Bioprocessing of Sargassum fusiforme via Lactobacillus Fermentation: Effects on Nutrient Composition, Organoleptic Properties, and In Vitro Antioxidant and Hypoglycemic Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of S. fusiforme Fermentation Supernatants
2.3. Assessment of pH Levels and Total Acidity
2.4. Determination of Total Phenolic Contents
2.5. Determination of Total Flavonoid Contents
2.6. Determination of Short-Chain Fatty Acids
2.7. Examination of the Volatile Components of S. fusiforme Supernatants
2.8. Assessment of the Antioxidant Activity of Fermented S. fusiforme
2.8.1. 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging
2.8.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid Free Radical Scavenging
2.8.3. Ferric Ion-Reducing Antioxidant Power
2.9. Enzyme Inhibition Assays
2.9.1. α-Amylase
2.9.2. α-Glucosidase
2.10. Statistical Analysis
3. Results and Discussion
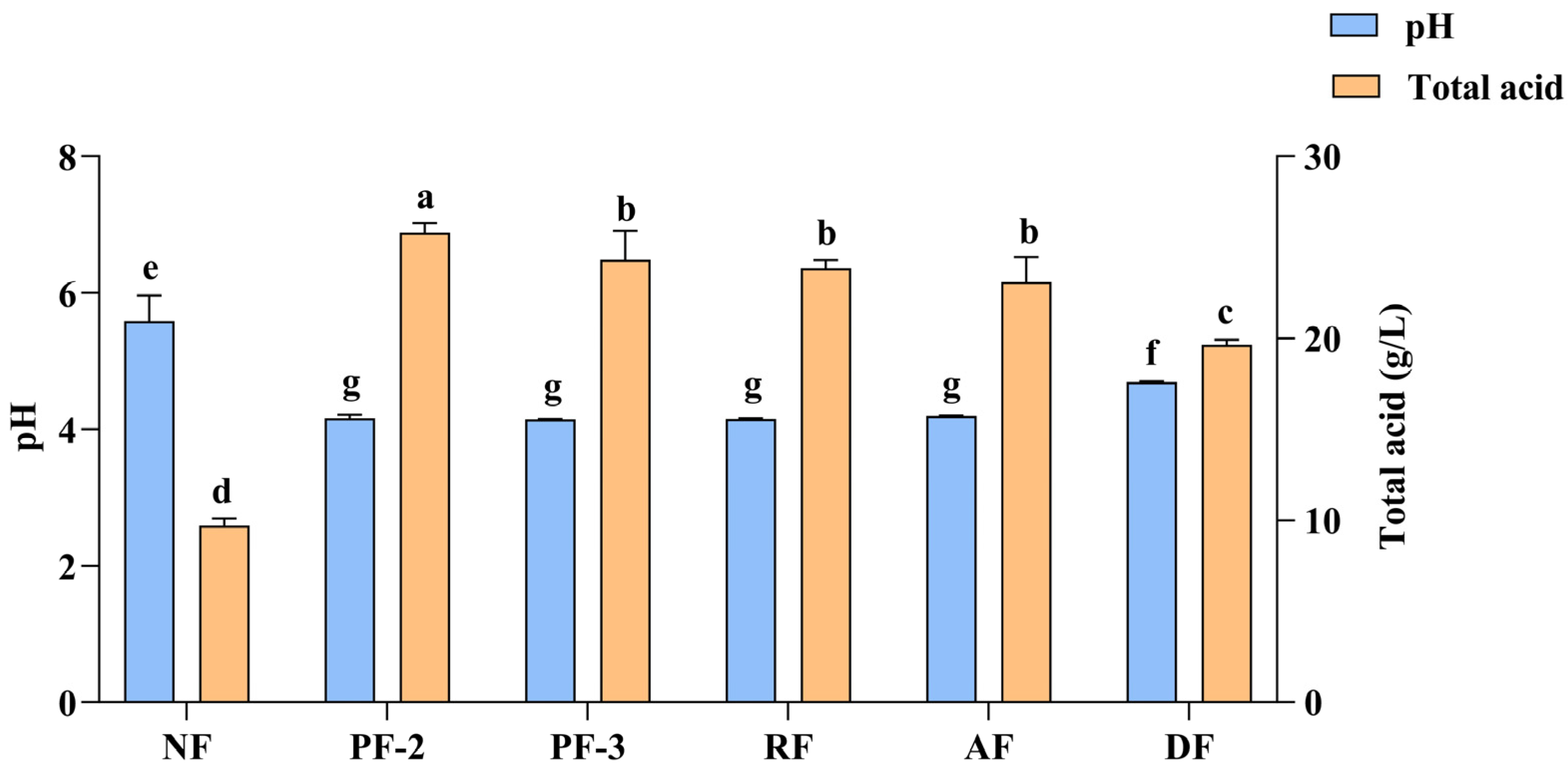
3.1. Changes in Lactobacillus Fermentation Based on the pH and Total Acid Content of S. fusiforme Supernatants
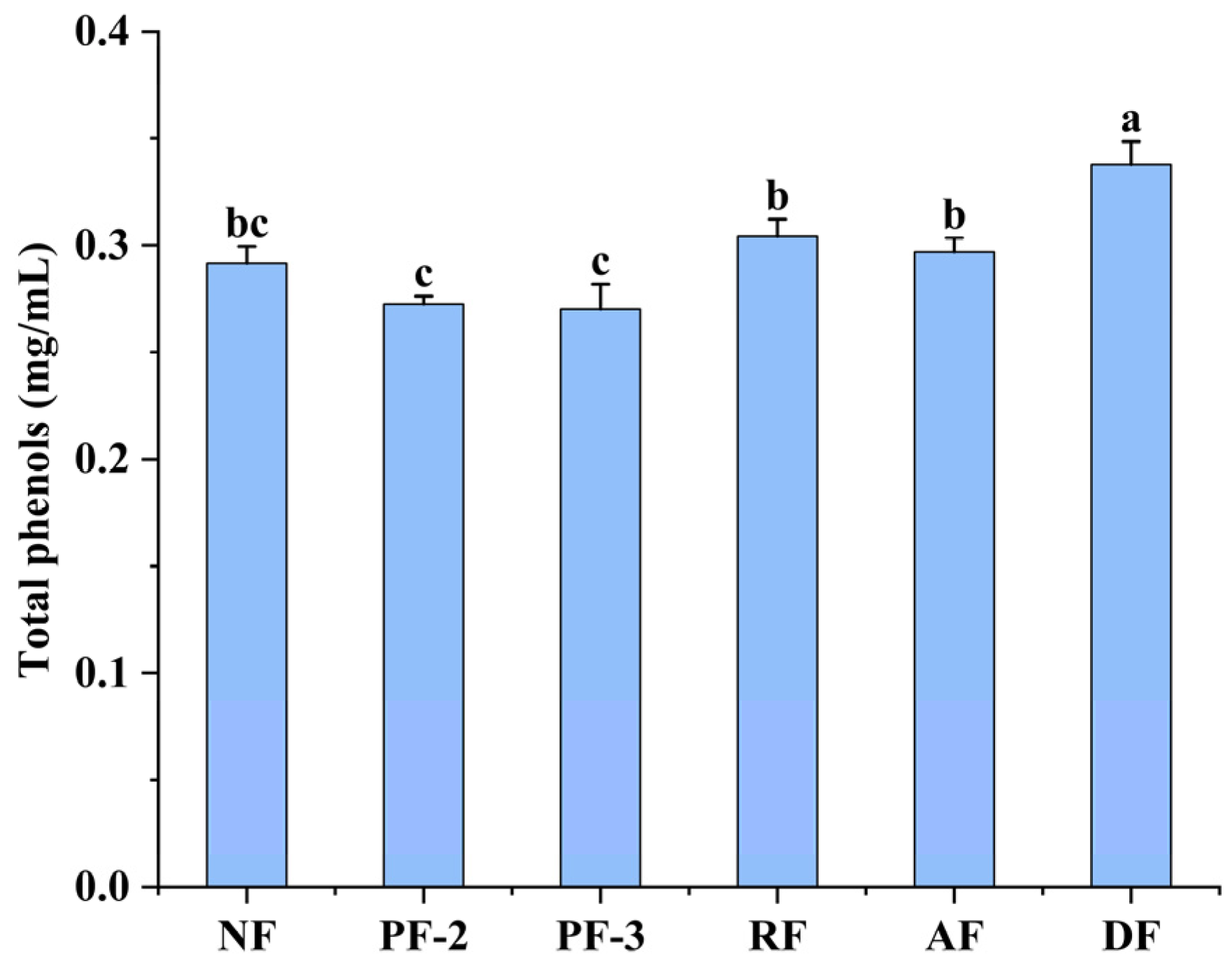
3.2. Effects of Lactobacillus Fermentation on the Total Phenolic Contents of S. fusiforme Fermentation Supernatants
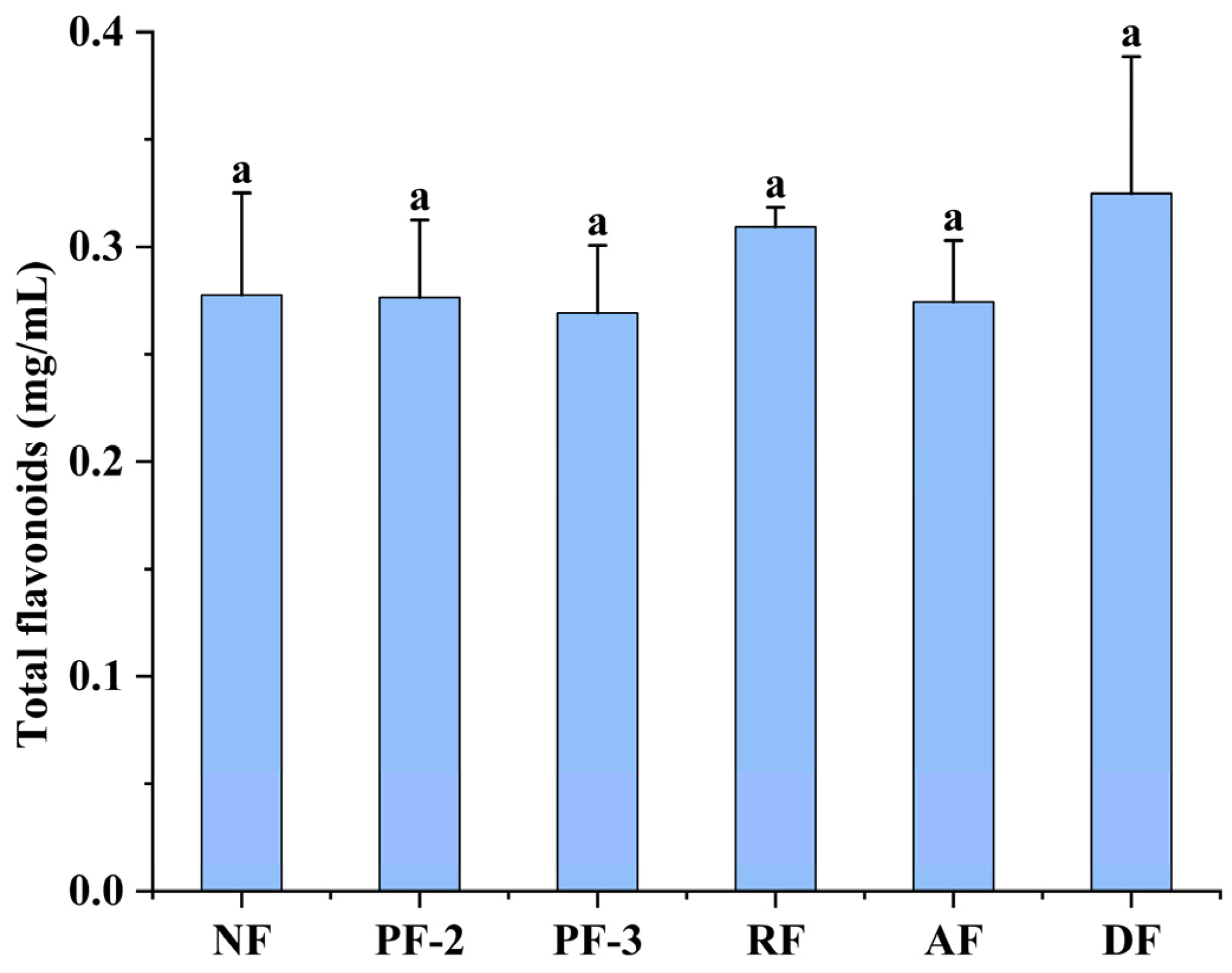
3.3. Effects of Lactobacillus Fermentation on the Total Flavonoid Contents in S. fusiforme Fermentation Supernatants
3.4. Analysis of Short-Chain Fatty Acids
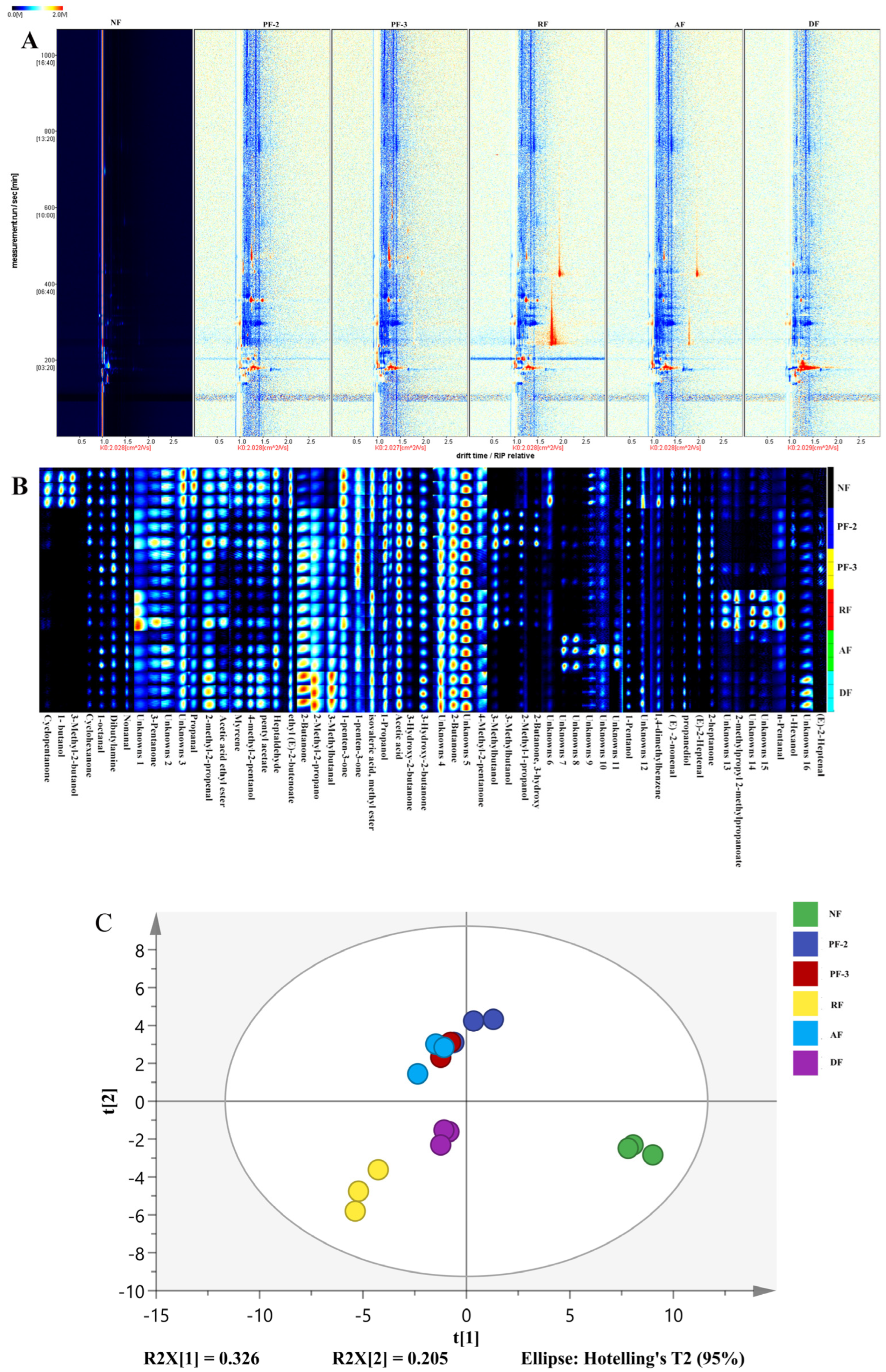
3.5. Effects of Lactobacillus Fermentation on the Volatile Constituents of S. fusiforme
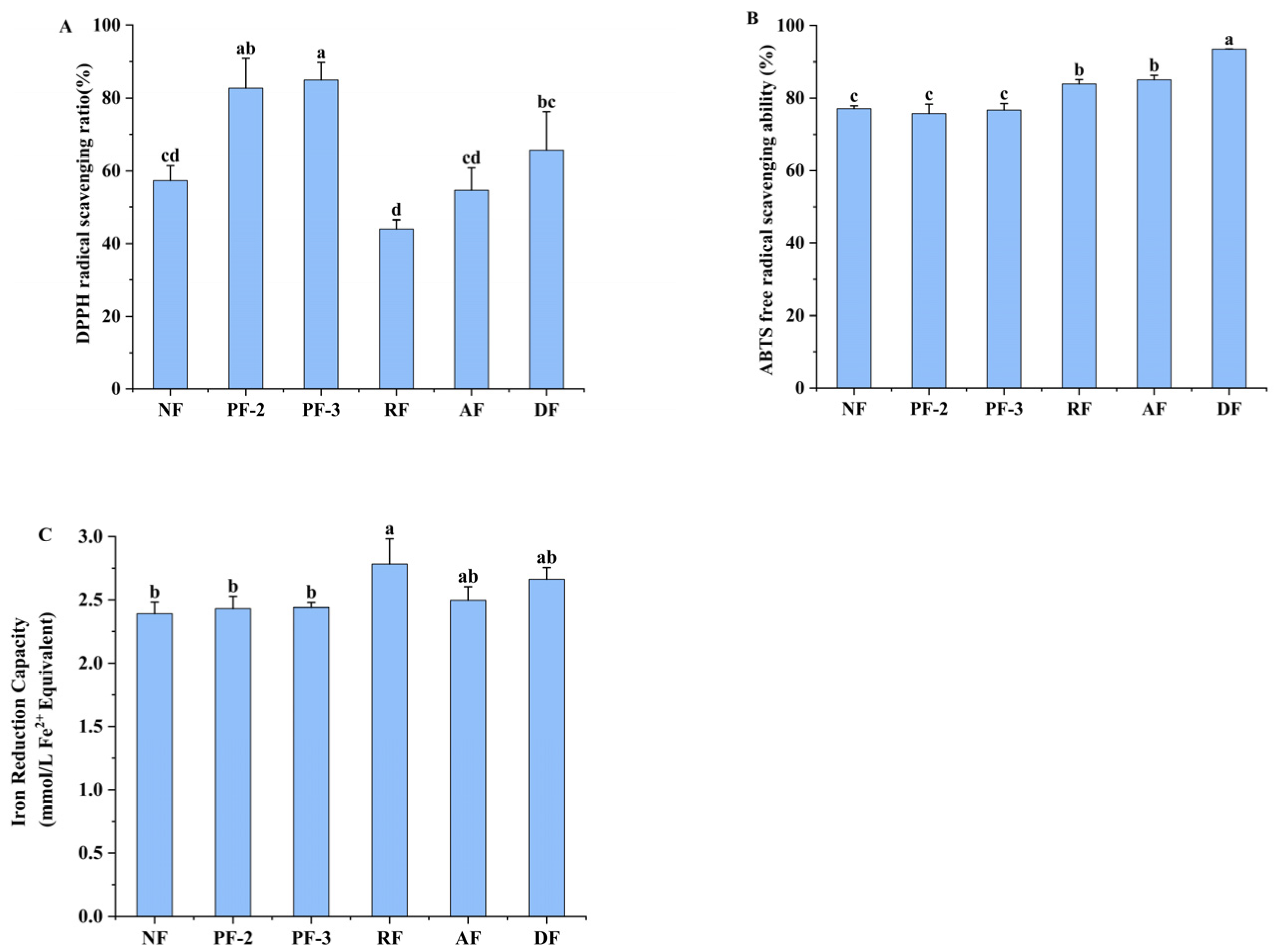
3.6. Effects of Lactobacillus Fermentation of S. fusiforme on Antioxidant Activity
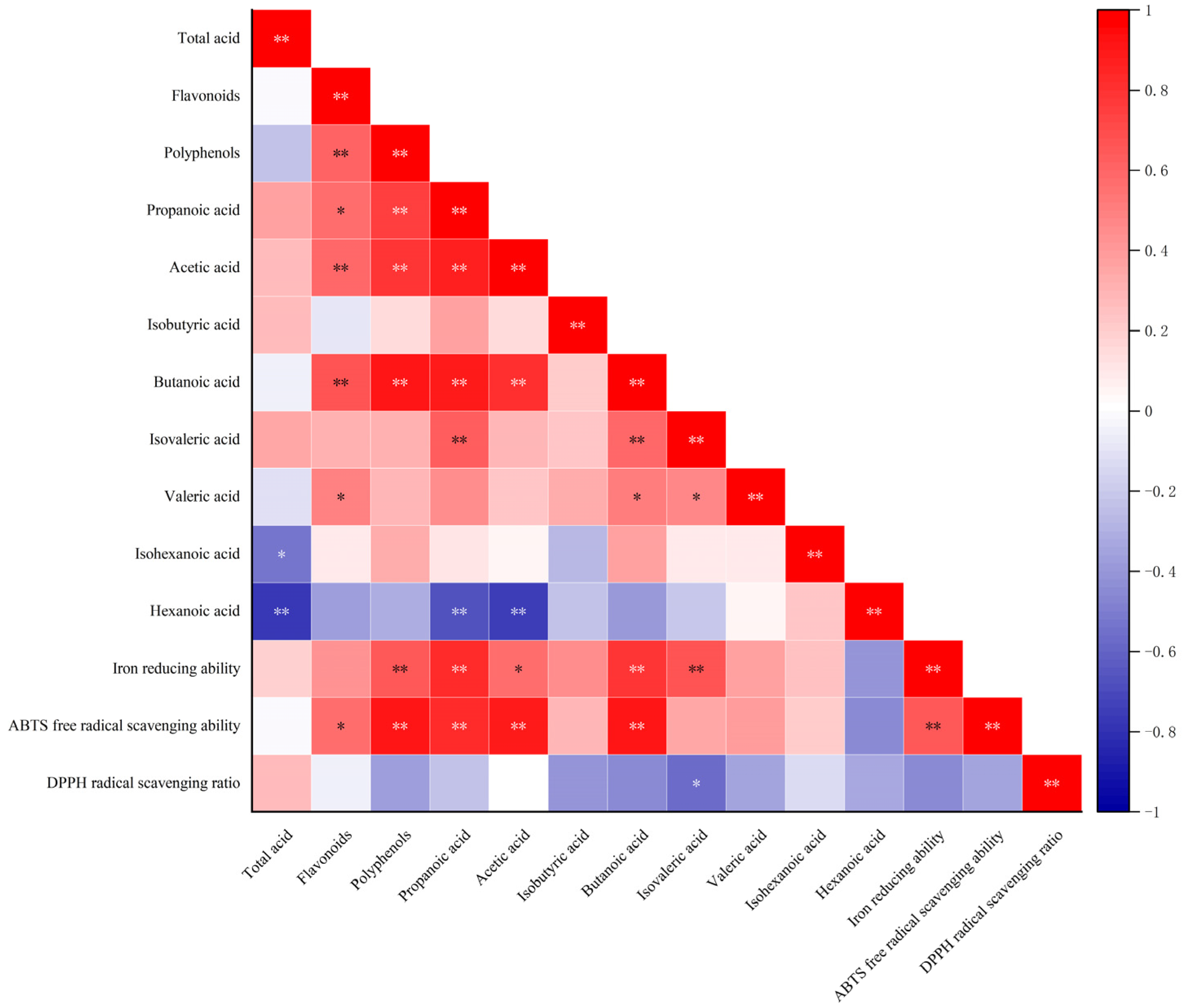
3.7. Correlations Between Antioxidant Activity and Active Components in S. fusiforme
