Simulated Gastrointestinal Digestion and In Vitro Fecal Fermentation of Purified Pyracantha fortuneana (Maxim.) Li Fruit Pectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
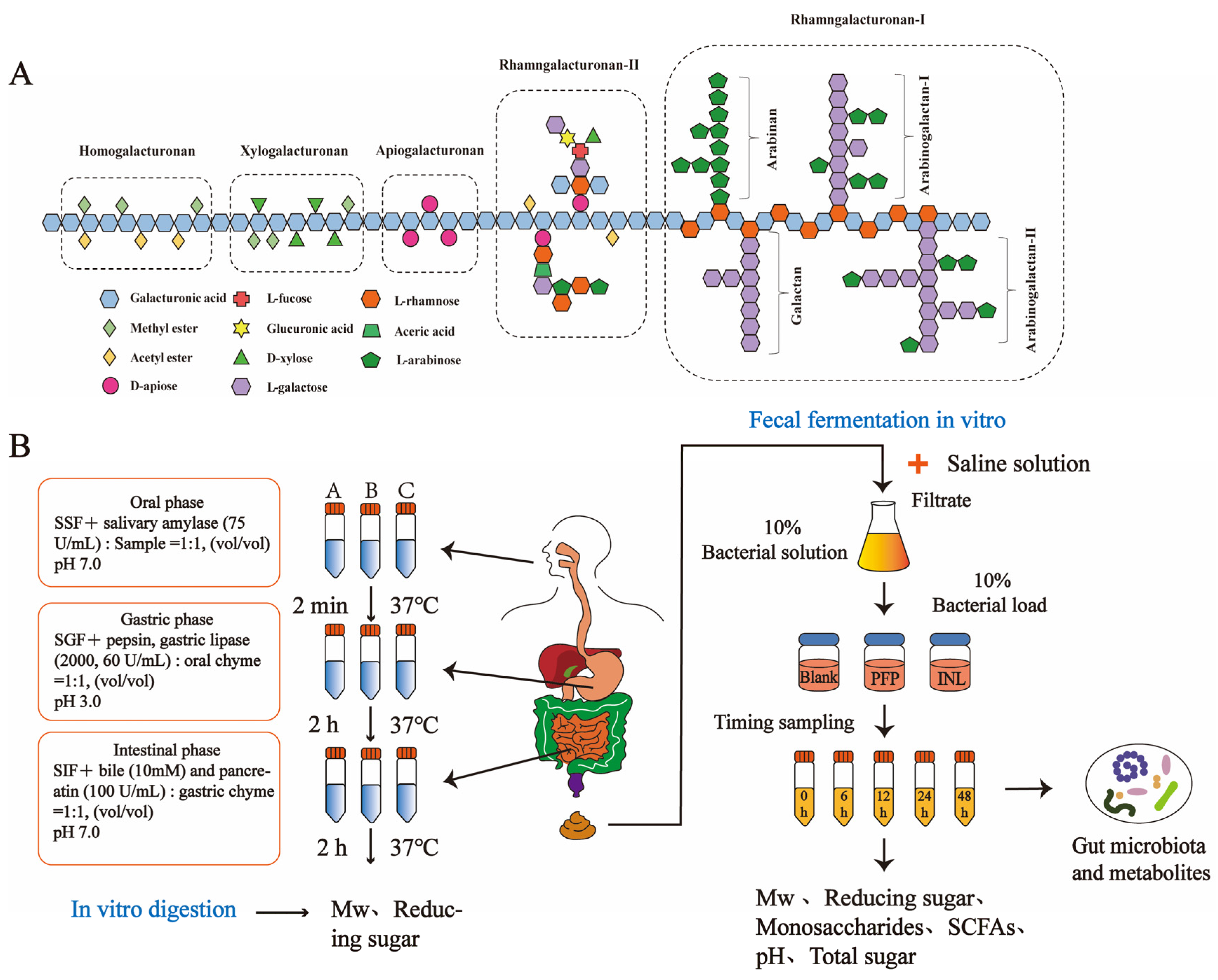
2.2. Extraction and Purification of PFP
2.3. Physicochemical Characterization of PFP
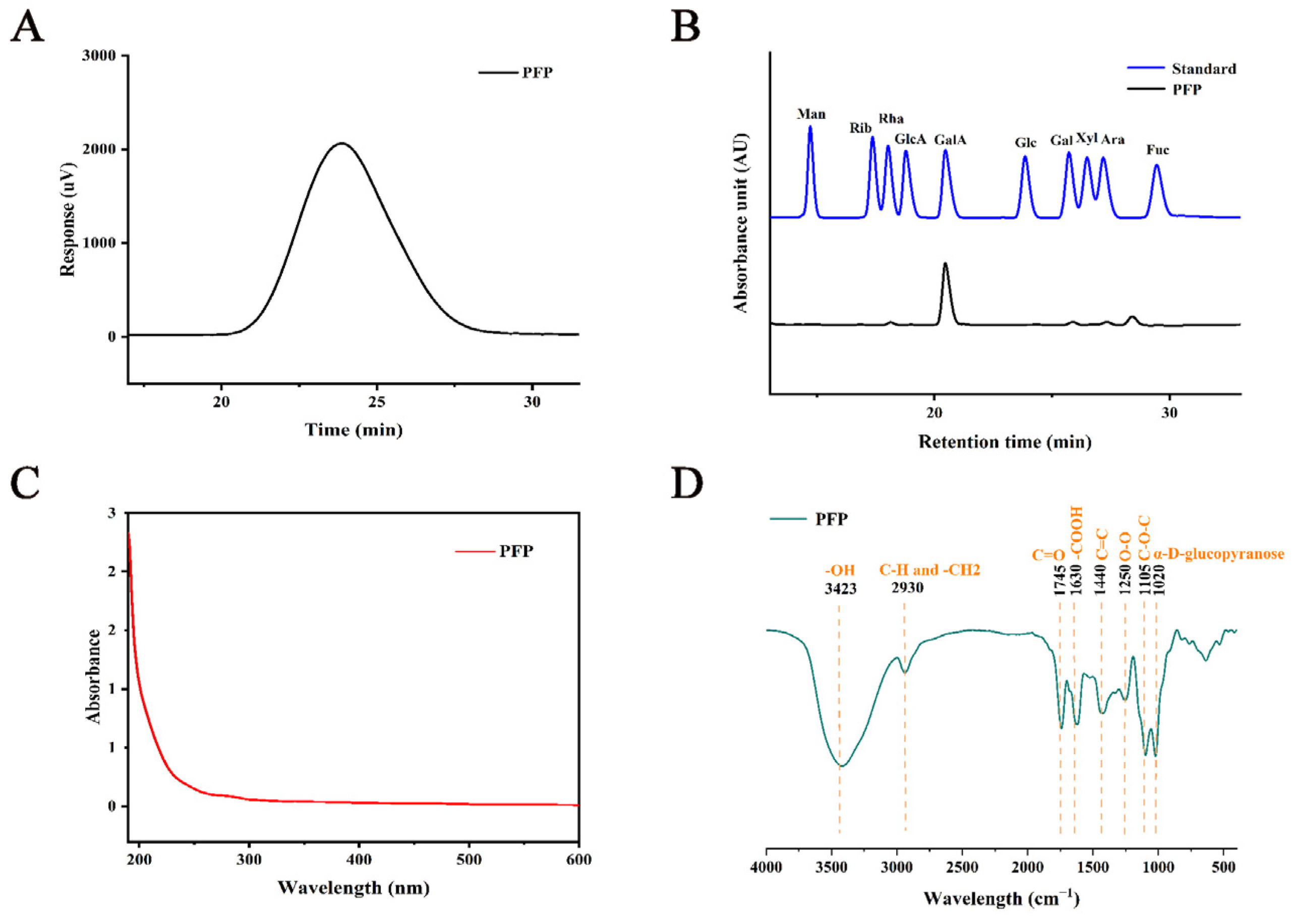
2.3.1. Composition of PFP
2.3.2. Fourier Transform Infrared (FT-IR) and Ultraviolet Spectroscopy (UV) Analysis of PFP
2.3.3. Determination of PFP Mw
2.3.4. Composition of Monosaccharide in PFP
2.4. In Vitro Digestion of PFP
2.4.1. In Vitro Saliva Digestion
2.4.2. In Vitro Gastric Digestion
2.4.3. In Vitro Small Intestinal Digestion
2.5. Determination of Physicochemical Properties of PFP During Digestion In Vitro
2.6. In Vitro Simulated Fecal Fermentation
2.7. Determination of Physical and Chemical Properties of PFP During In Vitro Fermentation
2.7.1. Changes in Basic Composition, Mw, and Monosaccharides
2.7.2. Determination of pH and SCFAs
2.7.3. Analysis of Gut Microbiota
2.7.4. Metabolomics Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties of PFP
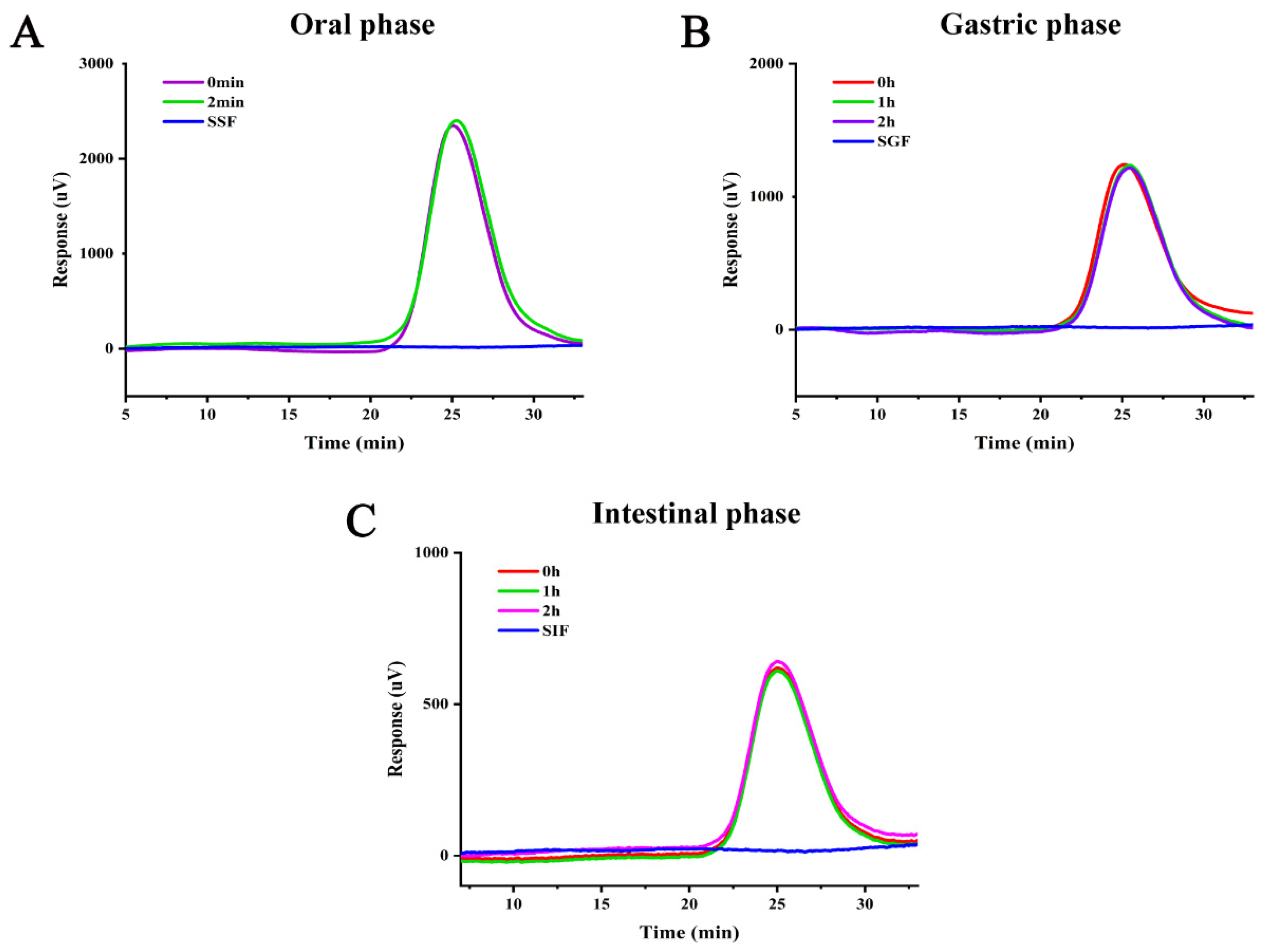
3.2. Simulated Digestive Characteristics of PFP In Vitro
3.2.1. Variations in Reducing Sugars
3.2.2. Mw Analysis
3.3. Characteristics of In Vitro Fermentation of PFP
3.3.1. Change of Mw
3.3.2. Carbohydrate Consumption During Fecal Fermentation
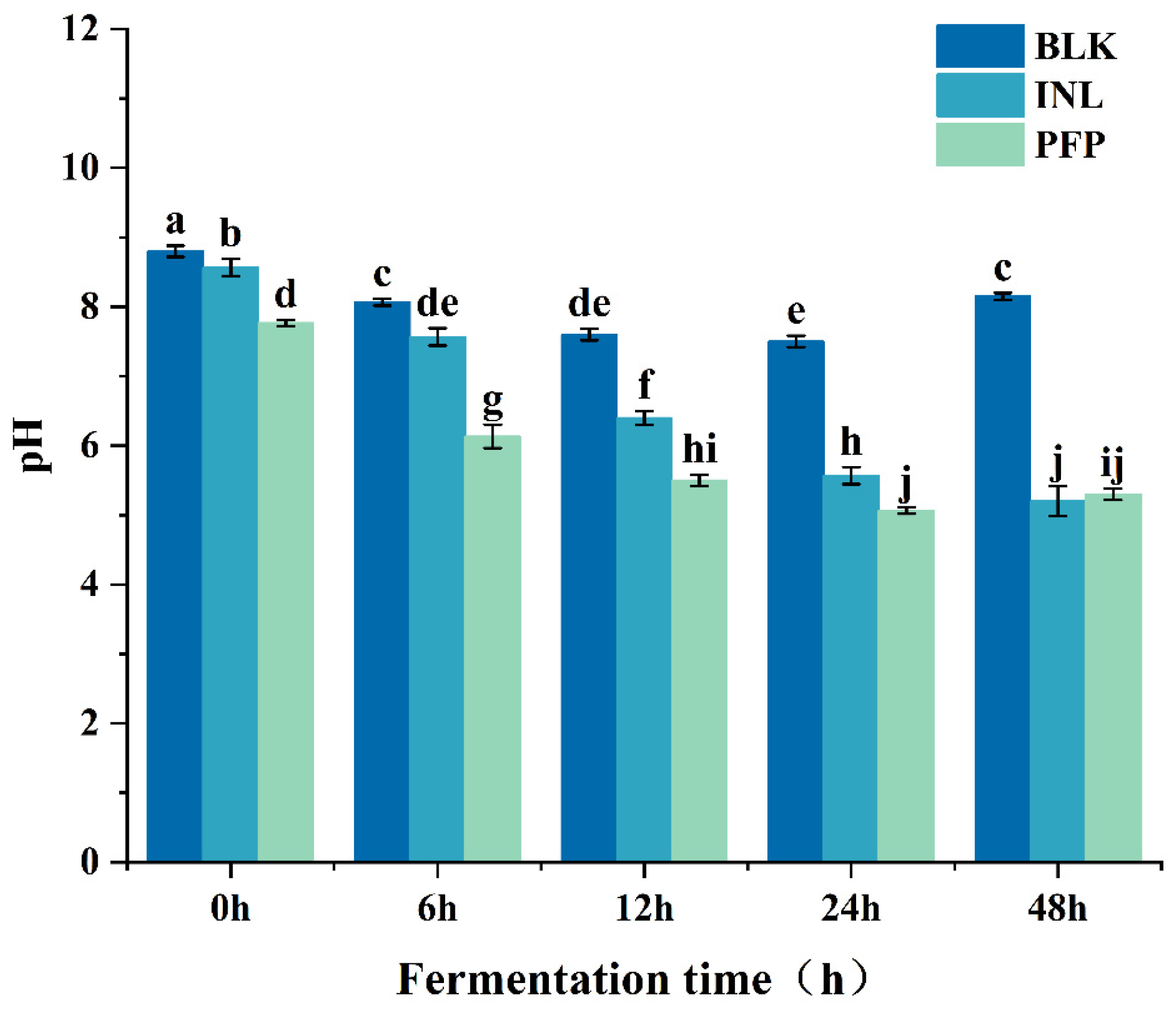
3.3.3. Change of pH and SCFAs
3.3.4. Effects of PFP Fermentation on Intestinal Microorganisms
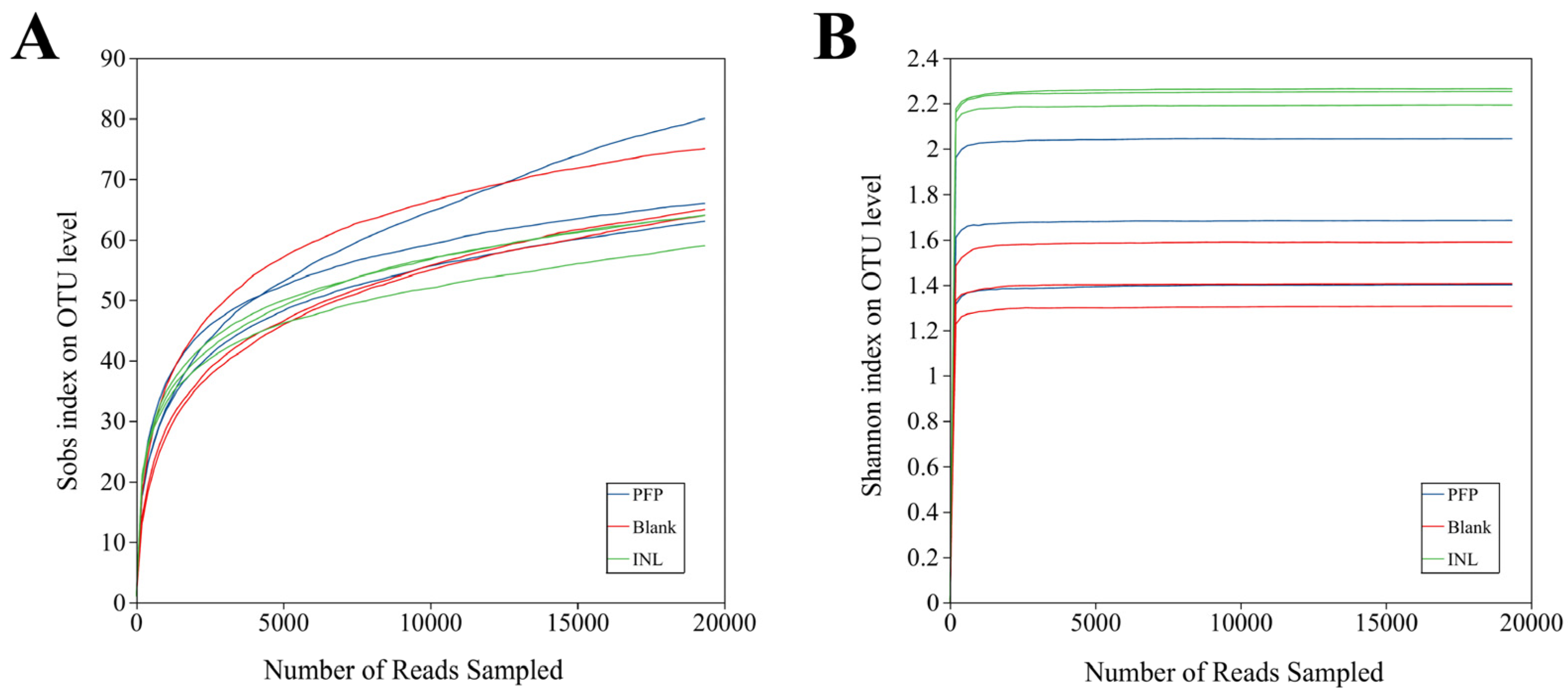
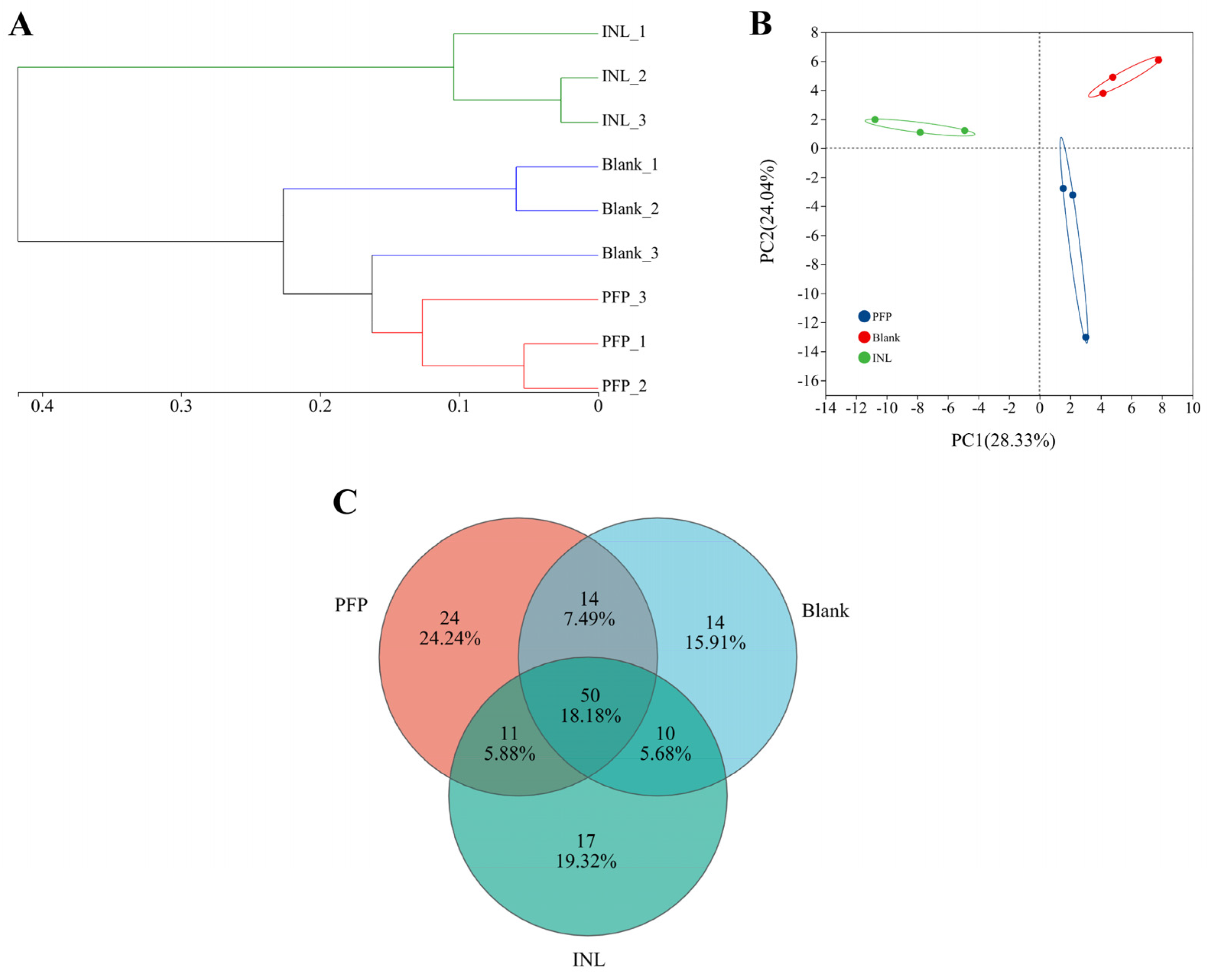
Microbial Diversity Analysis
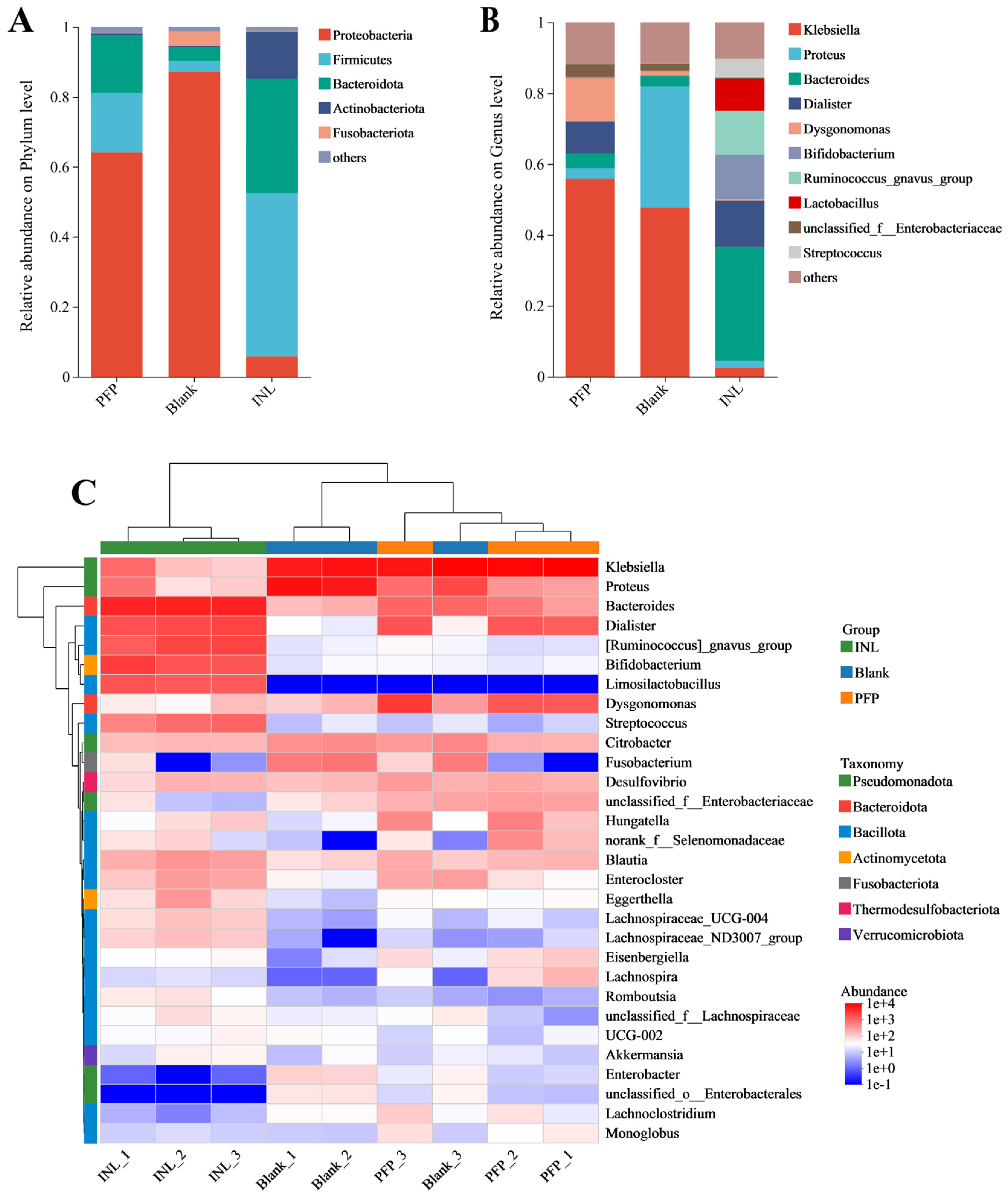
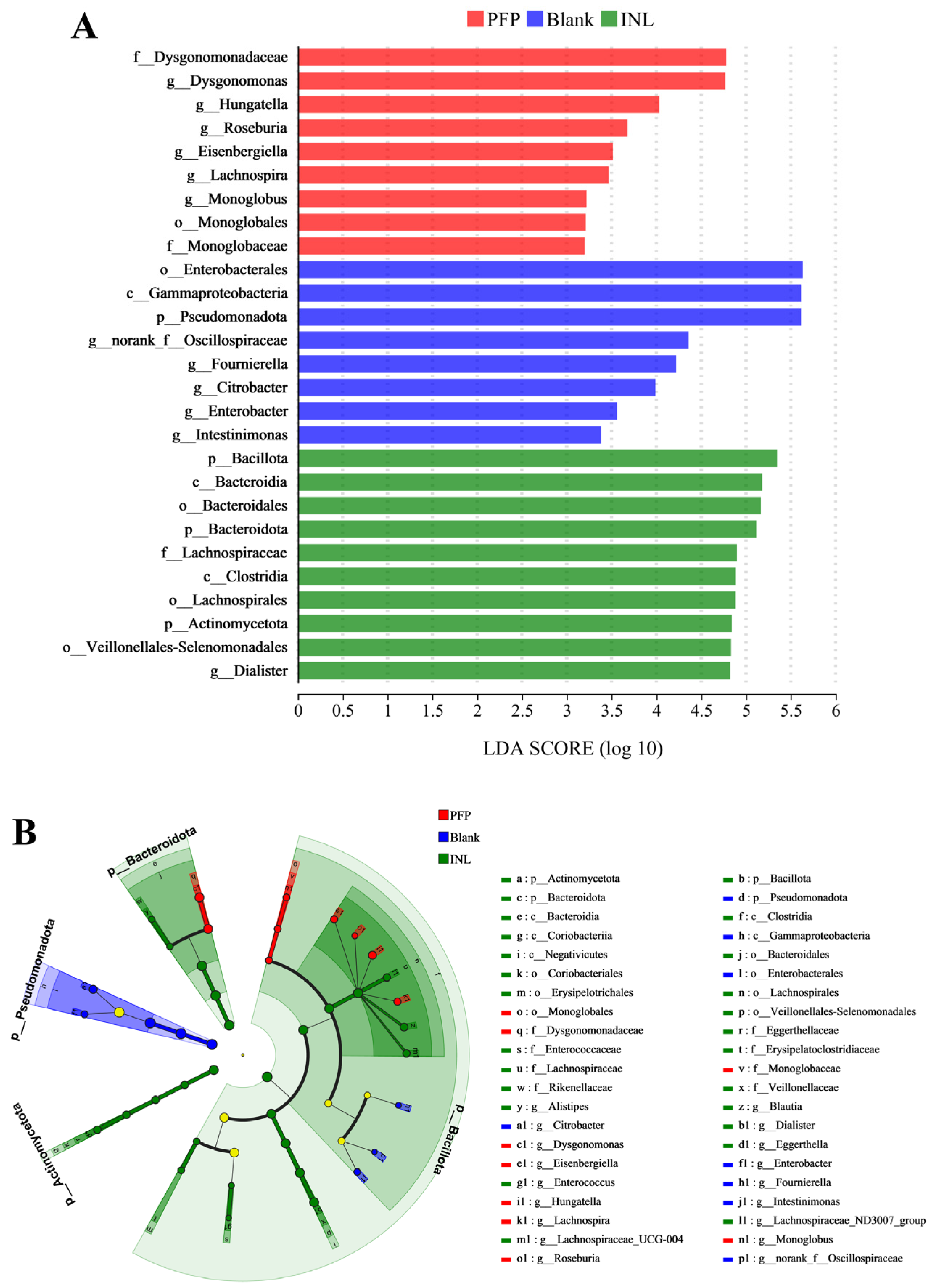
Effect of PFP on the Structure of the Gut Microbiota
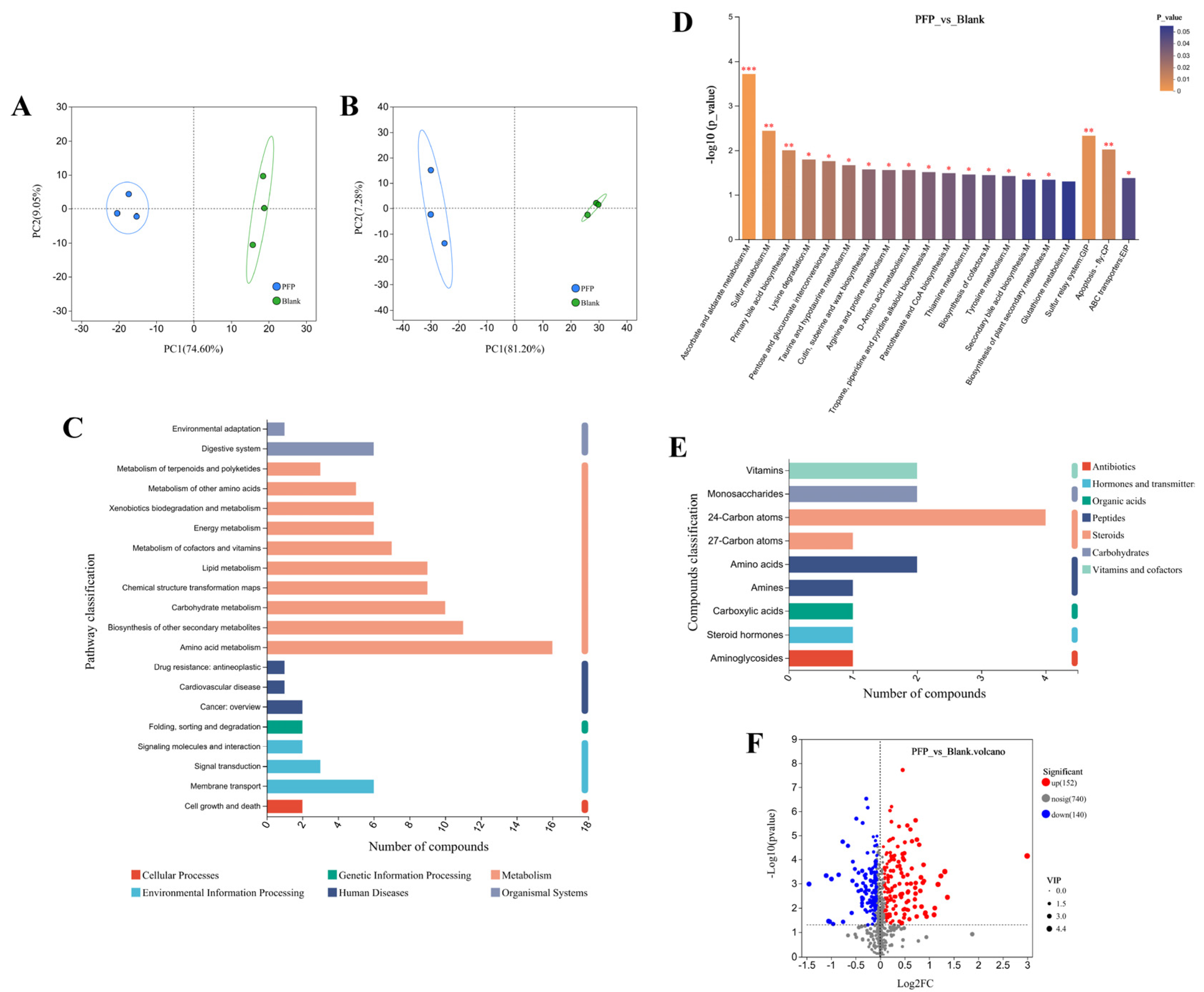
3.3.5. Microbial Metabolomics
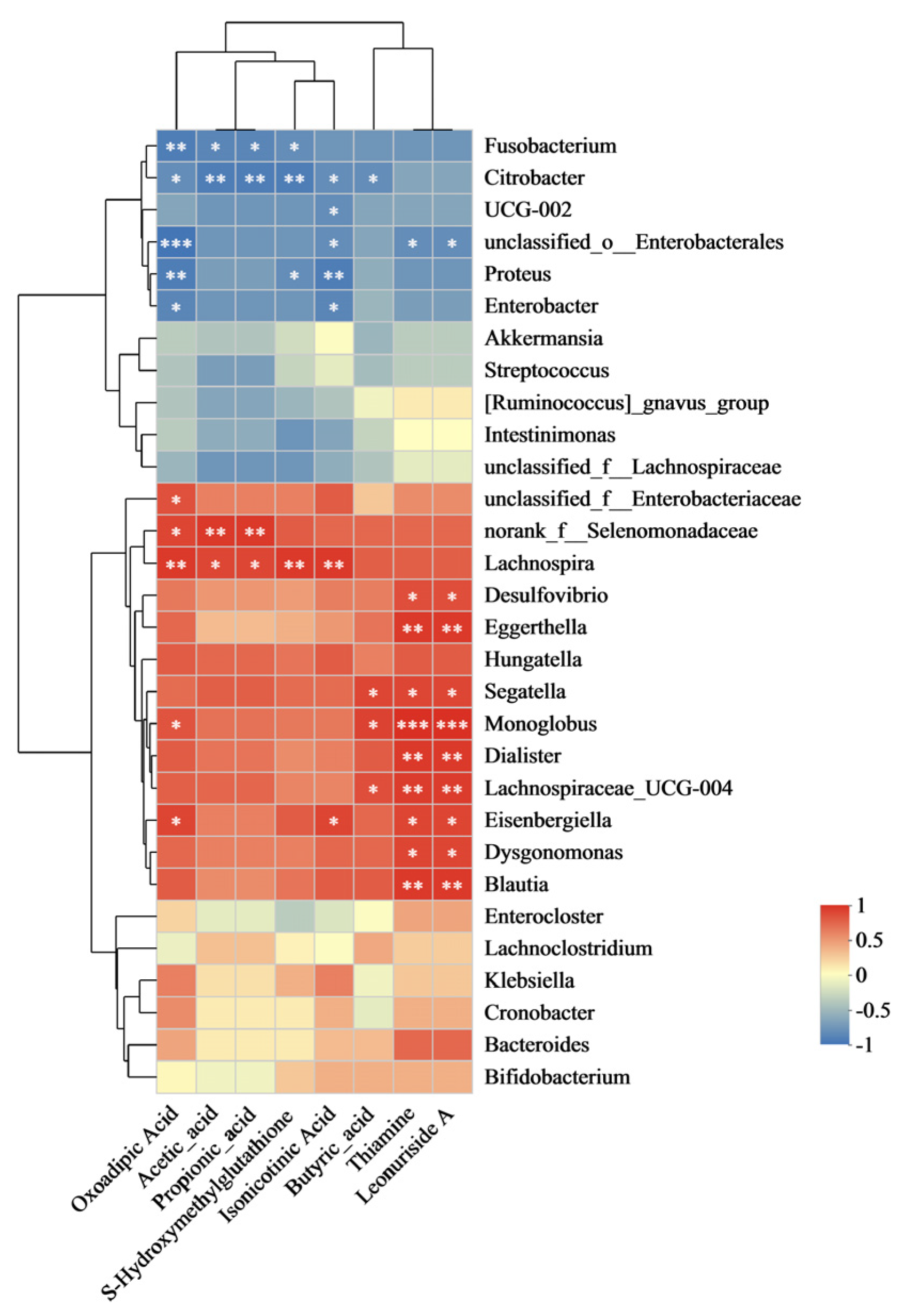
3.3.6. Correlation Between Microbiota and Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ara | Arabinose | Mw | Molecular weight |
| AGA | Apiogalacturonan | PFP | Pyracantha fortuneana polysaccharide |
| CAZymes | Carbohydrate active enzymes | PMP | 1-pheny-3-methyl-5-pyrazolone |
| DEAE | DEAE cellulose DE-23 | Rib | Ribose |
| FT-IR | Fourier transform infrared | Rha | Rhamnose |
| Fuc | Fucose | RG-I | Rhamnogalacturonan I |
| Glc | Glucose | RG-II | Rhamnogalacturonan II |
