 | 0.0610 | 0.1849 | |
| BbF | y = 0.0112x + 0.0047 | 0.9996 | 0.1440 | 0.4364 | |
| BaP | y = 0.0077x + 0.0040 | 0.9997 | 0.1534 | 0.4648 | |
| Infused tea (liquid) | BaA | y = 0.0079x + 0.0014 | 0.9995 | 0.0044 | 0.0132 |
| CHR | y = 0.0080x + 0.0010 | 0.9992 | 0.0064 | 0.0194 | |
| BbF | y = 0.0081x + 0.0009 | 0.9994 | 0.0037 | 0.0111 | |
| BaP | y = 0.0080x − 0.0001 | 0.9991 | 0.0035 | 0.0107 |
| Sample Type | 4PAHs | Recovery (%) (1) | ||||
|---|---|---|---|---|---|---|
| 1 (μg/kg) | 2 (μg/kg) | 5 (μg/kg) | 10 (μg/kg) | 20 (μg/kg) | ||
| Tea leaf (solid) | BaA | 87.39 ± 0.48 | 80.84 ± 0.73 | 106.64 ± 0.83 | 105.35 ± 0.48 | 107.92 ± 0.54 |
| CHR | 86.95 ± 0.70 | 84.35 ± 0.74 | 91.55 ± 0.69 | 88.80 ± 0.38 | 82.99 ± 0.28 | |
| BbF | 98.01 ± 0.74 | 90.83 ± 0.94 | 98.74 ± 0.21 | 103.89 ± 0.21 | 98.16 ± 0.12 | |
| BaP | 107.44 ± 0.07 | 84.21 ± 0.31 | 94.04 ± 0.26 | 100.55 ± 0.19 | 103.48 ± 0.21 | |
| Infused tea (liquid) | BaA | 100.02 ± 0.37 | 99.99 ± 0.08 | 100.00 ± 0.02 | 99.99 ± 0.09 | 100.00 ± 0.00 |
| CHR | 100.02 ± 0.14 | 99.99 ± 0.05 | 100.00 ± 0.09 | 99.99 ± 0.03 | 100.00 ± 0.07 | |
| BbF | 100.00 ± 0.28 | 100.00 ± 0.11 | 100.00 ± 0.04 | 100.00 ± 0.04 | 100.00 ± 0.05 | |
| BaP | 100.00 ± 0.28 | 100.00 ± 0.06 | 100.00 ± 0.23 | 100.00 ± 0.07 | 100.00 ± 0.03 | |
| Sample Type | 4PAHs | Interday (n = 3) | Intraday (n = 3) | |||
|---|---|---|---|---|---|---|
| Accuracy (%) (1) | RSD (%) (2) | Accuracy (%) | RSD (%) | |||
| Tea leaf (solid) | BaA | 1 | 85.20 | 12.62 | 87.39 | 0.55 |
| 2 | 81.90 | 2.12 | 80.84 | 0.90 | ||
| 5 | 105.50 | 4.97 | 106.64 | 0.78 | ||
| 10 | 105.63 | 1.84 | 105.35 | 0.45 | ||
| 20 | 105.57 | 4.26 | 107.92 | 0.50 | ||
| CHR | 1 | 86.75 | 5.98 | 86.95 | 0.81 | |
| 2 | 84.50 | 5.40 | 84.35 | 0.88 | ||
| 5 | 91.55 | 5.29 | 91.55 | 0.76 | ||
| 10 | 93.31 | 7.36 | 88.80 | 0.43 | ||
| 20 | 80.56 | 3.80 | 82.99 | 0.34 | ||
| BbF | 1 | 94.39 | 7.13 | 98.01 | 0.75 | |
| 2 | 87.18 | 5.40 | 90.83 | 1.03 | ||
| 5 | 97.59 | 2.29 | 98.74 | 0.21 | ||
| 10 | 105.10 | 8.35 | 103.89 | 0.20 | ||
| 20 | 97.61 | 0.92 | 98.16 | 0.12 | ||
| BaP | 1 | 107.26 | 1.53 | 107.44 | 0.06 | |
| 2 | 80.46 | 4.51 | 84.21 | 0.37 | ||
| 5 | 96.65 | 3.01 | 94.04 | 0.27 | ||
| 10 | 98.04 | 3.09 | 100.55 | 0.19 | ||
| 20 | 102.67 | 0.74 | 103.48 | 0.20 | ||
| Infused tea (liquid) | BaA | 1 | 99.99 | 0.26 | 100.02 | 0.37 |
| 2 | 100.35 | 0.35 | 99.99 | 0.08 | ||
| 5 | 99.77 | 0.22 | 100.00 | 0.02 | ||
| 10 | 99.94 | 0.09 | 99.99 | 0.09 | ||
| 20 | 100.05 | 0.06 | 100.00 | 0.00 | ||
