1. Introduction
Rare earth elements (REEs) are a group of lanthanides with atomic numbers ranging from 57 to 71, along with scandium (Sc) and yttrium (Y) [
1], which naturally coexist in mineral deposits. Based on their atomic structures and physicochemical properties, REEs are categorized into light REEs (LREEs) and heavy REES (HREEs). These elements are widely used in multiple industries due to their excellent magnetic, optical, and electrical properties [
2], essential for improving product performance and production efficiency. In addition, REEs are used in specific agricultural applications, such as in certain fertilizers and feed additives, possibly as trace elements or to promote specific growth characteristics [
3,
4]. Global REE production has surged since the 1950s, and the demand is projected to increase by 2600% over the next 25 years [
5]. As of 2024, China accounts for the largest share of the global REE supply, at 49% (44 million tons), with an annual production of 270 thousand tons [
6].
The increasing release of REEs into the environment due to their development and application has led to concentrations in various environmental media that may be several orders of magnitude higher than background levels [
7]. REE contamination has been found in the soil and river sediments of many mining areas, such as the Ganzhou mining area [
8], Amazon gold mining area [
9], Xiangjiang River [
10], Yellow River [
11], and Santos Estuary (Brazil) [
12]. REEs enter the environment through activities such as mining and fertilization, significantly elevating their levels in the soil, water, and air [
7,
13]. In soil, REEs are absorbed by crops, and they accumulate in the edible parts [
14], whereas in aquatic environments, they bioaccumulate in organisms, such as fish and shellfish, through water and sediment exposure [
15]. These findings underscore the potential risks of REEs entering the food chain and the need for ongoing monitoring and management [
16,
17].
Oral intake is a major route through which REEs enter the human body. After ingestion, REEs pass through the digestive tract and enter the bloodstream, where they are distributed to organs, such as the liver, kidneys, and heart, as well as the nervous and reproductive systems [
18]. REEs are mainly absorbed in their ionic form or as complexes with organic molecules such as amino acids and proteins [
19]. REEs can interact with cell membranes within these organs and affect cellular functions, leading to negative effects such as neurotoxicity, pulmonary toxicity, nephrotoxicity, and cytotoxicity [
20,
21,
22,
23]. Animal studies have demonstrated that high concentrations of REEs can damage the nervous system, lungs, kidneys, and cellular structures. In addition, several studies have suggested that maternal exposure to REEs may be associated with an increased risk of premature membrane rupture [
24], and early exposure to REEs may increase the risk of gestational diabetes [
25]. These findings emphasize the need for further research on the relationship between REE exposure and maternal health risks, especially through long-term epidemiological data, animal models, and multicenter collaborations, to delve deeper into this issue.
In China, REEs have been widely detected in various food products, including tea [
26], vegetables [
27], fruits [
28,
29], aquatic products [
15], and grains [
30]. For example, the average REE concentration in Chinese tea varies across regions, with Henan Province’s black tea at 2.960 mg/kg, Shanxi Province’s green tea at 2.500 mg/kg, Guizhou Province’s oolong tea at 3.870 mg/kg, and Hunan Province’s black tea at 2.955 mg/kg [
26]. Similarly, the REE content of vegetables and fruits differed between mining and non-mining areas [
28]. The average REE concentration in vegetables in the mining and non-mining areas was 92.90 μg/kg and 62.38 μg/kg, respectively. The REE content in fruits from mining areas was 12.90 μg/kg, compared to 11.89 μg/kg in fruits from non-mining areas. Aquatic products, such as mantis shrimp from Shandong Province [
31], have an average REE content of 9.60 μg/kg. Grains also reflect significant variations, with rice from Jiangxi mining areas containing 102.79 μg/kg of REEs, while rice from non-mining areas contains only 35.28 μg/kg [
32]. Furthermore, other studies have also investigated the REE levels in food samples from specific regions. For example, the concentration of ΣREE in edible mushrooms in Poland was 0.04 mg/kg [
33], the average level of ΣREE in samples from terrestrial (plant feed [containing unprocessed materials like barley, wheat, oats, bran, and fodder], fruits, honey, wild animal liver) sources in Italy was 0.46 mg/kg, and the average level of ΣREE in aquatic (seaweeds, zooplankton, bivalves, fishes) sources was 3.12 mg/kg [
34]. There are fewer studies on REEs in food products from abroad, and this may be due to the distribution of rare earth resources. These findings highlight the overall impact of mining activities on REE contamination levels [
28], with certain foods, such as tea, leafy vegetables, and aquatic products, showing notably higher REE accumulation and contributing significantly to dietary exposure.