| GlcA | Glucuronic acid | ROS | Reactive oxygen species |
| Gal | Galactose | SSF | Simulated salivary fluid |
| GalA | Galacturonic acid | SGF | Simulated gastric fluid |
| SUS | Sugar utilization system | SIF | Simulated intestinal fluid |
| QSAR | Quantitative structure–activity relationship | LC-MS | Liquid chromatography–mass spectrometry |
| HPLC | High performance liquid chromatography | HPGPC | High-performance gel permeation chromatography |
| HG | Homogalacturonan | UV | Ultraviolet–Visible |
| IBD | Inflammatory bowel disease | Xyl | Xylose |
| INL | inulin | XGA | Xylogalacturonan |
| LDA | Linear discriminant analysis | SCFAs | Short-chain fatty acids |
| KEGG | Kyoto Encyclopedia of Genes and Genomes | HMDB | Human Metabolome Database |
References
- Xu, H.; Zhao, C.F.; Tian, G.G.; Qian, L.R.; Yu, L.J. Characterization of active constituents in Pyracantha fortuneana fruit extract and their effects on hyperlipidaemia, obesity, and oxidative stress in rodents. J. Funct. Food. 2016, 22, 278–290. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Zhang, Q.; Liu, J.; Tao, T.; Zhang, T.; Wu, C.; Ren, Q.; Pu, X.; Peng, W. Pyracantha fortuneana (Maxim.) Li: A comprehensive review of its phytochemistry, pharmacological properties, and product development. Front. Sustain. Food Syst. 2022, 6, 940900. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, C.; Bu, Y.; Xiang, T.; Huang, X.; Wang, Z.; Yi, F.; Ren, G.; Liu, G.; Song, F. Antioxidative and immunoprotective effects of Pyracantha fortuneana (Maxim.) Li polysaccharides in mice. Immunol. Lett. 2010, 133, 14–18. [Google Scholar] [CrossRef]
- Yao, Y.; Shu, C.; Feng, G.; Wang, Q.; Yan, Y.; Yi, Y.; Wang, H.; Zhang, X.; Wang, L. Polysaccharides from Pyracantha fortuneana and its biological activity. Int. J. Biol. Macromol. 2020, 150, 1162–1174. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, L.; Qiu, M.; Li, L.; Qiu, S.; Liu, Y.; Wei, C. Physicochemical properties, structural characteristics and bioactivities of Pyracantha fortuneana polysaccharides prepared by six methods. Ind. Crop. Prod. 2024, 208, 117933. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ye, X.; Linhardt, R.J.; Liu, X.; Zhu, K.; Yu, C.; Ding, T.; Liu, D.; He, Q.; Chen, S. Dietary pectic substances enhance gut health by its polycomponent: A review. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 2015–2039. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Yeung, Y.K.; Kang, Y.; So, B.R.; Jung, S.K.; Chang, Y.H. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. 2021, 118, 106779. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Yi, J.; Wu, X.; Ma, Y.; Li, R. Pectin and homogalacturonan with small molecular mass modulate microbial community and generate high SCFAs via in vitro gut fermentation. Carbohydr. Polym. 2021, 269, 118326. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, Z.; Zhao, M.; Liu, P. Purification, Characterization, and Antioxidant Activity of Polysaccharides Isolated from Cortex Periplocae. Molecules 2017, 22, 1866. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.; Liu, W.; Su, Y.; Han, Q.; Zhao, L.; Zhang, Q.; Lin, D. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; He, L.; Cheng, J.; Li, J.; Tao, W.; Mao, G.; Zhang, H.; Linhardt, R.J.; Ye, X. Structural characterization and anti-proliferative activities of partially degraded polysaccharides from peach gum. Carbohydr. Polym. 2019, 203, 193–202. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Fang, C.; Chen, G.; Kan, J. Characterization and in vitro simulated gastrointestinal digestion and fermentation of Mentha haplocalyx polysaccharide. Int. J. Biol. Macromol. 2022, 222, 360–372. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Nie, X.; Gan, R.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Ye, K.; Fu, C.; Ma, S.; Du, H.; Chen, S.; Liu, D.; Ma, G.; Xiao, H. Comprehensive assessment of Hypsizygus marmoreus polysaccharides through simulated digestion and gut microbiota fermentation in vitro. Food Hydrocoll. 2023, 144, 108989. [Google Scholar] [CrossRef]
- Wei, C.; Liao, N.; Zhang, Y.; Ye, X.; Li, S.; Hu, Y.; Liu, D.; Linhardt, R.J.; Wang, X.; Chen, S. In vitro fermentation behaviors of fucosylated chondroitin sulfate from Pearsonothuria graeffei by human gut microflora. Int. J. Biol. Macromol. 2017, 102, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, W.; Lai, X.; Dai, H.; Sun, H.; Zhou, X.; Chen, T. Metabolic responses and their correlations with phytochelatins in Amaranthus hypochondriacus under cadmium stress. Environ. Pollut. 2019, 252, 1791–1800. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2020, 150, 1011–1019. [Google Scholar] [CrossRef]