3.8. Effects of Lactobacillus Fermentation on α-Glucosidase and α-Amylase Activities of S. fusiforme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Ling, X.; Li, Z.; Su, N.; Kong, L.; Han, S.; Song, D. Sargassum fusiforme and its fucoidan alleviates high-fat diet induced obesity associated with the improvement of the gut microbiota profile. J. Funct. Foods 2025, 125, 106686. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Xiang, J.; Huang, X.J.; Wei, X.; Zhang, Q.; Du, X.H.; Hu, J.S.; Wang, Y.M.; Zhang, C.X. Chemical Constituents from Sargassum fusiforme (Harv.) Setch. Chem. Biodivers. 2020, 17, e2000182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, C.; Fu, X.; Jeon, Y.J.; Mao, X.; Wang, L. Bioactivities of the popular edible brown seaweed Sargassum fusiforme: A review. J. Agric. Food Chem. 2023, 71, 16452–16468. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yin, C.; Liu, W.; Wu, M.; Su, L.; Chen, P. The Drying Method Affects the Physicochemical Properties, Antioxidant Activity, and Volatile Flavor of Sargassum fusiforme. J. Food Proc. Preserv. 2024, 2024, 4717136. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; Rodríguez, K.; López, J.; Scala, K.D. Evaluation of physicochemical composition and bioactivity of a red seaweed (Pyropia orbicularis) as affected by different drying technologies. Dry. Technol. 2020, 38, 1218–1230. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Luo, M.; Hu, K.; Zeng, Q.; Yang, X.; Wang, Y.; Dong, L.; Huang, F.; Zhang, R.; Su, D. Comparative analysis of the morphological properties and chemical composition of soluble and insoluble dietary fibers with bound phenolic compounds from different algae. Food Sci. 2000, 85, 3843–3851. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 2022, 8, 688. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.; Fan, X.; Zhao, T.; Wen, D.; Huang, X.; Li, B. Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease. Microorganisms 2024, 12, 1864. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating fermented: Health benefits of LAB-fermented foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Y.; Tong, Q.; Liu, Y.; Xu, W. Effects of different lactic acid bacteria on phenolic profiles, antioxidant capacities, and volatile compounds in purple sweet potato juice. J. Food. Sci. Technol. 2024, 61, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria–Potential for control of mold growth and mycotoxins: A review. Food Control. 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Zhang, Y.; Zheng, M.; Jiang, Z.; Zhu, Y.; Deng, S.; Li, Q.; Ni, H. Lactobacillus-fermentation enhances nutritional value and improves the inhibition on pancreatic lipase and oral pathogens of edible red seaweed Bangia fusco-purpurea. LWT 2023, 179, 114643. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of polyphenols and antioxidative activity in industrial beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, S.; Ge, L.; Wang, Y.; Liu, Y.; Chen, C.; Liu, X.; Han, Q. Reducing toxic constituents of ginkgolic acid content and improving bioactive flavonoid content from Ginkgo biloba leaves by high-temperature pretreatment processing. Food Sci. Nutr. 2023, 11, 838–852. [Google Scholar] [CrossRef]
- Zang, Z.; Li, L.; Yang, M.; Zhang, H.; Naeem, A.; Wu, Z.; Zheng, Q.; Song, Y.; Tao, L.; Wan, Z.; et al. Study on the ameliorative effect of honeysuckle on DSS-induced ulcerative colitis in mice. J. Ethnopharm 2024, 325, 117776. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Yang, B.; Zhang, W.; Cao, Z.; Zhao, P.; Dong, Z.; Ren, F.; Chen, L. Characterization of the flavor profile of Hulatang using GC-IMS coupled with sensory analysis. Front. Nutr. 2024, 11, 1461224. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Cheng, Z.; Meng, J.; Li, Y. Optimization of Polyphenols Release from Highland Barley Bran by Solid-State Fermentation and Antioxidant Activity Characterization. Fermentation 2024, 10, 438. [Google Scholar] [CrossRef]
- Yang, L.F.; Nie, W.; Cui, Y.P.; Yue, F.L.; Fan, X.T.; Sun, R.Y. Purification with macroporous resin and antioxidant activity of polyphenols from sweet potato leaves. Chem. Papers 2024, 78, 181–188. [Google Scholar] [CrossRef]
- Ferrera, T.S.; Heldwein, A.B.; Dos Santos, C.O.; Somavilla, J.C.; Sautter, C.K. Substâncias fenólicas, flavonoides e capacidade antioxidante em erveiras sob diferentes coberturas do solo e sombreamentos. Rev. Bras. Plant Med. 2016, 18, 588–596. [Google Scholar] [CrossRef]
- Liu, H.; Pei, Z.; Li, W. Hypoglycemic and antioxidative activity evaluation of phenolic compounds derived from walnut diaphragm produced in Xinjiang. J. Food Biochem. 2022, 46, e14403. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.; Xie, P.; Liu, L.; Zhang, C.; Cheng, J.; Zhang, Y.; Liu, Y.; Huang, L.; Jiang, J. Novel bioactive peptides from Ginkgo biloba seed protein and evaluation of their α-glucosidase inhibition activity. Food Chem. 2023, 404, 134481. [Google Scholar] [CrossRef]
- Li, W.; Mutuvulla, M.; Chen, X.; Jiang, M.; Dong, M. Isolation and identification of high viscosity-producing lactic acid bacteria from a traditional fermented milk in Xinjiang and its role in fermentation process. Eur. Food Res. Technol. 2012, 235, 497–505. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Kim, H.G.; Park, W.L.; Min, H.J.; Won, Y.S.; Seo, K.I. Antioxidant and anticancer effects of kiwi (Actinidia deliciosa) fermented beverage using Lactobacillus plantarum. Food Sci. Biotechnol. 