Due to the high detection rate and concentration of REEs in certain foods, dietary exposure assessment for specific foods has been conducted in some regions. Yang [
35] assessed the REE concentrations in 11 food items in the Chinese diet, showing that the average daily exposure to REEs from food was 1.62 μg/kg BW, accounting for 3.14% of the recommended intake, indicating a low health risk. Song [
36] used a simple distribution model (deterministic assessment) to calculate the daily exposure to REEs per kilogram of body weight for individuals. The results indicated that the average daily exposure from tea in the Borderlands was 0.668 μg/kg BW, which is within the safe range. One study [
37] employed Monte Carlo simulations to assess the probabilistic exposure to REEs in foods from a large mining area in Shandong Province. The study’s findings showed that the daily intake of REEs ranged from 3.80 to 5.40 μg/kg BW, indicating a low level with no health risks. Overall, the results of the dietary exposure assessments suggest that REE dietary exposure risk for the general population is safe. However, due to limited toxicological data, most studies have focused on assessing total REEs, with relatively few evaluations of individual REEs, particularly heavy REEs. Strengthening single-element assessments is essential for a more precise understanding of the potential health risks. In addition, dietary exposure assessments of REEs in the Zhejiang Province remain limited, highlighting the need for further research in this region to ensure food safety and protect public health.
Zhejiang is a coastal province in eastern China with a well-developed economy, strong industrial base, extensive applications of new energy, and rare earth resource exploitation. It is a major tea-producing region; tea plays a significant role in the local culture, and its consumption is widespread. In addition, using rare earth fertilizers in agriculture, particularly in southern China, increases the risk of REE accumulation in crops, such as vegetables. Furthermore, aquaculture activities near industrial zones facilitate the transfer of REEs from water sediments to aquatic organisms. These combined factors increase the risk of REE contamination in food products such as tea, vegetables, fruits, yellow wine, and shrimp, which are commonly consumed by Zhejiang residents, highlighting the need for continuous monitoring and risk management.
Given the increasing global use of REEs and the insufficient data on human dietary intake of these elements, particularly in Zhejiang Province, we aimed to monitor REE contamination levels in five major food categories—tea, vegetables, fruits, yellow wine, and shrimp—and assess their dietary exposure risk. In previous studies, the assessment of single REEs in food was insufficient because of insufficient toxicological information. In this study, based on the existing toxicological limits of animal experiments, we conducted a dietary assessment of five REEs in five food products after conversion by uncertainty factors to obtain more accurate results of exposure to REEs and scientifically assess the potential effects of REEs on the health of the population.
4. Discussion
In this study, we investigated REE contamination in food products in Zhejiang Province and evaluated the dietary exposure of different age groups in relation to the food consumption of the population and the concentration of contaminants in food products. REE detection rates in food were notably high, with tea and shrimp exhibiting the highest concentrations among the tested food categories. The dietary exposure of the population was lower than the reference dose, and the exposure of children was slightly higher than that of the other populations.
REE detection rate varied across different food types, with tea showing the highest detection rate (100%), which is consistent with findings from studies in Shanghai [
32], Lu’an [
53], and Zunyi [
54]. The high enrichment of REEs in tea can be attributed to its strong absorption capacity, the use of dry tea in the analysis, and specific processing methods such as roasting and fermentation, which may further concentrate REEs. The REE detection rate in shrimp was 100%, which is consistent with the findings of Shaoxing [
55] and Guilin [
56] (100% detection of REEs in seafood). A study conducted in Zhuhai found that the REE detection rate in shrimp (100%) was higher than that in fish [
57]. The high REE content in shrimp may be attributed to its shallow water habitat, which is more susceptible to contamination [
58,
59]. Moreover, using REE-containing feed additives in aquaculture may further contribute to REE accumulation in shrimp [
60]. The detection rates in vegetables generally agreed with those reported by Wang et al. [
32] (83.3% detection of REEs in vegetables), except for aquatic vegetables, which showed lower detection rates. This could be due to the shallow root system of aquatic vegetables, which limits their ability to uptake REE. The detection levels of REEs in fruits were similar to those in vegetables, likely because fruits and vegetables are plant foods, and they have similar REE uptake mechanisms from the soil during growth [
61].