- Vasilieva, T.; Sigarev, A.; Kosyakov, D.; Ul Yanovskii, N.; Anikeenko, E.; Chuhchin, D.; Ladesov, A.; Hein, A.M.; Miasnikov, V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017, 163, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wu, Y.; Liu, Y.; Lv, S.; You, Y.; Zhou, Z.; Chen, X.; Li, H. Structure and hypoglycemic effect of a neutral polysaccharide isolated from sea cucumber Stichopus japonicus. Int. J. Biol. Macromol. 2022, 216, 14–23. [Google Scholar] [CrossRef]
- Holloway, W.D.; Tasman-Jones, C.; Maher, K. Pectin digestion in humans. Am. J. Clin. Nutr. 1983, 37, 253–255. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; He, Y.; Xiang, P.; Wang, S.; Cao, Z.; Gou, T.; Shen, M.; Zhao, L.; Qin, W.; Gan, R.; et al. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of okra polysaccharides. Carbohydr. Polym. 2020, 238, 116183. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yao, L.; Zhang, L.; Zhang, Y.; Luo, Q.; Qiu, S.; Zeng, X.; Chen, S.; Ye, X. In Vitro Digestion and Fecal Fermentation of Peach Gum Polysaccharides with Different Molecular Weights and Their Impacts on Gut Microbiota. Foods 2022, 11, 3970. [Google Scholar] [CrossRef]
- Fu, C.; Ye, K.; Ma, S.; Du, H.; Chen, S.; Liu, D.; Ma, G.; Xiao, H. Simulated gastrointestinal digestion and gut microbiota fermentation of polysaccharides from Agaricus bisporus. Food Chem. 2023, 418, 135849. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Zhao, X.; Engelsen, S.B.; Wu, X.; Ma, Y.; Guo, Y.; Du, Q.; Yi, J. New insight into pectic fractions of cell wall: Impact of extraction on pectin structure and in vitro gut fermentation. Int. J. Biol. Macromol. 2023, 253, 127515. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Li, C.; Xie, M. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocoll. 2013, 33, 384–392. [Google Scholar] [CrossRef]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, T.; Smidt, H.; Scheurink, A.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Cheng, Y.; Guo, Y.; Hu, B.; Yao, W.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, Y.; Zhang, R.; Chen, Y.; Wang, F.; Zhang, M. Gut microbial fermentation promotes the intestinal anti-inflammatory activity of Chinese yam polysaccharides. Food Chem. 2023, 402, 134003. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrné, S.; Nyman, M. High-Fat Diet Reduces the Formation of Butyrate, but Increases Succinate, Inflammation, Liver Fat and Cholesterol in Rats, while Dietary Fibre Counteracts These Effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef]
