The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Chemical Analyses
2.2.1. Dry Matter
2.2.2. Vitamin C
2.2.3. Phenolic Compounds Extraction and Identification
2.3. Statistical Analyses
3. Results and Discussion
3.1. Dry Matter and Vitamin C
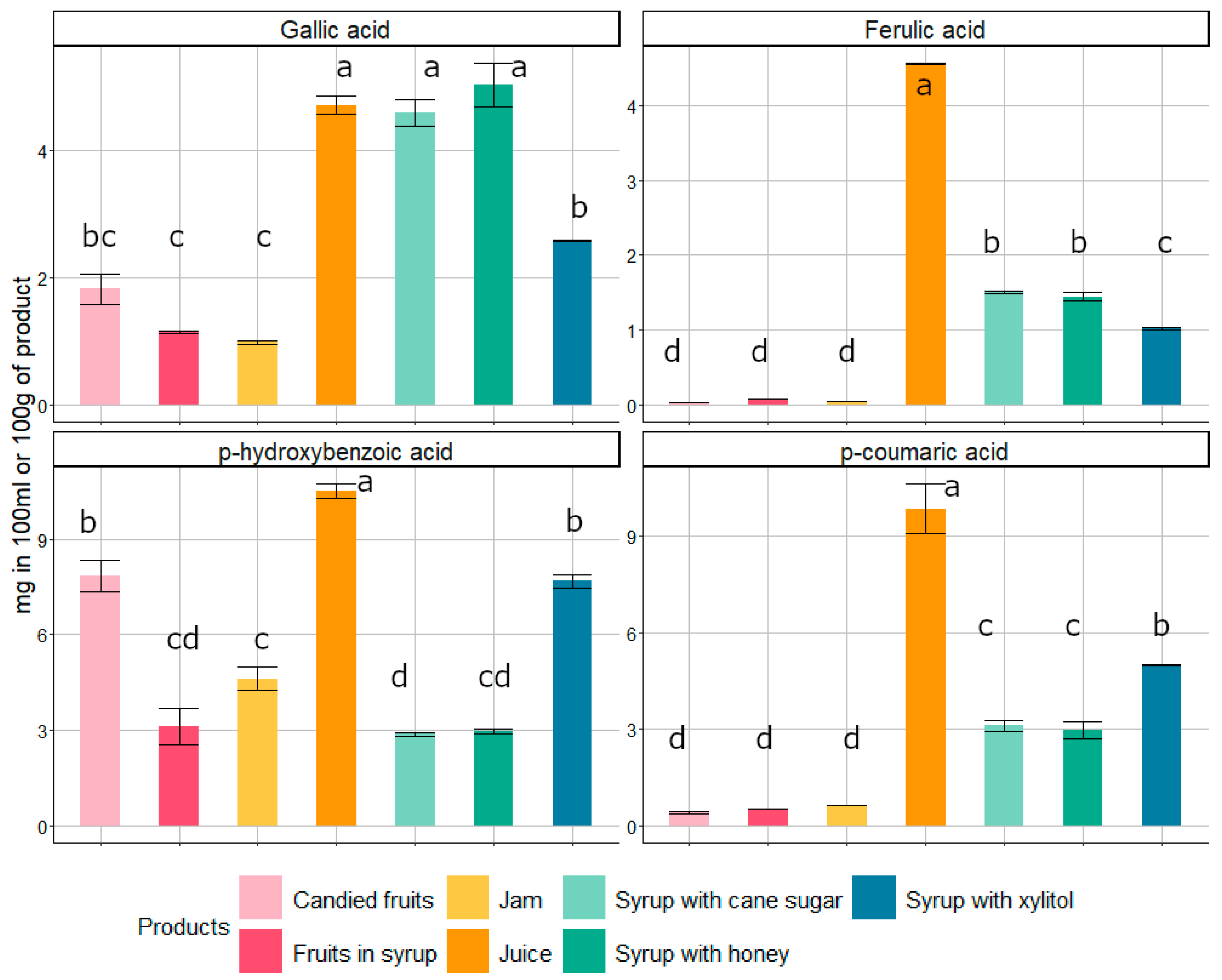
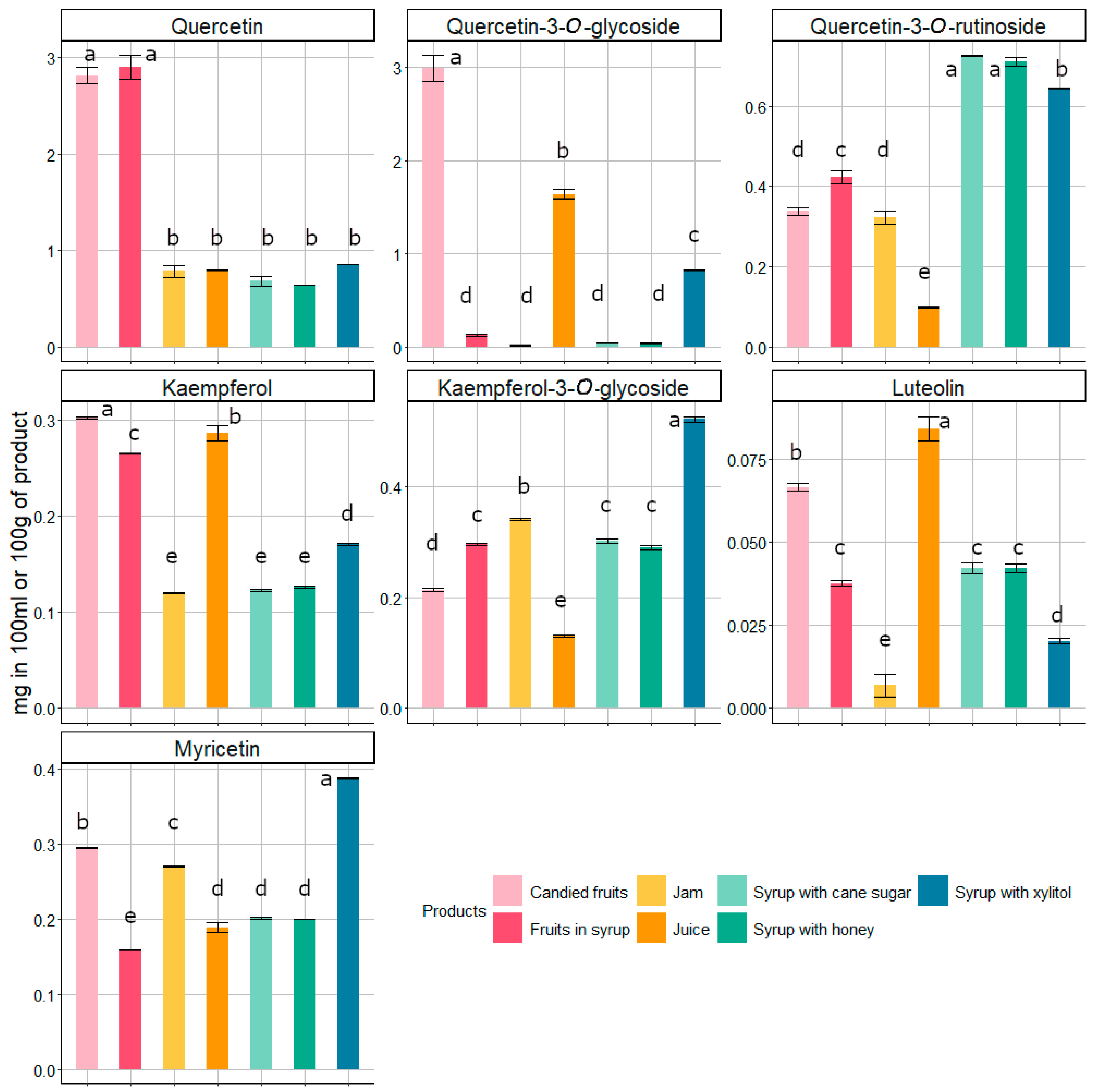
3.2. Phenolic Compounds
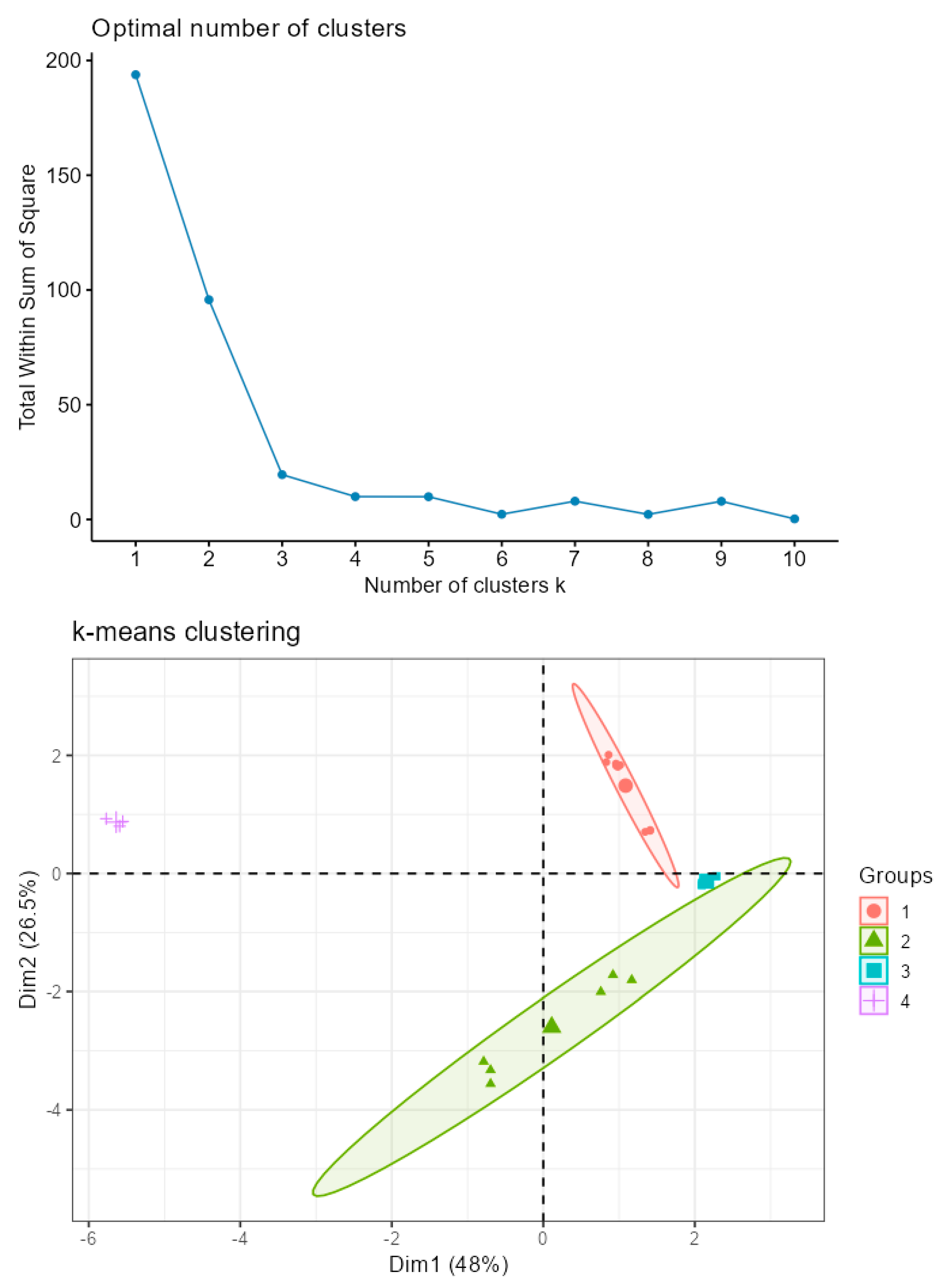
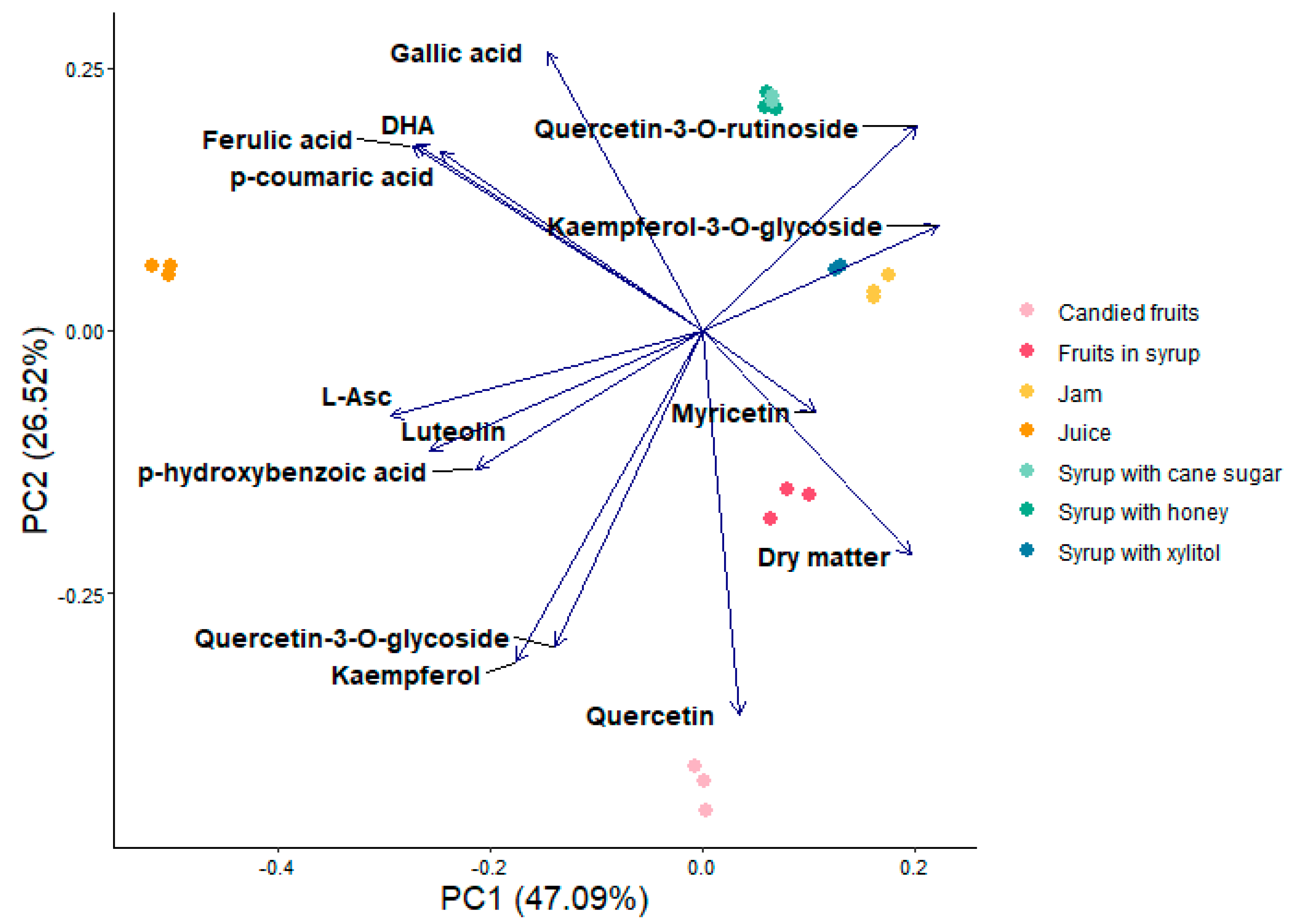
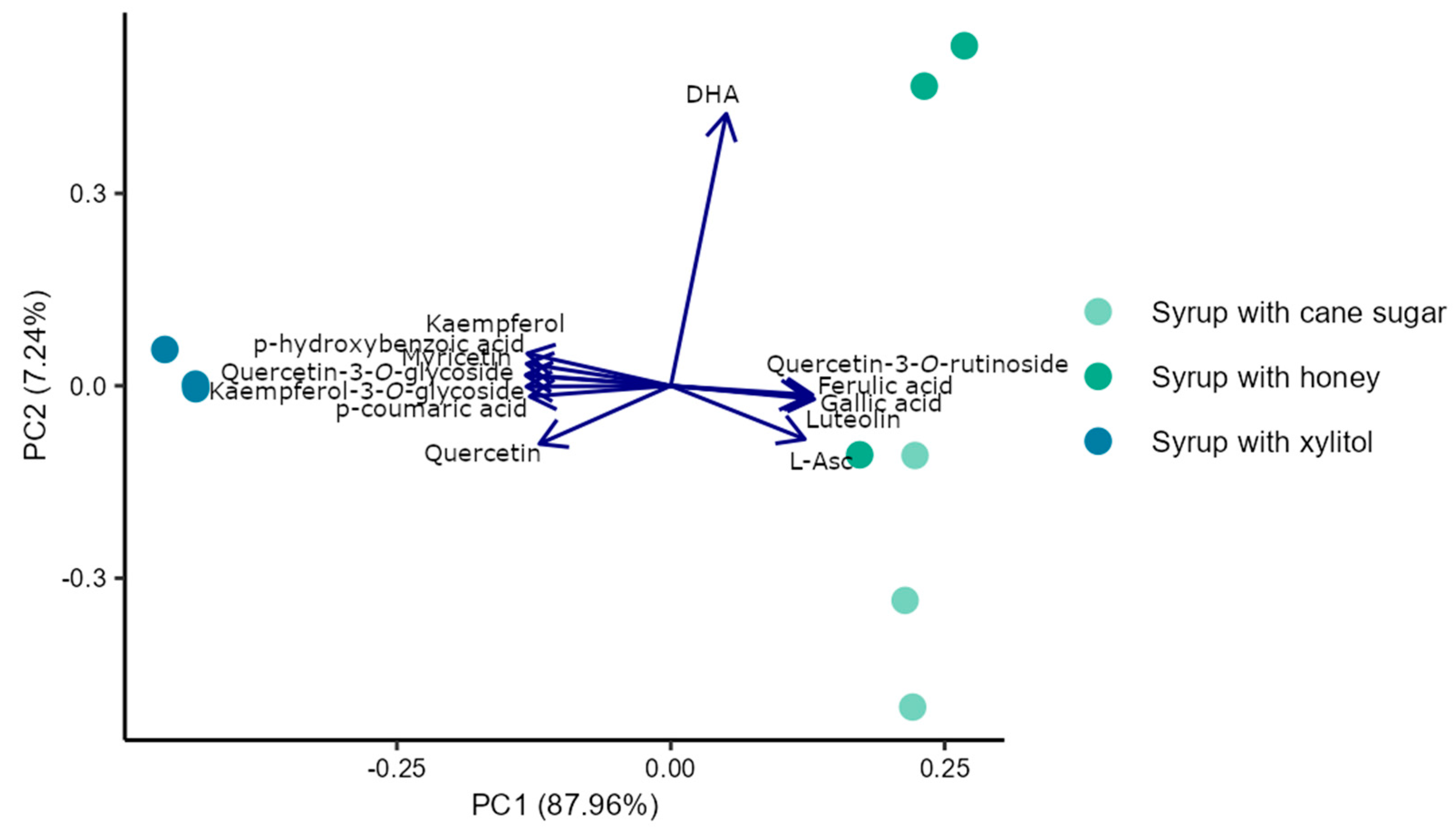
3.3. Principal Component Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem.—Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. Comparative Evaluation of the Nutritional Value and the Content of Bioactive Compounds in the Fruit of Individual Species of Chaenomeles and Quince. World Sci. News 2017, 73, 100–107. [Google Scholar]
- Du, H.; Wua, J.; Li, H.; Zhong, P.X.; Xu, Y.J.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and Triterpenes from Chaenomeles Fruits: Chemical Analysis and Antioxidant Activities Assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active Compounds, Antioxidant Activity and α-Glucosidase Inhibitory Activity of Different Varieties of Chaenomeles Fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Urbanaviciute, I.; Liaudanskas, M.; Seglina, D.; Viskelis, P. Japanese Quince Chaenomeles japonica (Thunb.) Lindl. Ex Spach Leaves a New Source of Antioxidants for Food. Int. J. Food Prop. 2019, 22, 795–803. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Golis, T.; Bąbelewski, P. ABTS On-Line Antioxidant, α-Amylase, α-Glucosidase, Pancreatic Lipase, Acetyl-and Butyrylcholinesterase Inhibition Activity of Chaenomeles Fruits Determined by Polyphenols and Other Chemical Compounds. Antioxidants 2020, 9, 60. [Google Scholar] [CrossRef]
- Byczkiewicz, S.; Szwajgier, D.; Kobus-Cisowska, J.; Szczepaniak, O.; Szulc, P. Comparative Examination of Bioactive Phytochemicals in Quince (Chaenomeles) Fruits and Their in Vitro Antioxidant Activity. Emir. J. Food Agric. 2021, 33, 293–302. [Google Scholar] [CrossRef]
- Zhang, R.; Li, S.; Zhu, Z.; He, J. Recent Advances in Valorization of Chaenomeles Fruit: A Review of Botanical Profile, Phytochemistry, Advanced Extraction Technologies and Bioactivities. Trends. Food Sci. Technol. 2019, 91, 467–482. [Google Scholar] [CrossRef]
- Urbanavičiūte, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgiene, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef]
- Chojnacka, K.; Sosnowska, D.; Polka, D.; Owczarek, K.; Gorlach-Lira, K.; Oliveira De Verasa, B.; Lewandowska, U. Comparison of Phenolic Compounds, Antioxidant and Cytotoxic Activity of Extracts Prepared from Japanese Quince (Chaenomeles japonica L.) Leaves. J. Physiol. Pharmacol. 2020, 71, 213–222. [Google Scholar] [CrossRef]
