The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review
Abstract
1. Introduction
2. Overview of AD
2.1. Pathogenesis of AD
2.2. AD, a Metabolic Syndrome?
2.3. Risk Factors in AD
3. The Gut Microbiome
3.1. Gut Microbiome Composition
3.2. Role and Funciton
3.2.1. Immunomodulatory and Metabolic Properties
3.2.2. Protective Role in Various Diseases
3.3. Dietary and Aging Factors Affecting Both the Gut Microbiome and AD
4. Gut Microbiome in AD
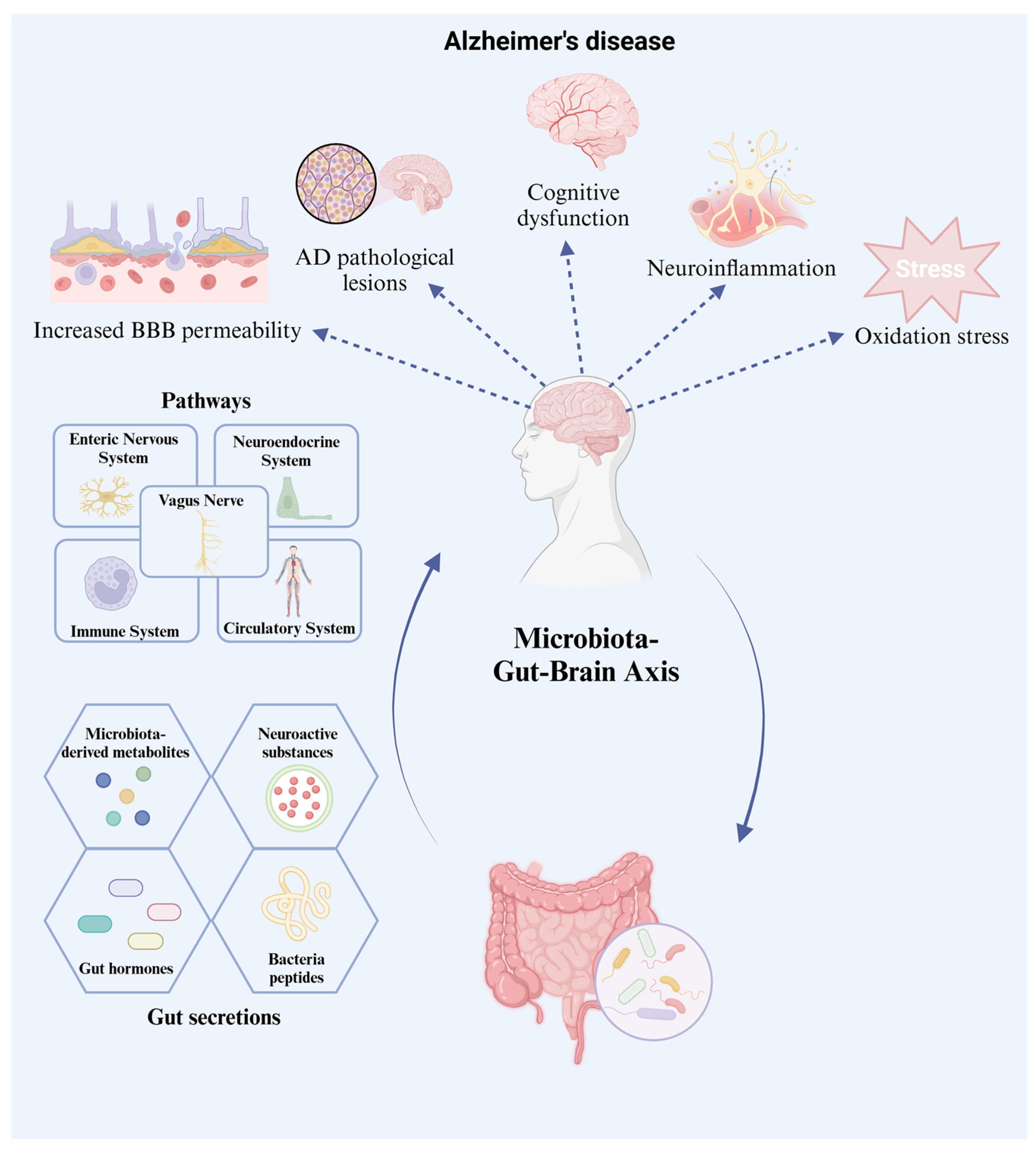
4.1. The Gut–Brain Axis
4.2. AD Pathogenesis: The Gut Microbiome Effect
4.2.1. Neuroactive Substances
4.2.2. Microbiome-Derived Metabolites
4.2.3. Ghrelin Hormone
5. The Interaction of Probiotics with GBA and Its Significance in AD
5.1. Functional Properties of Probiotics