- Tan, J.; Mckenzie, C.; Potamitis, M.; Thorburn, A.; Mackay, C.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, S.S.; Garcez, F.B.; Alencar, J.C.G.; Bastos, A.A.; Morley, J.E.; Cederholm, T.; Aprahamian, I.; de Souza, H.P.; Avelino-Silva, T.J.; Bindels, L.B.; et al. Gut microbiota disturbances in hospitalized older adults with malnutrition and clinical outcomes. Nutrition 2024, 122, 112369. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, X.; Tu, T.; Wang, L.; Mao, Y.; Luo, Y.; Qiu, S.; Song, A. Characterization and prebiotic potential of polysaccharides from Rosa roxburghii Tratt pomace by ultrasound-assisted extraction. Int. J. Biol. Macromol. 2024, 268, 131910. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Zhu, J.; Zhang, S.; Chen, S.; Liu, X.; Li, L.; Yan, J. Advances and challenges in interaction between heteroglycans and Bifidobacterium: Utilization strategies, intestinal health and future perspectives. Trends Food Sci. Technol. 2023, 134, 112–122. [Google Scholar] [CrossRef]
- Kmezik, C.; Mazurkewich, S.; Meents, T.; McKee, L.S.; Idström, A.; Armeni, M.; Savolainen, O.; Brändén, G.; Larsbrink, J. A polysaccharide utilization locus from the gut bacterium Dysgonomonas mossii encodes functionally distinct carbohydrate esterases. J. Biol. Chem. 2021, 296, 100500. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, L.; Liu, X.; Zhao, L.; Li, L.; Jin, M.; Yu, X.; Liu, F.; Li, Y.; Li, L.; et al. In vitro simulated digestion and fermentation characteristics of pectic polysaccharides from fresh passion fruit (Passiflora edulis f. flavicarpa L.) peel. Food Chem. 2024, 452, 139606. [Google Scholar] [CrossRef] [PubMed]
- Gamage, H.K.A.H.; Tetu, S.G.; Chong, R.W.W.; Ashton, J.; Packer, N.H.; Paulsen, I.T. Cereal products derived from wheat, sorghum, rice and oats alter the infant gut microbiota in vitro. Sci Rep. 2017, 7, 14312. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Xia, C.; Xu, X.; Zhang, R.; Su, D.; Jia, X.; Deng, M.; Lee, Y.; Zhang, M.; Huang, F. Effects of molecular weight on simulated digestion and fecal fermentation of polysaccharides from longan pulp in vitro. Int. J. Biol. Macromol. 2025, 306, 141711. [Google Scholar] [CrossRef]