- Byczkiewicz, S.; Szwajgier, D.; Baranowska-Wójcik, E.; Telichowska, A.; Szymandera-Buszka, K.; Wojtczak, J.; Kobus-Cisowska, J. Research on Application of Japanese Quince (Chaenomeles L.) and Pork Collagen in Dark Chocolate—Benefits in Prevention of Inflammation In Vitro Model. Nutrients 2024, 16, 1758. [Google Scholar] [CrossRef] [PubMed]
- Urbanavičiūtė, I.; Viškelis, P. Biochemical Composition of Japanese Quince (Chaenomeles japonica) and Its Promising Value for Food, Cosmetic, and Pharmaceutical Industries. In Fruit Industry; IntechOpen: London, UK, 2022. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Pogoń, P.; Machalica, J. Chaenomeles japonica, Cornus Mas, Morus Nigra Fruits Characteristics and Their Processing Potential. J. Food Sci. Technol. 2014, 51, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Codina, M.C.; González, E.J.; Molina, A.; Carmona, M.; Berruga, M.I. Bio-Based Films from Quince by-Products: A Sustainable Alternative for Biodegradable Food Packaging. Food Hydrocoll. 2024, 157, 110395. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Ifie, I.; Marshall, L.J. Food Processing and Its Impact on Phenolic Constituents in Food. Cogent Food Agric. 2018, 4, 1507782. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H. Editorial: Chemical and Biological Changes of Polyphenols Caused by Food Thermal Processing. Front. Nutr. 2022, 9, 948894. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and Mechanisms of Dietary Polyphenols Affected by Non-Thermal Processing Technology in Fruits and Vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Pineda, M.M. Antioxidative, Sensory and Volatile Profiles of Cookies Enriched with Freeze-Dried Japanese Quince (Chaenomeles japonica) Fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Viskelis, P.; Viškelis, J.; Bobinaitė, R.; Shalkevich, M.; Urbonaviciene, D. Biochemical Composition and Antioxidant Activity of Japanese Quince Fruits and Their Syrup and Candied Fruit Slices. In Proceedings of the 9th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 8 May 2014. [Google Scholar]
- Bieniasz, M.; Dziedzic, E.; Kaczmarczyk, E. The Effect of Storage and Processing on Vitamin C Content in Japanese Quince Fruit. Folia Hortic. 2017, 29, 83–93. [Google Scholar] [CrossRef]
- Savescu, P.; Matei, G. The Induced Influence of Food Sweeteners on Concentrations of Flavin-Mononucleotides From Natural Quince Juice. Ann. Univ. Craiova—Agric. Mont. Cadastre Ser. 2015, 45, 255–258. [Google Scholar]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Al-Zughbi, I.; Krayem, M. Quince Fruit Cydonia Oblonga Mill Nutritional Composition, Antioxidative Properties, Health Benefits and Consumers Preferences towards Some Industrial Quince Products: A Review. Food Chem. 2022, 393, 133362. [Google Scholar] [CrossRef] [PubMed]
- Polish Standard PN-R-04013; Analiza Chemiczno—Rolnicza Roślin. Oznaczanie Powietrznie Suchej i Suchej Masy. The Polish Committee for Standardization: Warsaw, Poland, 1988.