5.2. Commonly Used Probiotics
5.3. Prebiotics and Symbiotics
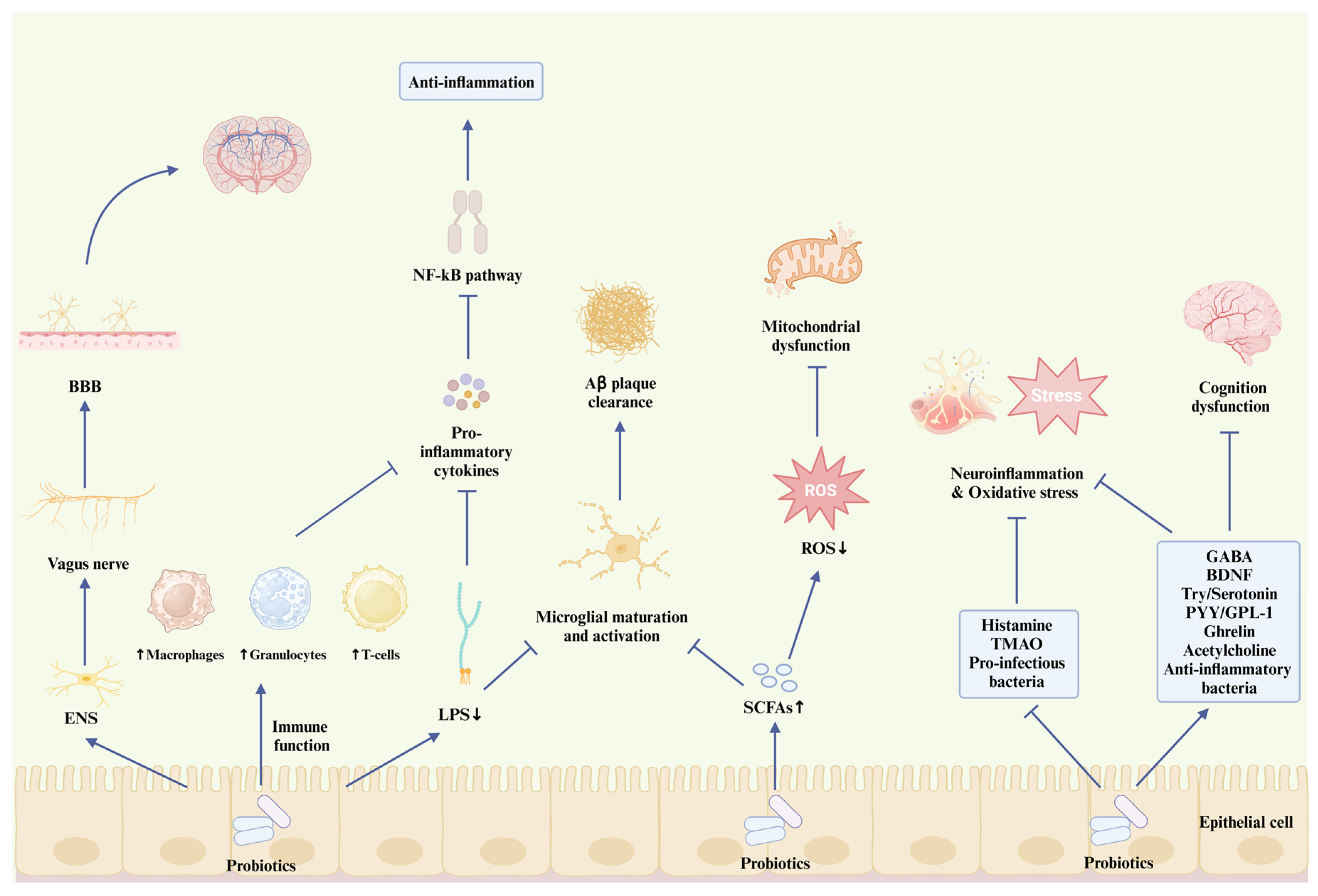
5.4. How Probiotics Interact with AD Pathogenesis
5.4.1. Probiotics and Aβ Plaque
5.4.2. Probiotics and Neuroinflammation
5.4.3. Probiotics and Mitochondrial Function
5.4.4. Probiotics and Other AD Pathological Hypotheses
6. Applications, Prospects, and Limitations of Probiotics
6.1. Different Intervention Conditions of Probiotcs
6.2. The Complexity in Designing an Effective Probiotic-Based Intervention for AD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s disease: Epidemiology and clinical progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Ageing and Health. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 October 2024).