- Rawat, P.S.; Li, Y.; Zhang, W.X.; Meng, X.F.; Liu, W.F. Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity. Appl. Environ. Microbiol. 2022, 88, e01546-22. [Google Scholar] [CrossRef]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; Scott, K.P.; Flint, H.J. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X.; et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef]
- Sun, T.; Liang, X.; Xu, X.; Wang, L.; Xiao, W.; Ma, Y.; Wang, R.; Gu, Y.; Li, S.; Qiu, Y.; et al. In vitro digestion and fecal fermentation of basidiospore-derived exopolysaccharides from Naematelia aurantialba. Int. J. Biol. Macromol. 2024, 261, 129756. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Yao, A.; Cui, W.; Wei, Y.; Guo, S.; Duan, J.; Kang, H.; Zhou, X.; Su, S.; et al. In vitro fermentation characteristics and interaction of neutral and acidic polysaccharides from Lycii fructus on human gut microbiota. Food Hydrocoll. 2024, 152, 109940. [Google Scholar] [CrossRef]
- Kim, C.C.; Kelly, W.J.; Patchett, M.L.; Tannock, G.W.; Jordens, Z.; Stoklosinski, H.M.; Taylor, J.W.; Sims, I.M.; Bell, T.J.; Rosendale, D.I. Monoglobus pectinilyticus gen. nov., sp nov., a pectinolytic bacterium isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2017, 67, 4992–4998. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Zhang, X.; Han, L.; Yin, S.; Peng, Q.; Wang, M. Microbiome-metabolomic analysis of the impact of Zizyphus jujuba cv. Muzao polysaccharides consumption on colorectal cancer mice fecal microbiota and metabolites. Int. J. Biol. Macromol. 2019, 131, 1067–1076. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Q.; Jia, L.; He, H.; Zhang, T.; Jia, W.; Zhu, L.; Qi, W.; Wang, N. Effects of in vitro fermentation of Atractylodes chinensis (DC.) Koidz. polysaccharide on fecal microbiota and metabolites in patients with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2023, 253, 126860. [Google Scholar] [CrossRef]
- Hong, Y.; Li, B.B.; Zheng, N.N.; Wu, G.S.; Ma, J.L.; Tao, X.; Chen, L.L.; Zhong, J.; Sheng, L.L.; Li, H.K. Integrated Metagenomic and Metabolomic Analyses of the Effect of Astragalus Polysaccharides on Alleviating High-Fat Diet-Induced Metabolic Disorders. Front. Pharmacol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, Y.; Ji, J.; Zhang, Y.; Feng, Y.; Sun, X. Polysaccharides extracted from Polygonatum sibiricum alleviate intestine-liver-kidney axis injury induced by citrinin and alcohol co-exposure in mice. Food Chem. Toxicol. 