- Kazimierczak, R.; Średnicka-Tober, D.; Barański, M.; Hallmann, E.; Góralska-Walczak, R.; Kopczyńska, K.; Rembiałkowska, E.; Górski, J.; Leifert, C.; Rempelos, L.; et al. The Effect of Different Fertilization Regimes on Yield, Selected Nutrients, and Bioactive Compounds Profiles of Onion. Agronomy 2021, 11, 883. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Srednicka-Tober, D.; Hallmann, E.; Kopczynska, K.; Zarzynska, K. The Impact of Organic vs. Conventional Agricultural Practices on Selected Quality Features of Eight Potato Cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of Innovative Technologies on the Content of Vitamin C and Its Bioavailability from Processed Fruit and Vegetable Products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef]
- Watychowicz, K.; Janda, K.; Jakubczyk, K.; Wolska, J. Chaenomeles—Health Promoting Benefits. Rocz. Panstw. Zakl. Hig. 2017, 68, 217–227. [Google Scholar] [PubMed]
- Klopotek, Y.; Otto, K.; Böhm, V. Processing Strawberries to Different Products Alters Contents of Vitamin C, Total Phenolics, Total Anthocyanins, and Antioxidant Capacity. J. Agric. Food Chem. 2005, 53, 5640–5646. [Google Scholar] [CrossRef]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional Comparison of Fresh, Frozen and Canned Fruits and Vegetables. Part 1. Vitamins C and B and Phenolic Compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Putri, M.P. Variation of Processed Fresh Pineapple on Vitamin C Content Using UV-VIS Spectrophotometry. J. Nat. Sci. Learn. 2023, 2, 29–33. [Google Scholar] [CrossRef]
- Rohaiza Ahmad, S.; Usamah Haji Sarbini, M. The Effect of Storage on Vitamin C Stability of Locally Grown Fruits in Brunei Darussalam. Innovare J. Food Sci. 2019, 7, 1–5. [Google Scholar]
- Giannakourou, M.C.; Taoukis, P.S. Effect of Alternative Preservation Steps and Storage on Vitamin c Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Van Bree, I.; Baetens, J.M.; Samapundo, S.; Devlieghere, F.; Laleman, R.; Vandekinderen, I.; Noseda, B.; Xhaferi, R.; De Baets, B.; De Meulenaer, B. Modelling the Degradation Kinetics of Vitamin C in Fruit Juice in Relation to the Initial Headspace Oxygen Concentration. Food Chem. 2012, 134, 207–214. [Google Scholar] [CrossRef]
- Nakilcioğlu-Taş, E.; Ötleş, S. Kinetic Modelling of Vitamin C Losses in Fresh Citrus Juices under Different Storage Conditions. Acad. Bras Cienc. 2020, 92, e20190328. [Google Scholar] [CrossRef]
- Pielak, M.; Czarniecka-Skubina, E.; Głuchowski, A. Effect of Sugar Substitution with Steviol Glycosides on Sensory Quality and Physicochemical Composition of Low-Sugar Apple Preserves. Foods 2020, 9, 293. [Google Scholar] [CrossRef]
- Uckiah, A.; Goburdhun, D.; Ruggoo, A. Vitamin C Content during Processing and Storage of Pineapple. Nutr. Food Sci. 2009, 39, 398–412. [Google Scholar] [CrossRef]
- Scrob, T.; Varodi, S.M.; Vintilă, G.A. Effects of Sweeteners and Storage on the Acidity, Soluble Solids and Sensorial Profile of Lingonberry Jams. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 97–107. [Google Scholar] [CrossRef]