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D.; Grinberg, L.T. Is Alzheimer disease a disease? Nat. Rev. Neurol. 2024, 20, 245–251. [Google Scholar] [CrossRef]
- Bao, W.-D.; Pang, P.; Zhou, X.-T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.-Z. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.-P.; Motherway, M.O.C.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Kocaadam-Bozkurt, B.; Bozkurt, O.; Sharma, H.; Esposito, R.; Özoğul, F.; Capasso, R. Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: The gut-health axis for a good mind. Biomed. Pharmacother. 2022, 153, 113430. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Patil, N.; Jain, M.; Kole, C.; Kaushik, P. Probiotics for neurodegenerative diseases: A systemic review. Microorganisms 2023, 11, 1083. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.-A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, J. Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front. Neurosci. 2020, 13, 1443. [Google Scholar] [CrossRef]
- Zheng, W.-H.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211. [Google Scholar] [CrossRef]
- Zotova, E.; Holmes, C.; Johnston, D.; Neal, J.W.; Nicoll, J.A.; Boche, D. Microglial alterations in human Alzheimer’s disease following Aβ42 immunization. Neuropathol. Appl. Neurobiol. 2011, 37, 513–524. [Google Scholar] [CrossRef]
- Brawek, B.; Garaschuk, O. Network-wide dysregulation of calcium homeostasis in Alzheimer’s disease. Cell Tissue Res. 2014, 357, 427–438. [Google Scholar] [CrossRef]
- Lee, C.D.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of microglia and astrocytes in Alzheimer’s disease: From neuroinflammation to Ca2+ homeostasis dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef]
- Aisen, P.S.; Schafer, K.A.; Grundman, M.; Pfeiffer, E.; Sano, M.; Davis, K.L.; Farlow, M.R.; Jin, S.; Thomas, R.G.; Thal, L.J. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 2003, 289, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar]
- Ingelsson, M.; Fukumoto, H.; Newell, K.; Growdon, J.; Hedley–Whyte, E.; Frosch, M.; Albert, M.; Hyman, B.; Irizarry, M. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004, 62, 925–931. [Google Scholar] [CrossRef]
- Dujardin, S.; Lécolle, K.; Caillierez, R.; Bégard, S.; Zommer, N.; Lachaud, C.; Carrier, S.; Dufour, N.; Aurégan, G.; Winderickx, J. Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: Relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2014, 2, 14. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Porras, C.A.; Rouault, T.A. Iron homeostasis in the CNS: An overview of the pathological consequences of iron metabolism disruption. Int. J. Mol. Sci. 2022, 23, 4490. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s disease puzzle: Delving into pathogenesis hypotheses. Aging Dis. 2024, 15, 43. [Google Scholar]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242, 124784. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, J.; Wang, Y.; Shao, J.; Li, Z.; Li, M. The regulation of volatile flavor compounds in fermented meat products mediated by microorganisms: A review. Food Biosci. 2024, 62, 105180. [Google Scholar] [CrossRef]
- Yue, X.; Li, M.; Liu, Y.; Zhang, X.; Zheng, Y. Microbial diversity and function of soybean paste in East Asia: What we know and what we don’t. Curr. Opin. Food Sci. 2021, 37, 145–152. [Google Scholar] [CrossRef]
- Shapira, M. Gut microbiotas and host evolution: Scaling up symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Jovel, J.; Dieleman, L.A.; Kao, D.; Mason, A.L.; Wine, E. The human gut microbiome in health and disease. Metagenomics 2018, 92, 197–213. [Google Scholar]
- Shen, X.; Xie, A.; Li, Z.; Jiang, C.; Wu, J.; Li, M.; Yue, X. Research Progress for probiotics regulating intestinal Flora to improve functional dyspepsia: A review. Foods 2024, 13, 151. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Jiang, C.; Xie, A.; Yue, X.; Li, M. A review of casein phosphopeptides: From enrichment identification to biological properties. Food Biosci. 2024, 59, 104217. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Li, Z.; Kanwal, R.; Yue, X.; Li, M.; Xie, A. Polyphenols and intestinal microorganisms: A review of their interactions and effects on human health. Food Biosci. 2024, 62, 105220. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R.J. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Sun, P.; Su, L.; Zhu, H.; Li, X.; Guo, Y.; Du, X.; Zhang, L.; Qin, C. Gut microbiota regulation and their implication in the development of neurodegenerative disease. Microorganisms 2021, 9, 2281. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Shao, J.; Yue, X.; Li, M. Maillard reaction-based conjugates as carrier strategies for delivery of bioactive compounds: A review. Curr. Opin. Food Sci. 2024, 61, 101260. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; De Giori, G.S.; Sesma, F.; Van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Hill, M. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Hong, R.; Yang, H.; Guo, Y.; Liu, Q.; Xu, N.; Xie, Y.; Li, M.; Yue, X. The application and mechanism of polysaccharides, proteins and their complexes on enhancing yogurt gel stability: A review. Food Sci. Anim. Prod. 2024, 2, 9240066. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Abdlla, R.; Chen, J.; Yue, X.; Quek, S.Y. Donkey whey proteins ameliorate dextran sulfate sodium-induced ulcerative colitis in mice by downregulating the S100A8-TRAF6-NF-κB axis-mediated inflammatory response. Food Sci. Hum. Wellness 2023, 12, 1809–1819. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.; Murphy, K.; Brooks, L.; Bewick, G.; Hanyaloglu, A.; Ghatei, M.; Bloom, S.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik Jr, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Sharma, M.K.; Jalewa, J.; Hölscher, C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY 5Y cells exposed to methylglyoxal stress. J. Neurochem. 2014, 128, 459–471. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Du, Y.-F.; Chen, L. Neuropeptides exert neuroprotective effects in Alzheimer’s disease. Front. Mol. Neurosci. 2019, 11, 493. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Parvaneh, M.; Karimi, G.; Jamaluddin, R.; Ng, M.H.; Zuriati, I.; Muhammad, S.I. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin. Interv. Aging 2018, 13, 1555–1564. [Google Scholar] [CrossRef]
- Masoumi, S.J.; Mehrabani, D.; Saberifiroozi, M.; Fattahi, M.R.; Moradi, F.; Najafi, M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci. Nutr. 2021, 9, 1704–1711. [Google Scholar] [CrossRef]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Xie, A.; Shen, X.; Hong, R.; Xie, Y.; Zhang, Y.; Chen, J.; Li, Z.; Li, M.; Yue, X.; Quek, S.Y. Unlocking the potential of donkey Milk: Nutritional composition, bioactive properties and future prospects. Food Res. Int. 2025, 209, 116307. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, Y.; Liu, X.; Tian, C.; Zhong, X.; Zhao, L. A systematic review and meta-analysis: Lactobacillus acidophilus for treating acute gastroenteritis in children. Nutrients 2022, 14, 682. [Google Scholar] [CrossRef]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut (microbiome) feeling about the brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar] [CrossRef]
- Farmer, A.D.; Randall, H.A.; Aziz, Q. It’s a gut feeling: How the gut microbiota affects the state of mind. J. Physiol. 2014, 592, 2981–2988. [Google Scholar] [CrossRef]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Anastassova, N.; Stefanova, D.; Hristova-Avakumova, N.; Georgieva, I.; Kondeva-Burdina, M.; Rangelov, M.; Todorova, N.; Tzoneva, R.; Yancheva, D. New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors. Antioxidants 2023, 12, 977. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary fiber intake and gut microbiota in human health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Hung, C.-C.; Chang, C.-C.; Huang, C.-W.; Nouchi, R.; Cheng, C.-H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477. [Google Scholar] [CrossRef]
- Verhaar, B.J.; Hendriksen, H.M.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M. Gut microbiota composition is related to AD pathology. Front. Immunol. 2022, 12, 794519. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Shen, Y.-E.; Wang, X.; Fu, S.; Zhang, X.; Zhang, Y.-N.; Wang, R.-T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging 2020, 12, 628. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Biagi, E.; Rampelli, S.; Turroni, S.; Quercia, S.; Candela, M.; Brigidi, P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 2017, 165, 180–184. [Google Scholar] [CrossRef]
- Corrada, M.M.; Brookmeyer, R.; Paganini-Hill, A.; Berlau, D.; Kawas, C.H. Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann. Neurol. 2010, 67, 114–121. [Google Scholar] [CrossRef]