In this study, tea contained the highest REE levels. Tea was also found to contain higher levels of REEs (1050 µg/kg) than other foods (grains, vegetables, meat, aquatic products, eggs) in Shanghai. In this study, the concentration of REEs in Tieguanyin tea was significantly higher than the concentration in green and black teas. A survey of REEs in tea in China [
62] showed that oolong tea had the highest concentration of REEs (2488.7 µg/kg), followed by black tea (1548.6 µg/kg), with green tea having the lowest (1023.7 µg/kg), which is consistent with our findings. This variation can be primarily attributed to the differences in moisture content. Tea leaves undergo roasting, which reduces their moisture content, leading to a higher concentration of REEs in the dry matter. In contrast, vegetables and fruits have a higher moisture content, which dilutes REE concentrations, resulting in lower levels. In addition, the deep root systems of tea plants enable them to absorb more REEs from the soil [
63,
64]. In comparison, vegetables typically have shallow root systems [
65,
66] that limit REE uptake. In aquatic environments, water-grown vegetables have low REE concentrations due to the lower REE levels in water compared to soil and their shallow root system. However, aquatic organisms, such as shrimp may accumulate REEs by ingesting sediment, with calcium and chitin in their shells further enhancing REE retention [
58,
59]. The REEs in rice wine are relatively low and vary greatly, mainly due to the high water content of wine and the adsorption of rare earth on fermentation lees during processing [
67]. Overall, the differences in REE concentrations across food types can be explained by factors such as moisture content, root system structure, processing methods, and species-specific bioaccumulation characteristics.
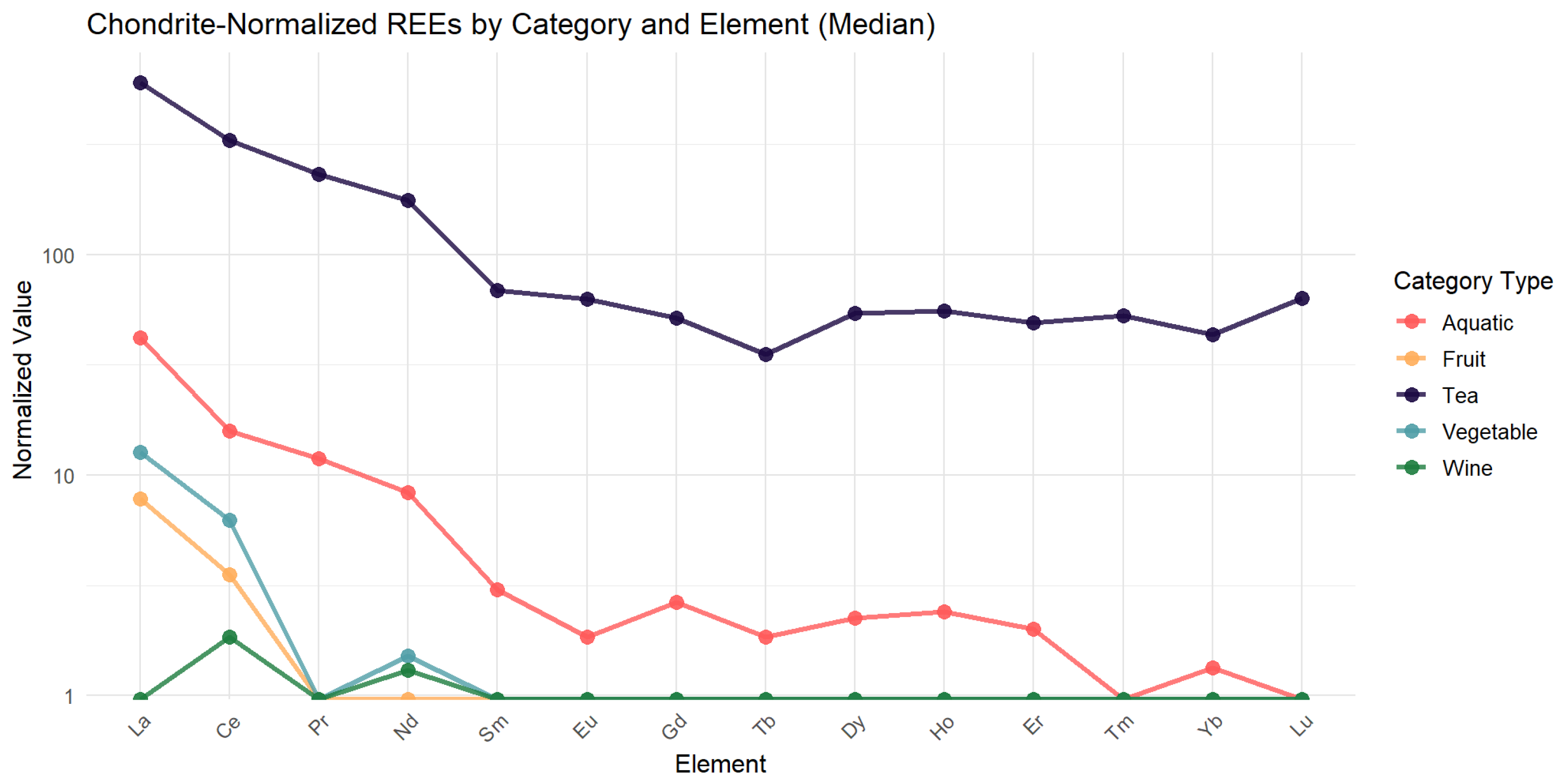
It is essential to consider the detection rates of specific elements and their natural abundance in the environment to further explore the distribution patterns of REEs. In our study, combined with the chondrite normalization curve and clustering heat map, we discovered that the REE with the highest detection rates in food were Ce, La, and Y (Ce > La > Y), followed by Nd, Sc, Pr, Gd, and Dy, and the detection rates of REEs were low. This pattern aligns with previous findings [