2025, 34, 207–216. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado leaf extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; El, S.N.; Kilinc, A.K.; Karakaya, S. Vegetable and fermented vegetable juices containing germinated seeds and sprouts of lentil and cowpea. Food Chem. 2014, 156, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Zhao, S.; Cheng, L.; Zhao, C. Effects of different fermentation methods on chemical composition, antioxidant activity, and enzymatic inhibition of fermented fig juice. CyTA-J. Food 2024, 22, 2326299. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, J.S.; Park, H.M.; Kim, S.; Paek, N.S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, C.; Li, Q.; Xu, Y.J.; Liu, Y. Different dietary lipid consumption affects the serum lipid profiles, colonic short chain fatty acid composition and the gut health of Sprague Dawley rats. Food Res. Int. 2020, 132, 109117. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.C.; Yeo, A.Y.; Yuan, W.; Lee, Y.Y.; Ikasari, L.; Dharmawan, J.; Delahunty, C.M. Flavour characterization of twelve species of edible algae. Algal Res. 2024, 80, 103540. [Google Scholar] [CrossRef]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef]
- Zhao, R.; Ran, J.; Ruan, X.; Du, H.; Li, G.; Zhao, L.; Sun, J.; Liang, X. Apple polyphenol biotransformation using probiotics in vitro and dynamic simulated digestion by bionic rats. J. Sci. Food Agric. 2023, 103, 5490–5499. [Google Scholar] [CrossRef]
- Rao, R.R.; Tiwari, A.K.; Reddy, P.P.; Babu, K.S.; Suresh, G.; Ali, A.Z.; Madhusudana, K.; Agawane, S.B.; Badrinarayan, P.; Sastry, G.N.; et al. Synthesis of antihyperglycemic, α-glucosidase inhibitory, and DPPH free radical scavenging furanochalcones. Med. Chem. Res. 2012, 21, 760–774. [Google Scholar]