- Harada, C.N.; Love, M.C.N.; Triebel, K. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737. [Google Scholar] [CrossRef]
- Mishra, V.; Yadav, D.; Solanki, K.S.; Koul, B.; Song, M. A Review on the Protective Effects of Probiotics against Alzheimer’s Disease. Biology 2023, 13, 8. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.; Fahnestock, M.; Moine, D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.; Barrett, E.; Grenham, S.; Fitzgerald, P.; Stanton, C.; Ross, R.; Quigley, E.; Cryan, J.; Dinan, T. BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes 2011, 2, 199–207. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Klöcker, N.; Kermer, P.; Weishaupt, J.H.; Labes, M.; Ankerhold, R.; Bähr, M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J. Neurosci. 2000, 20, 6962–6967. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent advances on the role of brain-derived neurotrophic factor (BDNF) in neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Bai, X.; Edden, R.A.; Gao, F.; Wang, G.; Wu, L.; Zhao, B.; Wang, M.; Chan, Q.; Chen, W.; Barker, P.B. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J. Magn. Reson. Imaging 2015, 41, 1326–1331. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Franco, A.; Fernández-Novoa, L.; Cacabelos, R. Blood levels of histamine, IL-1β, and TNF-α in patients with mild to moderate Alzheimer disease. Mol. Chem. Neuropathol. 1996, 29, 237–252. [Google Scholar] [CrossRef]
- Choi, H.; Mook-Jung, I. Functional effects of gut microbiota-derived metabolites in Alzheimer’s disease. Curr. Opin. Neurobiol. 2023, 81, 102730. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H. Exploring the serotonin-probiotics-gut health axis: A review of current evidence and potential mechanisms. Food Sci. Nutr. 2024, 12, 694–706. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef]
- Claeysen, S.; Bockaert, J.; Giannoni, P. Serotonin: A new hope in Alzheimer’s disease? ACS Chem. Neurosci. 2015, 6, 940–943. [Google Scholar] [CrossRef]
- Jeon, S.G.; Hong, S.B.; Nam, Y.; Tae, J.; Yoo, A.; Song, E.J.; Kim, K.I.; Lee, D.; Park, J.; Lee, S.M. Ghrelin in Alzheimer’s disease: Pathologic roles and therapeutic implications. Ageing Res. Rev. 2019, 55, 100945. [Google Scholar] [PubMed]
- Gahete, M.D.; Córdoba-Chacón, J.; Kineman, R.D.; Luque, R.M.; Castaño, J.P. Role of ghrelin system in neuroprotection and cognitive functions: Implications in Alzheimer’s disease. Peptides 2011, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C. Structure and physiological actions of ghrelin. Scientifica 2013, 2013, 518909. [Google Scholar] [CrossRef]
- Tóth, K.; László, K.; Lénárd, L. Role of intraamygdaloid acylated-ghrelin in spatial learning. Brain Res. Bull. 2010, 81, 33–37. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rubio, A.; Cordoba-Chacon, J.; Gracia-Navarro, F.; Kineman, R.D.; Avila, J.; Luque, R.M.; Castano, J.P. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2010, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Cha, M.-Y.; Mook-Jung, I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimer’s Dis. 2014, 41, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhu, M.; He, Y.; Chu, W.; Du, Y.; Du, H. Increased serum acylated ghrelin levels in patients with mild cognitive impairment. J. Alzheimer’s Dis. 2017, 61, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in medicine: A long debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef]
- Hu, S.; Tang, B.; Lu, C.; Wang, S.; Wu, L.; Lei, Y.; Tang, L.; Zhu, H.; Wang, D.; Yang, S. Lactobacillus rhamnosus gg ameliorates triptolide-induced liver injury through modulation of the bile acid-fxr axis. Pharmacol. Res. 2024, 206, 107275. [Google Scholar] [CrossRef]
- Fotiadis, C.I.; Stoidis, C.N.; Spyropoulos, B.G.; Zografos, E.D. Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J. Gastroenterol. WJG 2008, 14, 6453. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic bacteria: A promising tool in cancer prevention and therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 15611–15616. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn. Res. JCDR 2017, 11, KC01. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, K.-E.; Kim, J.-K.; Kim, D.-H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Kang, J.W.; Zivkovic, A.M. The potential utility of prebiotics to modulate Alzheimer’s disease: A review of the evidence. Microorganisms 2021, 9, 2310. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Bielecka, M. Prebiotic effectiveness of fructans of different degrees of polymerization. Trends Food Sci. Technol. 2004, 15, 170–175. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic effect of fructooligosaccharides from Morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota-gut-brain axis. Front. Aging Neurosci. 2017, 9, 403. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Zhao, Y.; Dua, P.; Lukiw, W. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease (AD). J. Alzheimer’s Dis. Park. 2015, 5, 177. [Google Scholar]