2025, 197, 115314. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Song, J.; Chen, Z.; Zhou, D.; Kong, Y.; Liu, Y.; Zhang, Y.; Bai, H.; Ding, Z. Dietary thiamine enhances thiamine transport, carbohydrate absorption, glycolysis, and antioxidant properties in Macrobrachium nipponense when fed a high-carbohydrate diet. Aquacult. Rep. 2024, 39, 102400. [Google Scholar] [CrossRef]
- Das, P.R.; Darwish, A.G.; Ismail, A.; Haikal, A.M.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Soliman, K.F.A.; Sherif, S.M.; et al. Diversity in blueberry genotypes and developmental stages enables discrepancy in the bioactive compounds, metabolites, and cytotoxicity. Food Chem. 2022, 374, 131632. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.; Huang, Z.; Zhang, X.; Rao, Z.; Xu, M. The maintenance of redox homeostasis to regulate efficient glutathione metabolism in Corynebacterium glutamicum. Chem. Eng. J. 2025, 506, 160237. [Google Scholar] [CrossRef]
- Sugaya, K.; Hashimoto, F.; Ono, M.; Ito, Y.; Masuoka, C.; Nohara, T. Anti-Oxidative Constituents from Leonurii Herba (Leonurus japonicus). Food Sci. Technol. Int. 1998, 4, 278–281. [Google Scholar] [CrossRef]
- Nie, H.; Huang, S.; Li, X.; Gong, J.; Wu, F.; Yin, J.; Liao, Y.; Wu, S.; Luo, Y. Identification of compounds from chufa (Eleocharis dulcis) peels with inhibitory acrylamide formation activity. Rev. Bras. Farmacogn. 2019, 29, 483–487. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Isonicotinic acid hydrazide derivatives: Synthesis, antimicrobial activity, and QSAR studies. Med. Chem. Res. 2012, 21, 1451–1470. [Google Scholar] [CrossRef]

| PFP | Data |
|---|---|
| Mw (Da) | 8.51 × 105 ± 0.19 |
| DM (%) | 69.93 ± 0.17 |
| Chemical composition (%) | |
| Total soluble sugars | 95.69 ± 1.9 |
| Uronic acid | 70.04 ± 1.75 |
| Monosaccharide composition (%) | |
| Rha | 3.59 ± 0.06 d |
| GalA | 82.53 ± 0.24 a |
| Glc | 1.99 ± 0.10 e |
| Gal | 4.89 ± 0.08 c |
| Ara | 7.00 ± 0.01 b |
| Process | Time | Reducing Sugar (mg/mL) |
|---|---|---|
| Saliva digestion | 0 min | 0.0659 ± 0.0017 b |
| 2 min | 0.0679 ± 0.0010 b | |
| Gastric juice digestion | 0 h | 0.0859 ± 0.0004 a |
| 1 h | 0.0870 ± 0.0007 a | |
| 2 h | 0.0870 ± 0.0001 a | |
| Small intestinal digestion | 0 h | 0.0463 ± 0.0004 c |