68] that reported REE* concentrations in food samples in the order La > Ce > Nd > Y. The higher detection rates of Ce, La, and Y are likely attributed to their natural abundance in the Earth’s crust, where they are present at concentrations of 31–66 µg/g, compared to much lower levels for elements, such as Tm and Lu (0.5–0.8 µg/g) [
69]. In the vast majority of foods, the LREE/HREE ratio is >2. The ratios of LREEs/HREEs for tea, vegetables, fruits, shrimp, and yellow wine were 2.7, 3.5, 3.9, 2.1, and 1.4, respectively, suggesting that fruits and vegetables have a high potential for LREE enrichment. The LREE/HREE ratio in Chinese soils ranged from 6.68 to 11.59, with an average of 8.89 [
70]. The LREE/HREE ratio in water sediments ranged from 6.93 to 11.97, with an average of 9.57 [
71]. The lower LREE/HREE ratio in food compared to soil and sediments suggests a relative enrichment of HREEs in certain food products. This accumulation raises concerns about bioaccumulation and potential toxicity, which may pose risks to food safety and human health.
At the single-element level, Ce was the most abundant REE in all food samples, followed by La, Y, and Nd, which differs from the results reported by Wang et al. [
72], who reported Ce to be the most abundant REE in marine wild fish. Y was more abundant than La in cephalopods and Sm was more abundant than La and Nd in milk, eggs, fish, and pork. In the present study, Ce and La had the highest REEs, but Yt exceeded Sm and Nd, which may explain the differences across food species. La, Ce, Nd, and Y have been identified as toxic after oral exposure, affecting several physiological systems. La is associated with learning and memory impairment [
73], abnormal calcium metabolism, and reduced bone mineral density [
74]. Ce exposure has been linked to placental dysfunction, fetal growth restriction, and anemia [
75]. Nd causes DNA damage, abnormal cardiovascular and cerebrovascular development [
76], and fetal neural tube defects [
77]. Y is associated with bone metabolism disorders, including decreased bone mineral density [
78].