- Han, L.; Fang, C.; Zhu, R.; Peng, Q.; Li, D.; Wang, M. Inhibitory effect of phloretin on α-glucosidase: Kinetics, interaction mechanism and molecular docking. Int. J. Biol. Macromol. 2017, 95, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Beiza, N.; Pérez-Correa, J.R.; Franco, W. Fermentation of Murta (Ugni molinae) Juice: Effect on Antioxidant Activity and Control of Enzymes Associated with Glucose Assimilation. Int. J. Mol. Sci. 2023, 24, 15197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, H.; Shi, S.; Peng, X. Mechanistic study and synergistic effect on inhibition of α-amylase by structurally similar flavonoids. J. Mol. Liq. 2022, 360, 119485. [Google Scholar] [CrossRef]
- Copeland, L. Assessing the glycemic impact of starch by in vitro methods. Food Biosci. 2024, 60, 104434. [Google Scholar] [CrossRef]

| NF | PF-2 | PF-3 | RF | AF | DF | |
|---|---|---|---|---|---|---|
| Acetic acid | 14.50 ± 1.97 e | 137.26 ± 12.31 c | 117.16 ± 3.05 d | 173.54 ± 0.32 b | 185.76 ± 6.71 b | 403.04 ± 3.38 a |
| Isohexanoic acid | 4.11 ± 0.01 a | 3.08 ± 0.47 b | 4.14 ± 0.46 a | 4.00 ± 0.49 ab | 3.20 ± 0.30 ab | 4.02 ± 0.21 ab |
| Propanoic acid | 0.37 ± 0.02 c | 0.50 ± 0.07 bc | 0.51 ± 0.03 bc | 0.74 ± 0.02 a | 0.54 ± 0.11 b | 0.80 ± 0.01 a |
| Isobutyric acid | 0.56 ± 0.06 a | 0.60 ± 0.13 a | 0.57 ± 0.01 a | 0.72 ± 0.10 a | 0.73 ± 0.20 a | 0.59 ± 0.03 a |
| Butanoic acid | 0.11 ± 0.02 d | 0.09 ± 0.00 d | 0.10 ± 0.00 d | 0.22 ± 0.01 b | 0.15 ± 0.01 c | 0.26 ± 0.01 a |
| Isovaleric acid | 0.19 ± 0.03 d | 0.24 ± 0.04 cd | 0.22 ± 0.00 cd | 0.55 ± 0.00 a | 0.27 ± 0.04 bc | 0.32 ± 0.01 b |
| Valeric acid | 0.05 ± 0.00 a | 0.03 ± 0.02 a | 0.03 ± 0.01 a | 0.08 ± 0.00 a | 0.05 ± 0.02 a | 0.08 ± 0.07 a |
