Mung Bean Peptides Alleviate Dextran-Sulfate-Sodium-Induced Colitis Symptoms in Mice by Protecting the Intestinal Mechanical Barrier and Regulating Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. MBPs Preparation
2.2. Scanning Electron Microscopy
2.3. Fourier Transform Infrared Spectroscopy
2.4. Determination of Amino Acid Composition
2.5. Determination of Relative Molecular Mass Distribution
2.6. Determination of Peptide Sequences
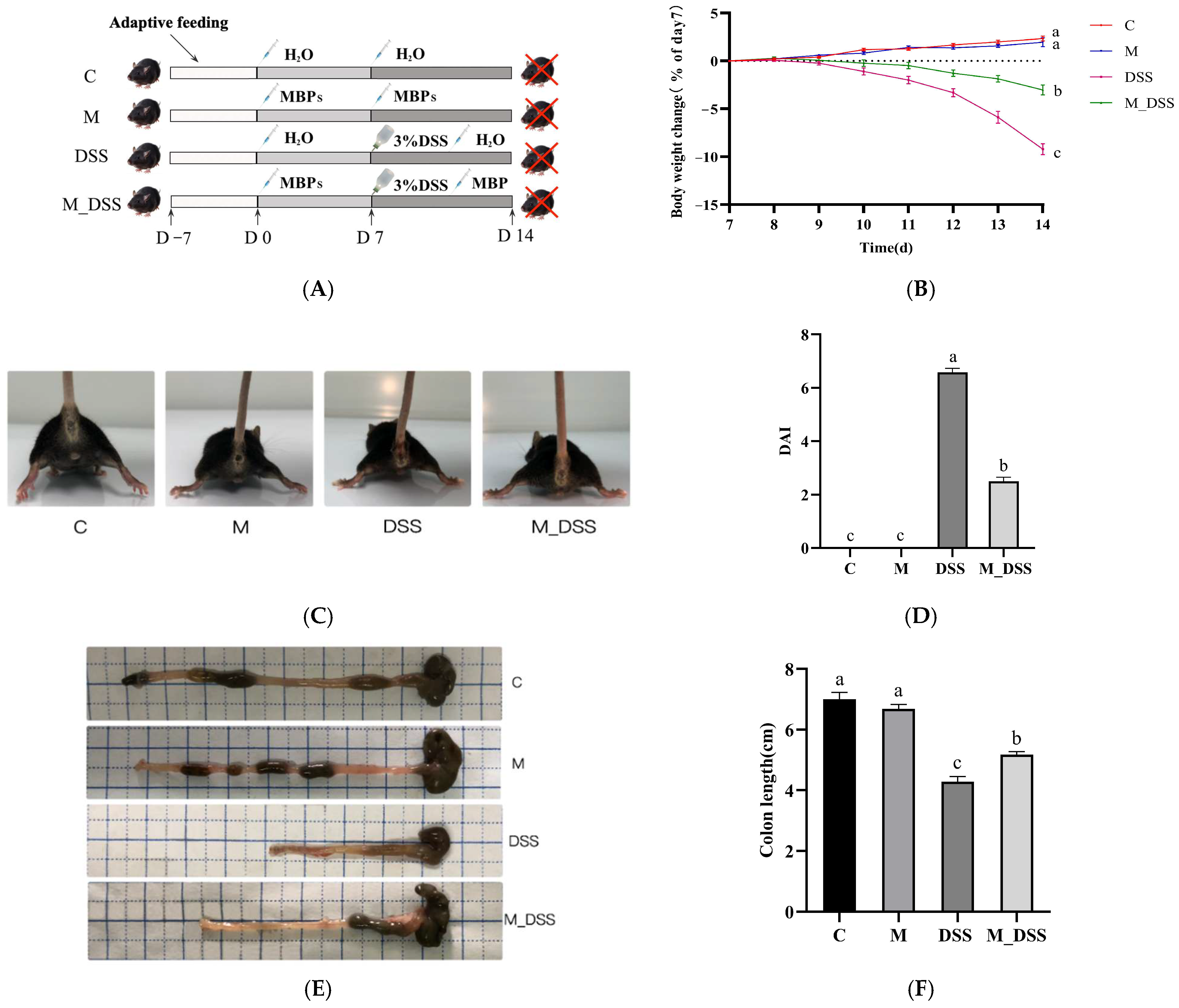
2.7. Animal Experimental Design
2.8. Disease Activity Index (DAI)