- Souza, P.B.A.; Santos, M.d.F.; Carneiro, J.d.D.S.; Pinto, V.R.A.; Carvalho, E.E.N. The Effect of Different Sugar Substitute Sweeteners on Sensory Aspects of Sweet Fruit Preserves: A Systematic Review. J. Food Process. Preserv. 2022, 46, e16291. [Google Scholar] [CrossRef]
- Jang, S.J.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Kim, Y.J.; Ham, Y.K.; Lim, Y.B.; Jeong, T.J.; Kim, S.Y.; Kim, C.J. Effects of Replacing Sucrose with Various Sugar Alcohols on Quality Properties of Semi-Dried Jerky. Korean J. Food Sci. Anim. Resour. 2015, 35, 622. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Albakry, B.S.S.; Artik, N.; Ayed, M.; Abdulaziz, O. Some Alternative Sweeteners (Xylitol, Sorbitol, Sucralose and Stevia): Review. Karaelmas Sci. Eng. J. 2014, 4, 63–70. [Google Scholar] [CrossRef]
- Jagannadha Rao, P.V.K.; Das, M.; Das, S.K. Changes in Physical and Thermo-Physical Properties of Sugarcane, Palmyra-Palm and Date-Palm Juices at Different Concentration of Sugar. J. Food Eng. 2009, 90, 559–566. [Google Scholar] [CrossRef]
- Son, Y.R.; Park, T.S.; Shim, S.M. Pharmacokinetics and Plasma Cellular Antioxidative Effects of Flavanols after Oral Intake of Green Tea Formulated with Vitamin C and Xylitol in Healthy Subjects. J. Med. Food 2016, 19, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kumaran, N. Studies on the Effects of Honey Incorporation on Quality and Shelf Life of Aonla Preserve. Cogent. Food Agric. 2015, 1, 1009334. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Verbeyst, L.; Bogaerts, R.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Modelling of Vitamin C Degradation during Thermal and High-Pressure Treatments of Red Fruit. Food Bioproc. Tech. 2013, 6, 1015–1023. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, X.; Li, J.; Jing, G.; Cai, L.; Ying, T. Integrated Application of Nitric Oxide and Modified Atmosphere Packaging to Improve Quality Retention of Button Mushroom (Agaricus bisporus). Food Chem. 2011, 126, 1693–1699. [Google Scholar] [CrossRef]
- Chetti, M.B.; Deepa, G.T.; Antony, R.T.; Khetagoudar, M.C.; Uppar, D.S.; Navalgatti, C.M. Influence of Vacuum Packaging and Long Term Storage on Quality of Whole Chilli (Capsicum annuum L.). J. Food Sci. Technol. 2014, 51, 2827–2832. [Google Scholar] [CrossRef]
- A, B.; Patil, A.; Sadanande, D. Post Harvest Technologies in Fruits and Vegetables; Lambert Publishing House: Saarbrücken, Germany, 2022; ISBN 978-620-2-52755. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic Composition, Antioxidant Activity, and Polyphenol Oxidase (PPO) Activity of Quince (Cydonia Oblonga Miller) Varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia Oblonga Miller) Fruit (Pulp, Peel, and Seed) and Jam: Antioxidant Activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic Profile of Quince Fruit (Cydonia Oblonga Miller) (Pulp and Peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Z.; Zhu, M.; Wan, X.; Zhang, L. Effects of Thermal Processing on Transformation of Polyphenols and Flavor Quality. Curr. Opin. Food Sci. 2023, 51, 101014. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Górnaś, P.; Segliņa, D.; Pilarska, A.; Jesionowski, T. Physical and Bioactive Properties of Muffins Enriched with Raspberry and Cranberry Pomace Powder: A Promising Application of Fruit By-Products Rich in Biocompounds. Plant Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Antoniewska, A.; Martinez-Pineda, M.; Nawirska-Olszańska, A.; Zbikowska, A.; Baranowski, D. Black Chokeberry Fruit Polyphenols: A Valuable Addition to Reduce Lipid Oxidation of Muffins Containing Xylitol. Antioxidants 2020, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Benedek, C.; Bodor, Z.; Merrill, V.T.; Kókai, Z.; Gere, A.; Kovacs, Z.; Dalmadi, I.; Abrankó, L. Effect of Sweeteners and Storage on Compositional and Sensory Properties of Blackberry Jams. Eur. Food Res. Technol. 2020, 246, 2187–2204. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Stability of Phenolic Compounds, Antioxidant Activity and Colour through Natural Sweeteners Addition during Storage of Sour Cherry Puree. Food Chem. 2016, 196, 925–934. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Tai, N. Do Quality Characteristics and Antioxidant Activities of Aronia Jam Replacing Sucrose with Different Sugar Substances. Korean J. Food Nutr. 2014, 27, 888–896. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, M.-K.; Lee, H.-I. Physicochemical and Sensory Properties and Antioxidant Activity of Xylitol Candies Containing Yuja (Citrus Junos) Peels or Pulp. Foods 2024, 13, 2396. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, H.-W.; Hwang, K.-E.; Kim, C.-J.; Lee, H.-M.; Kim, O.-K.; Choi, K.-S.; Chung, H.-J. Effects of Replacing Sugar with Xylitol and Sorbitol on the Textural Properties and Sonsory Characteristics of Injeolmi. Korean J. Food Cook. Sci. 2013, 29, 825–831. [Google Scholar] [CrossRef]
- Anish, R.; Mariappan, V.; Joybari, M.M.; Abdulateef, A.M. Performance Comparison of the Thermal Behavior of Xylitol and Erythritol in a Double Spiral Coil Latent Heat Storage System. Therm. Sci. Eng. Prog. 2020, 15, 100441. [Google Scholar] [CrossRef]
- Zayapor, M.N.; Abdullah, A.; Mustapha, W.A.W. Influence of Sugar Concentration and Sugar Type on the Polyphenol Content and Antioxidant Activity in Spiced Syrup Preparation. Ital. J. Food Sci. 2021, 33, 96. [Google Scholar] [CrossRef]
- Zawawi, N.; Chong, P.J.; Tom, N.N.M.; Anuar, N.S.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef]
- Hadagali, M.D.; Chua, L.S. The Anti-Inflammatory and Wound Healing Properties of Honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of Various Polyphenols from a Diet Containing Moderate Amounts of Berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Effects of Fruit Juice Concentrate Production on Individual Anthocyanin Species in Elderberry. Int. J. Food Sci. Technol. 2016, 51, 641–648. [Google Scholar] [CrossRef]