| Hexanoic acid | 0.20 ± 0.01 a | 0.16 ± 0.00 bc | 0.16 ± 0.01 bc | 0.16 ± 0.00 b | 0.16 ± 0.00 bc | 0.15 ± 0.00 c |
| Count | Class | Name | CAS | Odor Description |
|---|---|---|---|---|
| 1 | Ketones | Cyclopentanone | 120-92-3 | Minty |
| 2 | Cyclohexanone | 108-94-1 | Minty acetone | |
| 3 | 2-Butanone, 3-hydroxy | 513-86-0 | Sweet, buttery, creamy | |
| 4 | 3-Pentanone | 96-22-0 | Ethereal acetone | |
| 5 | 2-Butanone | 78-93-3 | Ethereal, fruity | |
| 6 | 1-Penten-3-one | 1629-58-9 | Pungent, peppery | |
| 7 | 4-Methyl-2-pentanone | 108-10-1 | Fruity | |
| 8 | 2-heptanone | 110-43-0 | Fruity, spicy | |
| 9 | Alcohols | 3-Methylbutanol | 123-51-3 | Slight smell of alcohol |
| 10 | 1-Pentanol | 71-41-0 | Pungent, bready | |
| 11 | 2-Methyl-1-propanol | 78-83-1 | Ethereal, winey | |
| 12 | 1-Butanol | 71-36-3 | Strong smell of alcohol | |
| 13 | 3-Methyl-2-butanol | 598-75-4 | Fruity | |
| 14 | 4-Methyl-2-pentanol | 108-11-2 | Pungent alcohol | |
| 15 | 2-Methyl-2-propanol | 75-65-0 | Camphor | |
| 16 | 1-Propanol | 71-23-8 | Alcoholic, fermented | |
| 17 | Propanediol | 57-55-6 | Odorless with a very slight alcoholic aroma | |
| 18 | 3-Methyl butanol | 123-51-3 | Alcoholic, pungent, ethereal, fruity | |
| 19 | 1-Hexanol | 111-27-3 | Pungent, fruity | |
| 20 | Aldehydes | 1-Octanal | 124-13-0 | Fruity |
| 21 | 1-Nonanal | 124-19-6 | Waxy, aldehydic, citrus, | |
| 22 | Propanal | 123-38-6 | Pungent | |
| 23 | 2-Methyl-2-propenal | 78-85-3 | Floral | |
| 24 | Heptaldehyde | 111-71-7 | Fresh | |
| 25 | 3-Methylbutanal | 590-86-3 | Ethereal, chocolate, peach | |
| 26 | (E)-2-nonenal | 18829-56-6 | Fats, cucumber, aldehydes, citrus | |
| 27 | (E)-2-Heptenal | 18829-55-5 | Fruity overtones | |