2.9. Histological Analysis of the Colon
2.10. Immunohistochemistry Staining
2.11. Measurement of Inflammatory Cytokines in the Serum
2.12. Analysis of Gut Microbiota in Colon Content
2.13. Statistical Analysis
3. Results
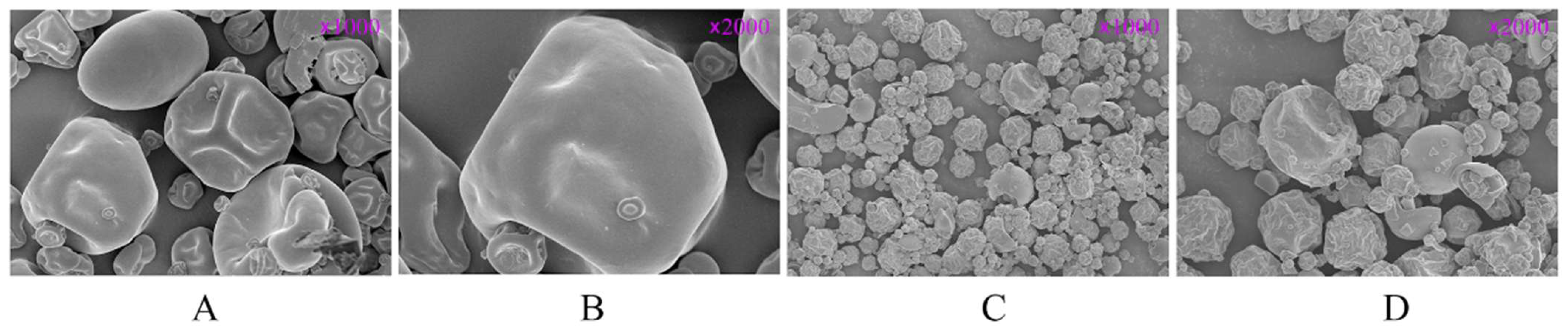
3.1. Scanning Electron Microscope Analysis