- Bhattacharjee, S.; Lukiw, W.J. Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef]
- Alonso, R.; Pisa, D.; Marina, A.I.; Morato, E.; Rábano, A.; Carrasco, L. Fungal infection in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 301–311. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J. Alzheimer’s Dis. 2014, 39, 169–179. [Google Scholar] [CrossRef]
- Schwartz, K.; Boles, B.R. Microbial amyloids–functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Yeon, S.-W.; You, Y.S.; Kwon, H.-S.; Yang, E.H.; Ryu, J.-S.; Kang, B.H.; Kang, J.-H. Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J. Funct. Foods 2010, 2, 143–152. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Lv, H.; Dong, Q.; Li, J.; Geng, W.; Wang, J.; Liu, F.; Jia, L.; Wang, Y. Modulation of the gut microbiota and glycometabolism by a probiotic to alleviate amyloid accumulation and cognitive impairments in AD rats. Mol. Nutr. Food Res. 2022, 66, 2200265. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Green, M.; Prakash, S. The microbiome and Alzheimer’s disease: Potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef]
- Guo, L.; Xu, J.; Du, Y.; Wu, W.; Nie, W.; Zhang, D.; Luo, Y.; Lu, H.; Lei, M.; Xiao, S. Effects of gut microbiota and probiotics on Alzheimer’s disease. Transl. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar]
- Streit, W.J.; Sammons, N.W.; Kuhns, A.J.; Sparks, D.L. Dystrophic microglia in the aging human brain. Glia 2004, 45, 208–212. [Google Scholar] [CrossRef]
- Kim, Y.J.; Mun, B.-R.; Choi, K.Y.; Choi, W.-S. Oral Administration of Probiotic Bacteria Alleviates Tau Phosphorylation, Aβ Accumulation, Microglia Activation, and Memory Loss in 5xFAD Mice. Brain Sci. 2024, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Caraveo, A.; Sayd, A.; Maus, S.R.; Caso, J.R.; Madrigal, J.L.; García-Bueno, B.; Leza, J.C. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci. Rep. 2017, 7, 13113. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sahu, K.; Singh, C.; Singh, A. Lipopolysaccharide induced altered signaling pathways in various neurological disorders. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 285–294. [Google Scholar] [CrossRef]
- Lekchand Dasriya, V.; Samtiya, M.; Dhewa, T.; Puniya, M.; Kumar, S.; Ranveer, S.; Chaudhary, V.; Vij, S.; Behare, P.; Singh, N. Etiology and management of Alzheimer’s disease: Potential role of gut microbiota modulation with probiotics supplementation. J. Food Biochem. 2022, 46, e14043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Woo, J.Y.; Kim, K.A.; Han, M.; Kim, D.H. Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent memory impairment in Fischer 344 rats. Lett. Appl. Microbiol. 2015, 60, 307–314. [Google Scholar]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Dirksen, R.T.; Wojtovich, A.P. Iron dysregulation in mitochondrial dysfunction and Alzheimer’s disease. Antioxidants 2022, 11, 692. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Bush, A.I. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: Targets for therapeutics. J. Neurochem. 2016, 139, 179–197. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Han, X.; Cao, W.; Wang, Y.; Ding, W.; Cao, M.; Zhang, Y.; Xu, Q.; Zhou, Y. Characterizing brain iron deposition in patients with subcortical vascular mild cognitive impairment using quantitative susceptibility mapping: A potential biomarker. Front. Aging Neurosci. 2017, 9, 81. [Google Scholar] [CrossRef]
- Van Bergen, J.M.; Li, X.; Quevenco, F.-C.; Gietl, A.; Treyer, V.; Meyer, R.; Buck, A.; Kaufmann, P.A.; Nitsch, R.M.; van Zijl, P.C. Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. Neuroimage 2018, 174, 308–316. [Google Scholar] [CrossRef]
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: Implications for neuronal damage. Hum. Mol. Genet. 2011, 20, 2495–2509. [Google Scholar] [CrossRef]