| 1 h | 0.0475 ± 0.0011 c | |
| 2 h | 0.0492 ± 0.0008 c |
| Fermentation Time (h) | Remaining Total Soluble Sugar (% Initial) | Residual Uronic Acid (% Initial) | Reducing Sugar (mg/mL) | |
|---|---|---|---|---|
| PFP | INL | |||
| 0 | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 0.24 ± 0.001 e |
| 6 | 80.91 ± 0.58 b | 95.67 ± 1.74 b | 77.59 ± 1.32 b | 0.29 ± 0.002 c |
| 12 | 80.16 ± 0.56 c | 88.04 ± 1.51 c | 69.23 ± 2.15 c | 0.33 ± 0.002 b |
| 24 | 59.46 ± 0.49 d | 53.57 ± 0.85 d | 62.91 ± 0.39 d | 0.36 ± 0.001 a |
| 48 | 26.47 ± 0.13 e | 22.79 ± 0.45 e | 48.07 ± 1.2 e | 0.27 ± 0 d |
| Sample | Acetic Acid (mmol/L) | Propionic Acid (mmol/L) | Isobutyric Acid (mmol/L) | Butyric Acid (mmol/L) | Isovaleric Acid (mmol/L) | Valeric Acid (mmol/L) | Total SCFAs (mmol/L) |
|---|---|---|---|---|---|---|---|
| Blank | 8.12 ± 1.15 aD | 4.16 ± 0.48 bF | 0.98 ± 0.03 cH | 1.38 ± 0.22 cH | 0.81 ± 0.08 cH | 0.78 ± 0.05 cH | 16.23 |
| INL | 14.29 ± 0.41 aB | 6.50 ± 0.42 cE | 0.91 ± 0.00 dH | 8.93 ± 0.66 bC | 0.66 ± 0.02 dH | 0.78 ± 0.01 dH | 32.07 |
| PFP | 29.18 ± 0.64 aA | 6.92 ± 0.11 bE | 1.00 ± 0.01 dH | 3.24 ± 0.08 cG | 0.87 ± 0.02 dH | 1.23 ± 0.02 dH | 42.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Lv, Y.; Yuan, X.; Huang, G.; Guo, Z.; Tan, J.; Qiu, S.; Wang, X.; Wei, C. Simulated Gastrointestinal Digestion and In Vitro Fecal Fermentation of Purified Pyracantha fortuneana (Maxim.) Li Fruit Pectin. Foods 2025, 14, 1529. https://doi.org/10.3390/foods14091529
Xu Q, Lv Y, Yuan X, Huang G, Guo Z, Tan J, Qiu S, Wang X, Wei C. Simulated Gastrointestinal Digestion and In Vitro Fecal Fermentation of Purified Pyracantha fortuneana (Maxim.) Li Fruit Pectin. Foods. 2025; 14(9):1529. https://doi.org/10.3390/foods14091529
Chicago/Turabian StyleXu, Qingrui, Yiyi Lv, Xiaohui Yuan, Guichun Huang, Zhongxia Guo, Jiana Tan, Shuyi Qiu, Xiaodan Wang, and Chaoyang Wei. 2025. "Simulated Gastrointestinal Digestion and In Vitro Fecal Fermentation of Purified Pyracantha fortuneana (Maxim.) Li Fruit Pectin" Foods 14, no. 9: 1529. https://doi.org/10.3390/foods14091529
APA StyleXu, Q., Lv, Y., Yuan, X., Huang, G., Guo, Z., Tan, J., Qiu, S., Wang, X., & Wei, C. (2025). Simulated Gastrointestinal Digestion and In Vitro Fecal Fermentation of Purified Pyracantha fortuneana (Maxim.) Li Fruit Pectin. Foods, 14(9), 1529. https://doi.org/10.3390/foods14091529