Combining the pollutant concentration and consumption data, we discovered that the risk of REE exposure in the five types of food examined in Zhejiang Province was within the safe range. Dietary REEs have also been assessed in other regions of China. Dai [
79] found that total dietary concentrations of REEs averaged 64.95 µg/kg ww, and the health risk was considered to be acceptable in a study of marketed food products (including grains, vegetables, meat, dairy products, and aquatic products) from 33 cities in China. Zhao et al. [
68] studied representative food samples from the Bayan Ebo mining area in Inner Mongolia, China, and measured an average Estimated Daily Intake of 0.275 µg/kg/day for REEs, which is lower than the recommended daily intake (70 µg/kg/day), again indicating an acceptable risk. However, studies have shown that the influence of REEs is associated with decreased intelligence quotient and memory during neurodevelopment in children [
80]. Due to their lower body weight, children have a lower tolerance for pollutants. In this study, the EDI of children (aged ≤6) was higher than that of adults, further indicating a higher risk of REE exposure. In the Shandong [
27,
30] and Guangdong regions [
81], children were at a higher risk of ingesting food with REEs than other populations. Therefore, more attention should be paid to the neurological effects of sustained exposure to low REE levels in children.
In this study, the dietary exposure to five REEs in five food types was assessed based on existing toxicological limits. The limits causing impaired immunity in offspring were as follows: La (0.4277 mg/kg BW/day), Ce (0.086 mg/kg BW/day), Y (0.065 mg/kg BW/day) [
45,
46,
47]. The sub-chronic oral toxicity limits were as follows: Y (29 mg/kg/day) [
48]. The genotoxicity threshold for acute exposure was Nd (4.57 mg/kg/day) [
49]. The limit causing a significant decline in brain dopamine (DA), 5-hydroxytryptamine (5-HT), and NOS activity were Sm (22.36 mg/kg/day) [
50] and the limit of impairment to learning and memory was Sm (2.24 mg/kg/day) [
50]. Dietary assessments under different exposure scenarios demonstrated that under extreme conditions (95th percentile concentration and consumption), children aged ≤ 6 years exhibited an HI for Ce beyond 1, suggesting a potential risk of impaired immune function in offspring. However, this reference dose relies on studies in pregnant and lactating mice and might not be directly applicable to young children. For all other age groups, the HI values remained below the safety threshold across all scenarios. Therefore, at the single-element level, the intake of these five rare earth elements through the five assessed food categories does not represent a significant health risk. Among all age groups, children aged ≤ 6 years consistently exhibited the highest HI values, primarily due to their lower body weight, resulting in higher expo-sure per body mass unit. Furthermore, vegetables retained the highest HQ values for Y, Ce, Nd, and Sm, whereas fruits displayed the same for La. This pattern could be attributed to the concentration distributions observed across the five food categories; vegetables generally contained substantially higher Y, Ce, Nd, and Sm levels compared to fruits, while La concentrations were markedly increased in fruits relative to vegetables.
Our study found that tea contains high REE concentrations. Given the widespread tea consumption in Zhejiang Province, we assessed the intake required to reach the safety limit under different scenarios. Our findings showed a significant difference between the current level of tea consumption and the amount required to pose a risk. In Zhejiang Province, residents primarily consume tea in brewed form, which means that the actual intake of REEs is further reduced due to the leaching rate. Even at high levels of contamination and high levels of consumption (for people who consume tea directly), the daily tea intake is still well below the level that could threaten safety. Overall, REEs in tea and other foods were within acceptable limits for all age groups.
This study had some limitations. The analyzed food types were limited, and consumption data (2015–2017) and contaminant concentrations (2018–2019) were collected at different times, potentially affecting the exposure estimates. Moreover, the lack of established maximum allowable limits for individual REEs restricts the accuracy of the risk assessment.
Future research should investigate the long-term health effects of low-level REE exposure, particularly in children [
82], and elevated risks in mining-area populations [
83]. Assessments of individual REEs, especially HREEs, are needed to address their potential toxicity. Furthermore, comprehensive evaluations of dietary REE intake from food and drinking water remain insufficient, requiring systematic risk assessments [
84].