- Pérez, M.J.; Ponce, D.P.; Aranguiz, A.; Behrens, M.I.; Quintanilla, R.A. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018, 19, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Symbiosis and evolution. Sci. Am. 1971, 225, 48–61. [Google Scholar] [CrossRef]
- Franco-Obregón, A.; Gilbert, J.A. The microbiome-mitochondrion connection: Common ancestries, common mechanisms, common goals. Msystems 2017, 2, e00018-17. [Google Scholar] [CrossRef] [PubMed]
- Beltagy, D.M.; Nawar, N.F.; Mohamed, T.M.; Tousson, E.; El-Keey, M.M. Beneficial consequences of probiotic on mitochondrial hippocampus in Alzheimer’s disease. J. Complement. Integr. Med. 2021, 18, 761–767. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C. Oxidative stress and dementia in Alzheimer’s patients: Effects of synbiotic supplementation. Oxidative Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Majeed, A.B.A.; Ramasamy, K. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- de Moreno de Leblanc, A.; LeBlanc, J.G.; Perdigon, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J. Med. Microbiol. 2008, 57, 100–105. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, K.T.; Chung, M.Y.; Cho, D.H.; Park, C.S. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): Role for a metal ion chelating effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Yue, M.; Wei, J.; Chen, W.; Hong, D.; Chen, T.; Fang, X. Neurotrophic role of the next-generation probiotic strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson’s disease via inhibiting ferroptosis. Nutrients 2022, 14, 4886. [Google Scholar] [CrossRef]
- Sobol, C.; Belostotskaya, G. Product fermented by Lactobacilli induces changes in intracellular calcium dynamics in rat brain neurons. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 37–45. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Bowen, D.M.; Smith, C.B.; White, P.; Davison, A.N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain J. Neurol. 1976, 99, 459–496. [Google Scholar] [CrossRef]
- Shamsipour, S.; Sharifi, G.; Taghian, F. An 8-week administration of Bifidobacterium bifidum and Lactobacillus plantarum combined with exercise training alleviates neurotoxicity of Aβ and spatial learning via acetylcholine in Alzheimer rat model. J. Mol. Neurosci. 2021, 71, 1495–1505. [Google Scholar] [CrossRef]
- Qadah, A.M.; Amr, A.; Yehia, H. Novel use of probiotic as acetylcholine esterase inhibitor and a new strategy for activity optimization as a biotherapeutic agent. J. App. Biol. Biotechnol. 2023, 11, 202–215. [Google Scholar] [CrossRef]
- Kapasi, A.; Schneider, J.A. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 878–886. [Google Scholar] [CrossRef]
- Tini, G.; Scagliola, R.; Monacelli, F.; La Malfa, G.; Porto, I.; Brunelli, C.; Rosa, G.M. Alzheimer’s disease and cardiovascular disease: A particular association. Cardiol. Res. Pract. 2020, 2020, 2617970. [Google Scholar] [CrossRef]
- Zhu, X.; Smith, M.A.; Honda, K.; Aliev, G.; Moreira, P.I.; Nunomura, A.; Casadesus, G.; Harris, P.L.; Siedlak, S.L.; Perry, G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007, 257, 240–246. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, X.; Yang, Y. Effect of probiotics on lipid profiles in hypercholesterolaemic adults: A meta-analysis of randomized controlled trials. Med. Clín. (Engl. Ed.) 2019, 152, 473–481. [Google Scholar]
- Sjögren, M.; Mielke, M.; Gustafson, D.; Zandi, P.; Skoog, I. Cholesterol and Alzheimer’s disease—Is there a relation? Mech. Ageing Dev. 2006, 127, 138–147. [Google Scholar] [CrossRef]
- Tao, Y.-W.; Gu, Y.-L.; Mao, X.-Q.; Zhang, L.; Pei, Y.-F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.; Tercero-Lozano, M.; Arraiza-Irigoyen, C.; Del Castillo-Codes, I.; Olza, J.; Plaza-Díaz, J.; Olivares, M.; Gil, Á.; Gómez-Llorente, C. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation and cardiovascular risk in obese adults with metabolic syndrome: A randomized clinical trial (PROSIR). Clin. Nutr. 2018, 37, S15. [Google Scholar] [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s disease: A systematic review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Huang, Y.-Y.; Tsai, S.-Y.; Kuo, Y.-W.; Lin, J.-H.; Ho, H.-H.; Chen, J.-F.; Hsia, K.-C.; Sun, Y. Efficacy of probiotic supplements on brain-derived neurotrophic factor, inflammatory biomarkers, oxidative stress and cognitive function in patients with Alzheimer’s dementia: A 12-week randomized, double-blind active-controlled study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.-Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef]