| CHR | 1 | 101.48 | 1.28 | 100.02 | 0.14 | |
| 2 | 100.09 | 0.10 | 99.99 | 0.05 | ||
| 5 | 100.20 | 0.27 | 100.00 | 0.09 | ||
| 10 | 100.25 | 0.36 | 99.99 | 0.03 | ||
| 20 | 100.58 | 0.65 | 100.00 | 0.07 | ||
| BbF | 1 | 100.54 | 0.99 | 100.00 | 0.28 | |
| 2 | 100.68 | 0.69 | 100.00 | 0.11 | ||
| 5 | 99.79 | 0.69 | 100.00 | 0.04 | ||
| 10 | 99.74 | 0.79 | 100.00 | 0.04 | ||
| 20 | 99.66 | 0.42 | 100.00 | 0.05 | ||
| BaP | 1 | 99.55 | 0.99 | 100.00 | 0.28 | |
| 2 | 101.14 | 1.31 | 100.00 | 0.06 | ||
| 5 | 100.51 | 0.33 | 100.00 | 0.23 | ||
| 10 | 99.50 | 0.79 | 100.00 | 0.07 | ||
| 20 | 98.86 | 1.32 | 100.00 | 0.03 | ||
| Sample | N (1) | BaA (μg/kg) | CHR (μg/kg) | BbF (μg/kg) | BaP (μg/kg) | 4PAHs (μg/kg) |
|---|---|---|---|---|---|---|
| Cassia tora tea | 9 | <LOQ | 0.33 ± 0.02 | <LOQ | <LOQ | 0.33 ± 0.02 |
| Green tea | 9 | 0.68 ± 0.10 | 1.36 ± 0.30 | <LOQ | <LOQ | 2.04 ± 0.39 |
| Buckwheat tea | 8 | <LOQ | 0.32 ± 0.03 | <LOQ | <LOQ | 0.32 ± 0.03 |
| Barley tea | 10 | 0.54 ± 0.06 | <LOQ | <LOQ | <LOQ | 0.54 ± 0.06 |
| Black tea | 9 | 3.30 ± 0.17 | 6.99 ± 0.29 | 2.83 ± 0.19 | 1.12 ± 0.23 | 14.24 ± 0.87 |
| Polygonatum odoratum tea | 8 | <LOQ | 0.25 ± 0.02 | <LOQ | <LOQ | 0.25 ± 0.02 |
| Yerba mate tea | 10 | 7.70 ± 0.72 | 19.70 ± 1.88 | 3.69 ± 0.84 | 2.49 ± 0.46 | 33.58 ± 3.90 |
| Corn tea | 10 | <LOQ | 0.24 ± 0.03 | <LOQ | <LOQ | 0.24 ± 0.03 |
| Brown rice tea | 9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Herbal tea | 9 | 8.13 ± 0.28 | 9.32 ± 0.22 | 4.31 ± 0.34 | 2.29 ± 0.20 | 24.05 ± 1.04 |
| Oolong tea | 9 | 6.83 ± 0.64 | 13.31 ± 1.04 | 1.87 ± 0.31 | 1.19 ± 0.29 | 23.21 ± 2.28 |
| TEQBaP Value (2) (μg-TEQBaP/kg) | |||||
|---|---|---|---|---|---|
| TEF (1) | BaA | CHR | BbF | BaP | 4PAHs |
| 0.10 | 0.01 | 0.10 | 1.00 | ||
| Yerba mate tea | 0.77 | 0.20 | 0.37 | 2.49 | 3.82 |
| Herbal tea | 0.81 | 0.09 | 0.43 | 2.29 | 3.63 |
| Oolong tea | 0.68 | 0.13 | 0.19 | 1.19 | 2.19 |
| Black tea | 0.33 | 0.07 | 0.28 | 1.12 | 1.80 |
| Green tea | 0.07 | 0.01 | 0.00 | 0.00 | 0.08 |
| Barley tea | 0.05 | 0.00 | 0.00 | 0.00 | 0.05 |
| Cassia tora tea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Buckwheat tea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Polygonatum odoratum tea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Corn tea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Brown rice tea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PAHs Concentrations in the Infusions (μg/kg) | |||||
|---|---|---|---|---|---|