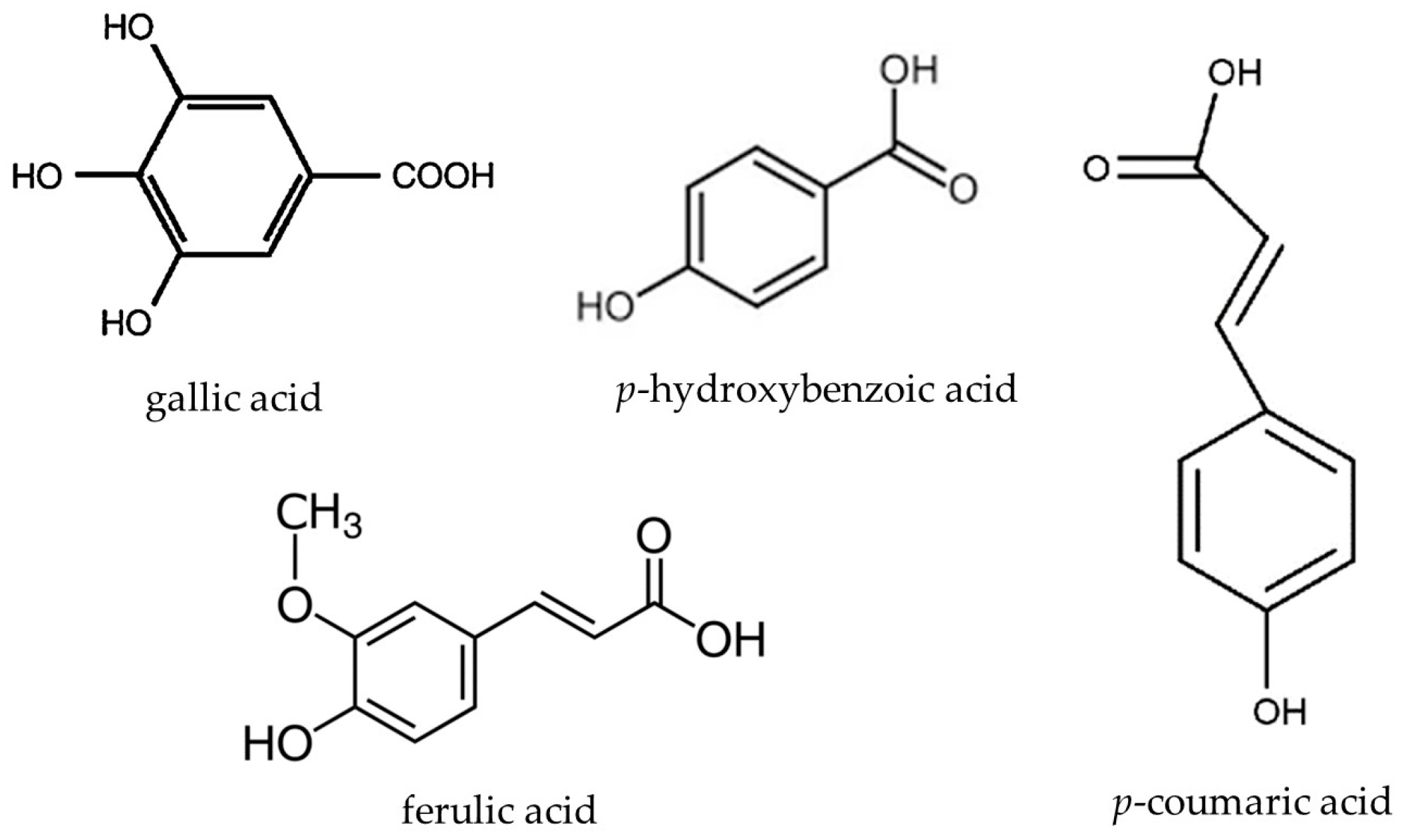
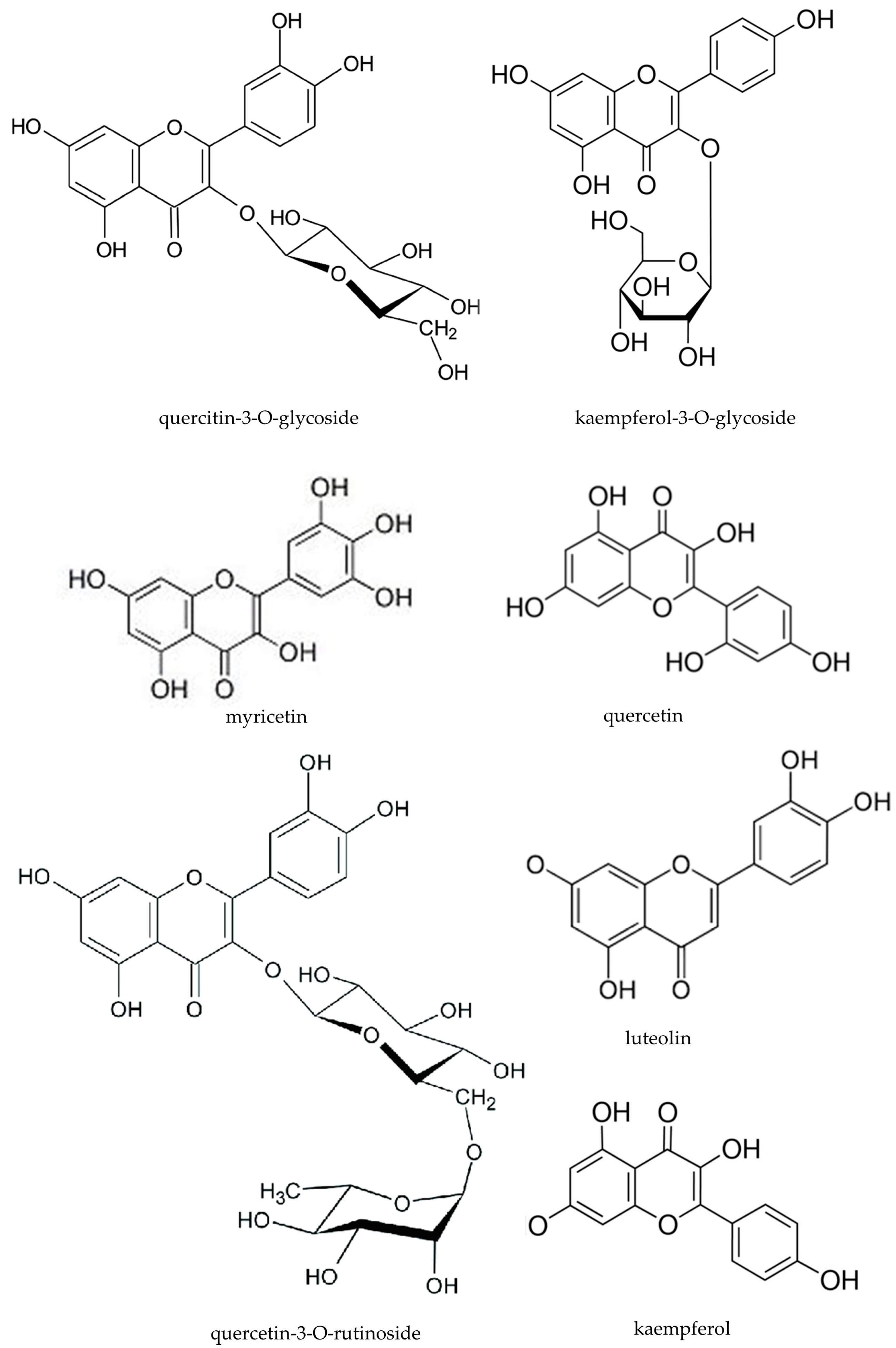
| Compounds | mg in 100 g f.w. | |
|---|---|---|
| LOD | LOQ | |
| gallic acid | 0.276 | 0.827 |
| p-hydroxybenzoic acid | 0.459 | 1.378 |
| p-coumaric acid | 0.147 | 0.441 |
| ferulic acid | 0.007 | 0.022 |
| quercitin-3-O-glycoside | 0.010 | 0.020 |
| kaempferol-3-O-glycoside | 0.037 | 0.110 |
| myricetin | 0.037 | 0.110 |
| quercetin | 0.092 | 0.276 |
| quercetin-3-O-rutinoside | 0.018 | 0.055 |
| luteolin | 0.002 | 0.006 |
| kaempferol | 0.037 | 0.110 |
| Quince Products | Dry Matter | Vitamin C (DHA + L-Asc) | DHA | L-Asc |
|---|---|---|---|---|
| Candied fruits | 70.83 ± 0.59 a | 11.27 ± 0.03 b | 1.91 ± 0.04 d | 9.36 ± 0.01 b |
| Fruits in syrup | 37.52 ± 1.22 d | 11.70 ± 0.24 b | 2.46 ± 0.24 c | 9.24 ± 0.01 b |
| Jam | 33.45 ± 0.10 e | 5.78 ± 0.05 c | 2.59 ± 0.04 c | 3.19 ± 0.01 c |
| Pressed juice | 6.80 ± 0.04 f | 34.48 ± 0.99 a | 8.22 ± 0.09 a | 26.26 ± 0.91 a |
| Syrup with cane sugar | 37.83 ± 0.99 d | 6.95 ± 0.02 c | 3.79 ± 0.02 b | 3.16 ± 0.00 c |
| Syrup with honey | 43.20 ± 0.03 c | 6.95 ± 0.08 c | 3.89 ± 0.04 b | 3.05 ± 0.04 c |
| Syrup with xylitol | 53.74 ± 0.11 b | 6.62 ± 0.04 c | 3.80 ± 0.00 b | 2.82 ± 0.04 c |
| ANOVA p-values | <0.001 | <0.001 | <0.001 | <0.001 |
| Quince Products | Polyphenols (Phenolic Acids + Flavonoids) | Phenolic Acids (sum) | Flavonoids (sum) |
|---|---|---|---|
| Candied fruits | 17.14 ± 0.53 c | 10.11 ± 0.68 c | 7.03 ± 0.23 a |
| Fruits in syrup | 9.09 ± 0.65 e | 4.88 ± 0.58 d | 4.21 ± 0.12 b |
| Jam | 8.17 ± 0.46 e | 6.31 ± 0.39 d | 1.86 ± 0.08 d |
| Pressed juice | 32.84 ± 0.98 a | 29.61 ± 0.93 a | 3.22 ± 0.06 c |
| Syrup with cane sugar | 14.21 ± 0.07 d | 12.09 ± 0.02 c | 2.12 ± 0.05 d |
| Syrup with honey | 14.48 ± 0.25 d | 12.43 ± 0.26 c | 2.05 ± 0.01 d |
| Syrup with xylitol | 19.71 ± 0.21 b | 16.28 ± 0.22 b | 3.43 ± 0.01 c |
| ANOVA p-values | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, R.; Kopczyńska, K.; Ponder, A.; Hallmann, E.; Żebrowska-Krasuska, M.; Średnicka-Tober, D. The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves. Foods 2025, 14, 1369. https://doi.org/10.3390/foods14081369
Kazimierczak R, Kopczyńska K, Ponder A, Hallmann E, Żebrowska-Krasuska M, Średnicka-Tober D. The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves. Foods. 2025; 14(8):1369. https://doi.org/10.3390/foods14081369
Chicago/Turabian StyleKazimierczak, Renata, Klaudia Kopczyńska, Alicja Ponder, Ewelina Hallmann, Małgorzata Żebrowska-Krasuska, and Dominika Średnicka-Tober. 2025. "The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves" Foods 14, no. 8: 1369. https://doi.org/10.3390/foods14081369
APA StyleKazimierczak, R., Kopczyńska, K., Ponder, A., Hallmann, E., Żebrowska-Krasuska, M., & Średnicka-Tober, D. (2025). The Concentrations of Phenolic Compounds and Vitamin C in Japanese Quince (Chaenomeles japonica) Preserves. Foods, 14(8), 1369. https://doi.org/10.3390/foods14081369