- de Rijke, T.J.; Doting, M.E.; van Hemert, S.; De Deyn, P.P.; van Munster, B.C.; Harmsen, H.J.; Sommer, I.E. A systematic review on the effects of different types of probiotics in animal Alzheimer’s disease studies. Front. Psychiatry 2022, 13, 879491. [Google Scholar] [CrossRef]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, gut microbiome, and Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 8243–8250. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]

| Probiotics | Model or Subjects | Duration | Significant Effect on AD | Source |
|---|---|---|---|---|
| 200 mL/d of fermentum milk contained Lactobacillus acidophilus, L. casei, L. fermentum and Bifidobacterium bifidum (2 × 109 CFU/g for each) | AD patients | 12 weeks | Cognitive function | Akbari, 2016 [177] |
| Bifidobacterium longum subsp. Infantis, B. breve, B. animalis subsp. Lactis, B. bifidum and Lactobacillus plantarum (1 capsule contained 1 × 1010 CFU/d) | AD patients | 12 weeks | ↑BDNF Inhibiting oxidative stress and inflammation | Hsu, 2023 [178] |
| Combination treatment of selenium (200 mg/day) and Lactobacillus acidophilus, L. casei, L. fermentum and Bifidobacterium bifidum (2 × 109 CFU/g for each) | AD patients | 12 weeks | Ameliorating the cognitive function, oxidative stress and inflammation | Akbari, 2016 [177] |
| Bifidobacterium brevis A1 (2 capsules contained > 2.0 × 1010 CFU/d) | MCI patients | 12 weeks | Improving the immediate memory | Kobayashi, 2019 [179] |
| 2 mL/kg/d fermented milk contained Acetobacter aceti, A. sp., Lactobacillus delbrueckii, L. fermentum, L. fructivorans, L. kefiranofaciens, Enterococcus faecium, Leuconostoc spp., Candida famata and C. krusei (probiotics dosage is not mentioned) | AD patients | 3 months | Inhibiting the mitochondrial dysfunction, DNA damage and apoptosis Mitigating the oxidative stress and inflammation Improving cognitive deficits | Ton, 2020 [157] |
| Bifidobacterium brevis A1 (probiotics dosage is not mentioned) | Aβ25–35-injected mice | 10 days | Ameliorating Cognitive function Increasing the expression of the immune response gene ↑plasma acetate levels | Kobayashi, 2017 [133] |
| Lactobacillus plantarum (1 × 108 CFU/kg/d) | D-galactose/AlCl3 induced AD rats | 12 weeks | Reshaping the gut microbiome composition Ameliorating Cognitive function ↓Aβ accumulation | Wang, 2022 [136] |
| 8 mL sterilized water of Bifidobacterium lactis, Limosilactobacillus fermentum and Levilactobacillus brevis (8 × 107 CFU/d) | 5xFAD mice model | 3 months | Attenuating the microglial activation ↓Aβ accumulation ↓Tau phosphorylation Modifying the gut microbiome composition Improving the spatial and recognition memories | Kim, 2024 [142] |
| Lactobacillus pentosus var. plantarum C29 (2 × 109 CFU/d) | Aged Fischer 344 rats | 8 weeks | Inhibiting NF-κB signalling pathway ↓the expression of BDNF Enhancing the aging-impaired memory | Jeong, 2015 [146] |
| 2 g (1 × 1010 CFU/g) of Lactobacillus acidophilus, L. fermentum, Bifidobacterium lactis and B. longum | Aβ1-42-injected mice | 8 weeks | Ameliorating memory deficit and oxidative stress Modifying the microbiome composition | Athari Nik Azm, 2018 [117] |
| Combination treatment of Memantine (1 mg/mL) and Lactobacillus plantarum (1 × 109 CFU/mL) | APP/PS1 mice | 12 weeks | Potentiating the drug therapy: Inhibiting amyloid plaque and neuroinflammation Protecting neurons and the hippocampal plasticity ↓TMAO | Wang, 2020 [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wu, X.; Zhang, Y.; Hu, A.; Zhou, Q.; Yue, X.; Liu, Z.; Li, M. The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review. Foods 2025, 14, 1531. https://doi.org/10.3390/foods14091531
Dong Y, Wu X, Zhang Y, Hu A, Zhou Q, Yue X, Liu Z, Li M. The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review. Foods. 2025; 14(9):1531. https://doi.org/10.3390/foods14091531
Chicago/Turabian StyleDong, Yushi, Xilin Wu, Yumeng Zhang, Adi Hu, Qian Zhou, Xiqing Yue, Zhenmin Liu, and Mohan Li. 2025. "The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review" Foods 14, no. 9: 1531. https://doi.org/10.3390/foods14091531
APA StyleDong, Y., Wu, X., Zhang, Y., Hu, A., Zhou, Q., Yue, X., Liu, Z., & Li, M. (2025). The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review. Foods, 14(9), 1531. https://doi.org/10.3390/foods14091531