| 28 | n-Pentanal | 110-62-3 | Fermented, bready | |
| 29 | Esters | 2-methylpropyl 2-methylpropanoate | 97-85-8 | Ethereal, chocolate |
| 30 | Acetic acid ethyl ester | 141-78-6 | Fruity—grape, sweet, rum-like | |
| 31 | Pentyl acetate | 628-63-7 | Fruity | |
| 32 | Ethyl (E)-2-butenoate | 623-70-1 | Pungent, chemical, diffusive | |
| 33 | Isovaleric acid, methyl ester | 556-24-1 | Apple, fruity | |
| 34 | Amines | Dibutylamine | 111-92-2 | Weak smell of ammonia |
| 35 | Olenes | Myrcene | 123-35-3 | Anise, grape, fruity |
| 36 | Acids | Acetic acid | 64-19-7 | Sour vinegar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, H.; Lin, S.; Su, L. Bioprocessing of Sargassum fusiforme via Lactobacillus Fermentation: Effects on Nutrient Composition, Organoleptic Properties, and In Vitro Antioxidant and Hypoglycemic Activities. Foods 2025, 14, 1385. https://doi.org/10.3390/foods14081385
Zhang C, Zhang H, Lin S, Su L. Bioprocessing of Sargassum fusiforme via Lactobacillus Fermentation: Effects on Nutrient Composition, Organoleptic Properties, and In Vitro Antioxidant and Hypoglycemic Activities. Foods. 2025; 14(8):1385. https://doi.org/10.3390/foods14081385
Chicago/Turabian StyleZhang, Chao, Houyun Zhang, Shengli Lin, and Laijin Su. 2025. "Bioprocessing of Sargassum fusiforme via Lactobacillus Fermentation: Effects on Nutrient Composition, Organoleptic Properties, and In Vitro Antioxidant and Hypoglycemic Activities" Foods 14, no. 8: 1385. https://doi.org/10.3390/foods14081385
APA StyleZhang, C., Zhang, H., Lin, S., & Su, L. (2025). Bioprocessing of Sargassum fusiforme via Lactobacillus Fermentation: Effects on Nutrient Composition, Organoleptic Properties, and In Vitro Antioxidant and Hypoglycemic Activities. Foods, 14(8), 1385. https://doi.org/10.3390/foods14081385