3.2. FTIR Spectroscopy Analysis
3.3. Amino Acid Composition of MBPs
3.4. Molecular Weight Distribution of MBPs
3.5. MBPs Peptide Sequences
3.6. Effects of MBPs on Colitis Symptoms
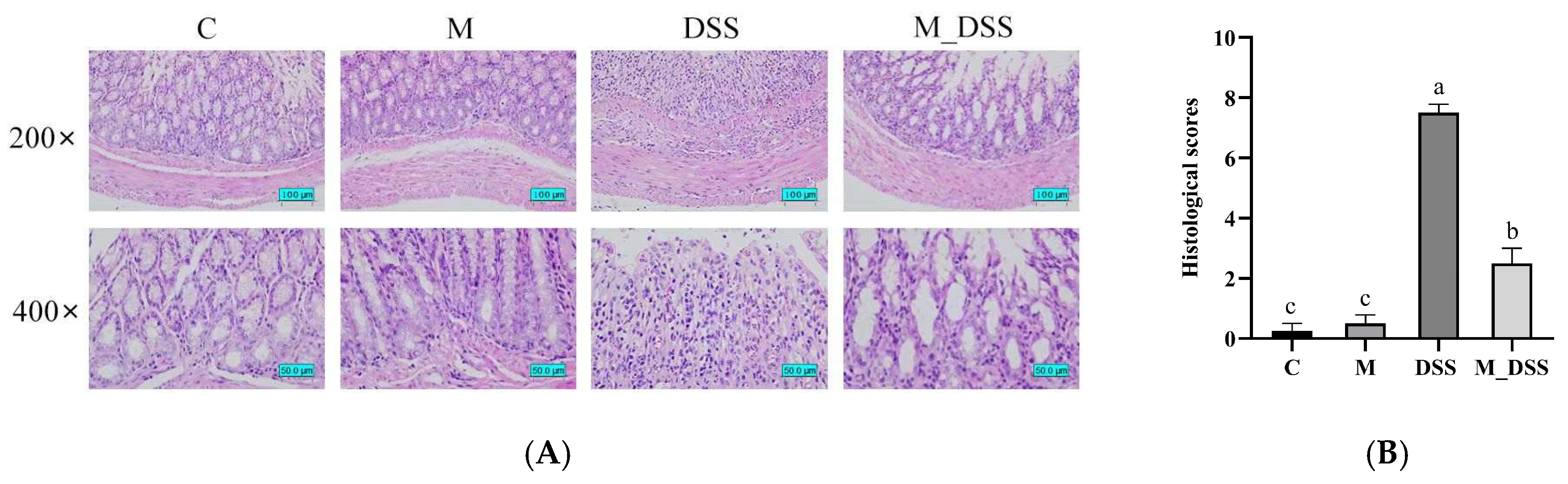
3.7. Effects of MBPs on Colon Tissue Damage