| PAHs | BaA | CHR | BbF | BaP | 4PAH |
| Yerba mate tea | 0.0324 | 0.0756 | 0.0144 | 0.0162 | 0.1386 |
| Herbal tea | 0.0395 | 0.0410 | 0.0163 | 0.0195 | 0.1163 |
| Oolong tea | 0.0142 | 0.0267 | 0.0121 | 0.0120 | 0.0650 |
| Black tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Green tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Barley tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Polygonatum odoratum tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Corn tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Cassia tora tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Buckwheat tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Brown rice tea | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Tea Type | BaP | 4PAHs | ||
|---|---|---|---|---|
| Conc. in Tea | MOEs | Conc. in Tea | MOEs | |
| Yerba mate tea | 0.0162 | 397,000 | 0.1386 | 227,000 |
| Herbal tea | 0.0195 | 329,000 | 0.1163 | 270,000 |
| Oolong tea | 0.0120 | 535,000 | 0.0650 | 484,000 |
| Black tea | ND | - | ND | - |
| Green tea | ND | - | ND | - |
| Barley tea | ND | - | ND | - |
| Cassia tora tea | ND | - | ND | - |
| Buckwheat tea | ND | - | ND | - |
| Polygonatum odoratum tea | ND | - | ND | - |
| Corn tea | ND | - | ND | - |
| Brown rice tea | ND | - | ND | - |
| Tea (Solid) | BaA/(BaA + CHR) |
|---|---|
| Yerba mate tea | 0.28 |
| Herbal tea | 0.47 |
| Oolong tea | 0.34 |
| Black tea | 0.32 |
| Green tea | 0.33 |
| Barley tea | 1.00 |
| Cassia tora tea | 0.00 |
| Buckwheat tea | 0.00 |
| Polygonatum odoratum tea | 0.00 |
| Corn tea | 0.00 |
| Brown rice tea | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-J.; Lee, Y.-H.; Shin, H.-S. Evaluation of Polycyclic Aromatic Hydrocarbons Content and Risk Assessment of Tea Products in South Korea. Foods 2025, 14, 1530. https://doi.org/10.3390/foods14091530
Lim K-J, Lee Y-H, Shin H-S. Evaluation of Polycyclic Aromatic Hydrocarbons Content and Risk Assessment of Tea Products in South Korea. Foods. 2025; 14(9):1530. https://doi.org/10.3390/foods14091530
Chicago/Turabian StyleLim, Kyung-Jik, Yoon-Hee Lee, and Han-Seung Shin. 2025. "Evaluation of Polycyclic Aromatic Hydrocarbons Content and Risk Assessment of Tea Products in South Korea" Foods 14, no. 9: 1530. https://doi.org/10.3390/foods14091530
APA StyleLim, K.-J., Lee, Y.-H., & Shin, H.-S. (2025). Evaluation of Polycyclic Aromatic Hydrocarbons Content and Risk Assessment of Tea Products in South Korea. Foods, 14(9), 1530. https://doi.org/10.3390/foods14091530