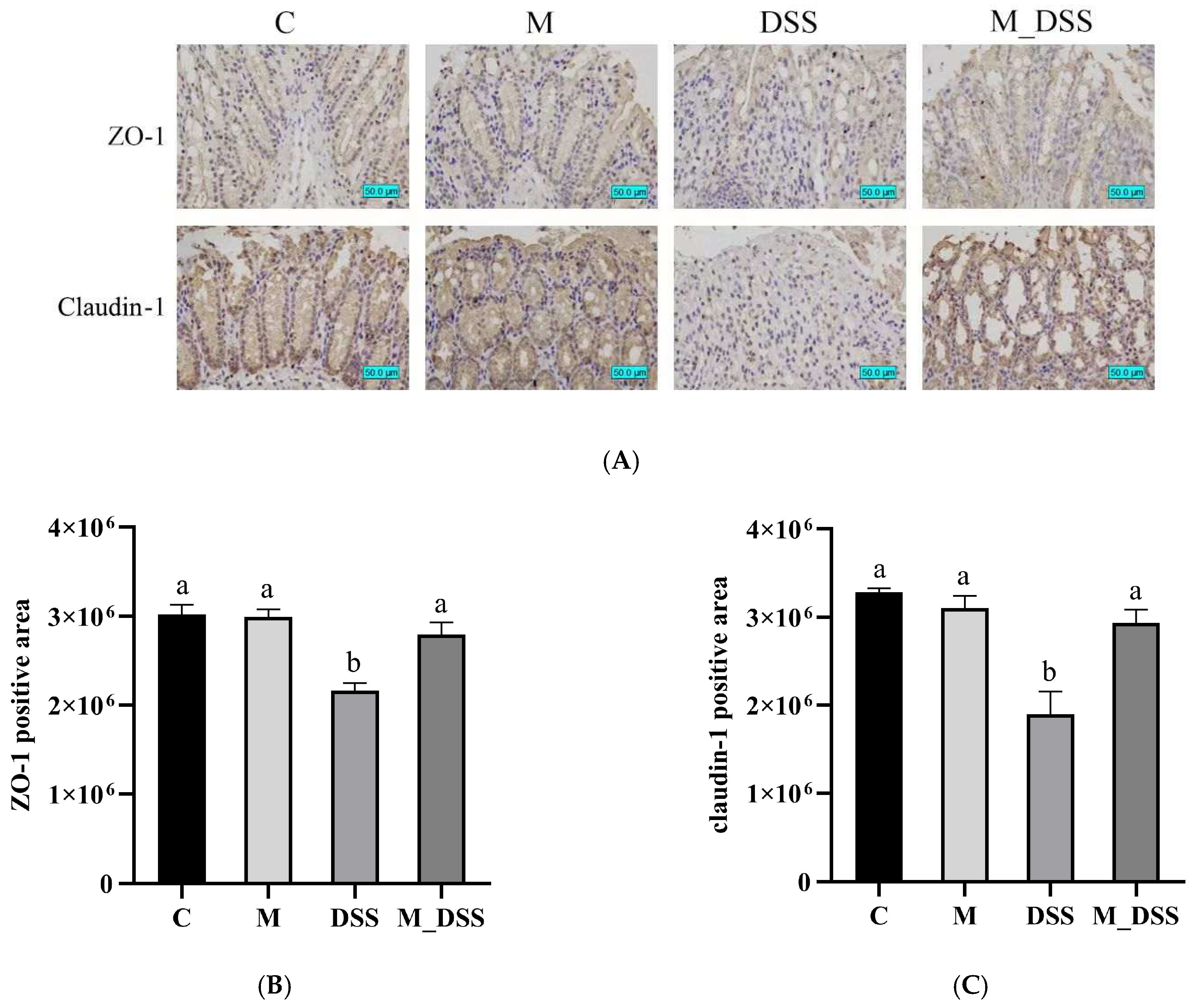
3.8. Effects of MBPs on Intestinal Epithelial Barrier Function
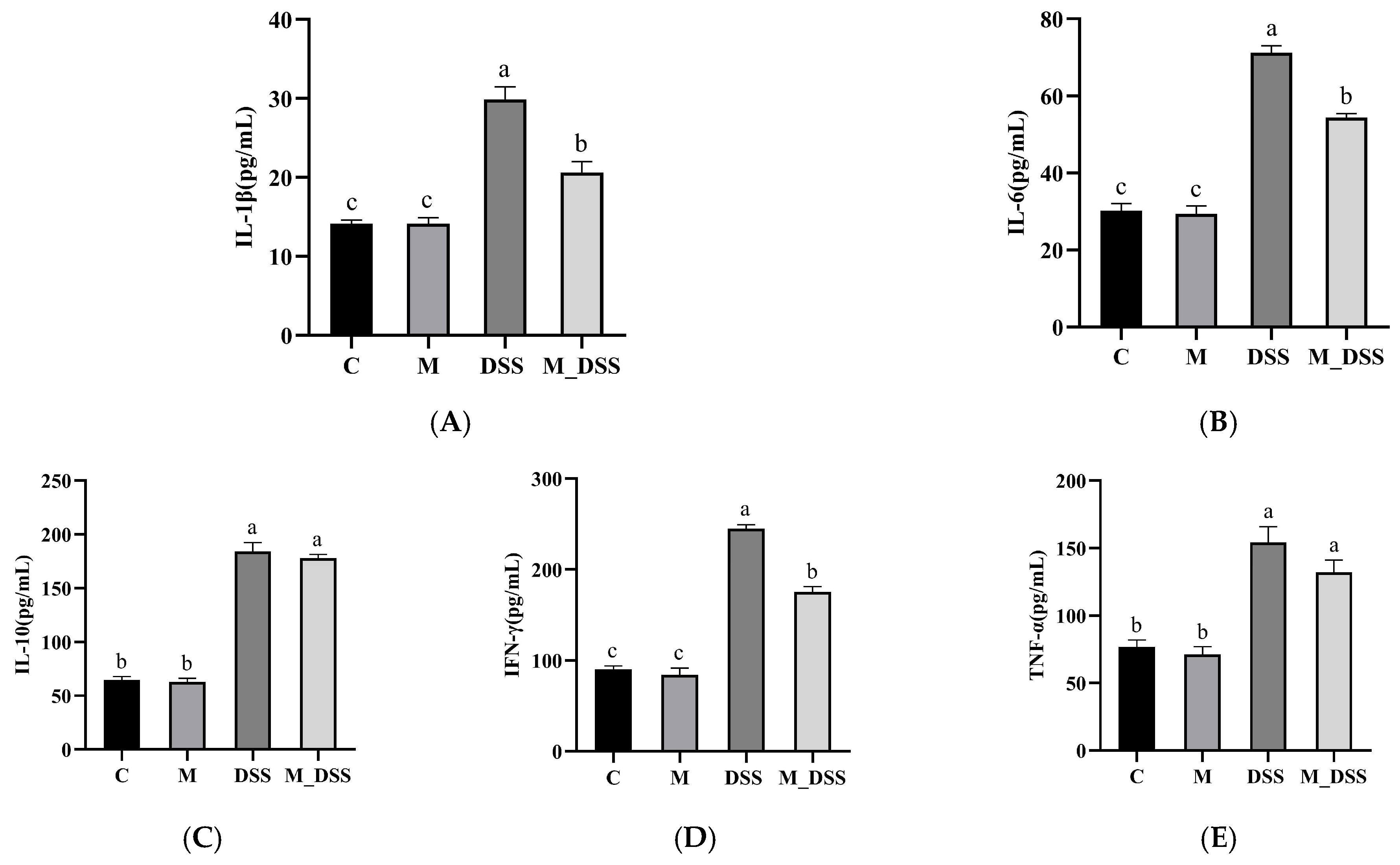
3.9. Effects of MBPs on Inflammatory Cytokines in Serum
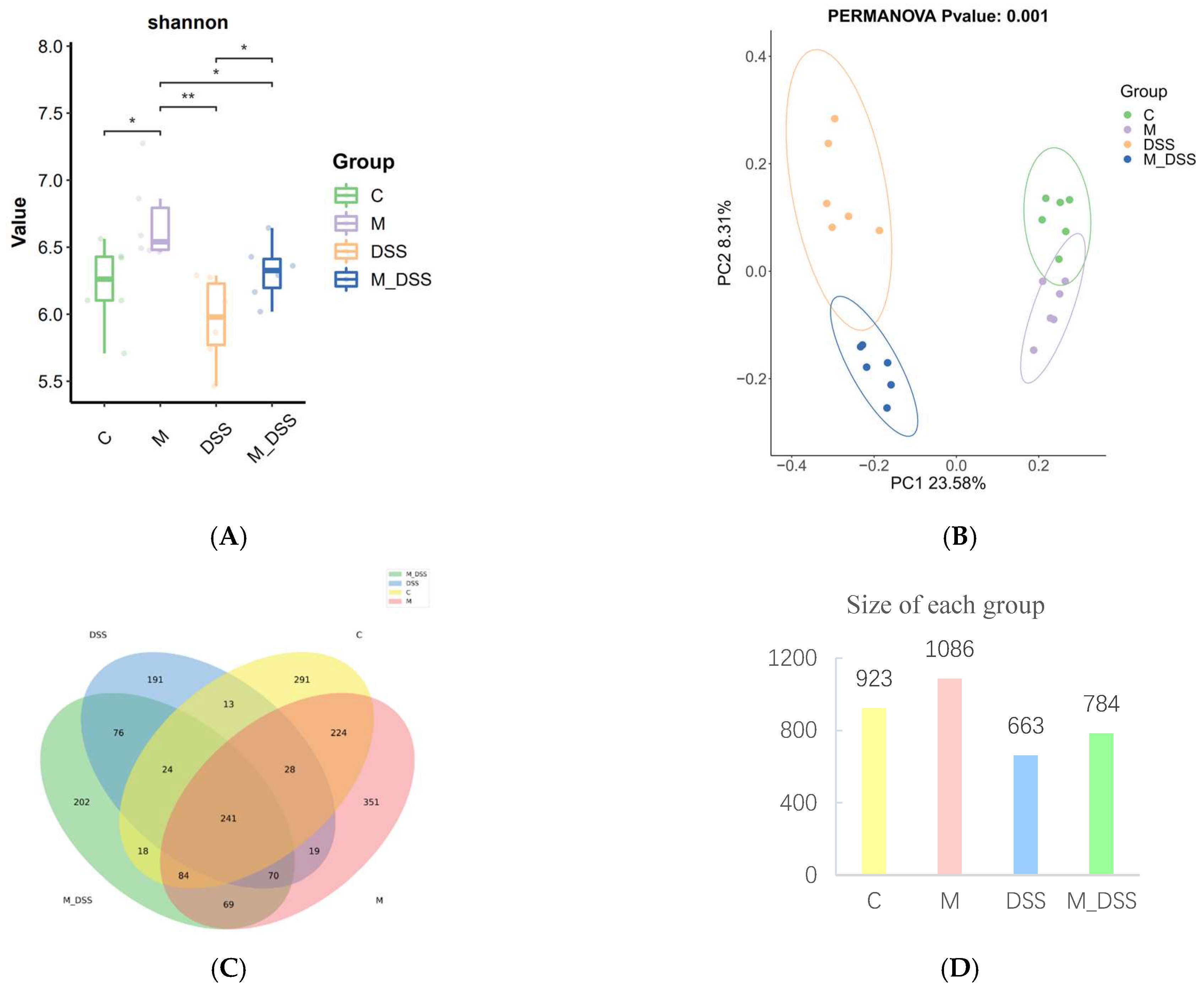
3.10. Effects of MBPs on Gut Microbiota Diversity
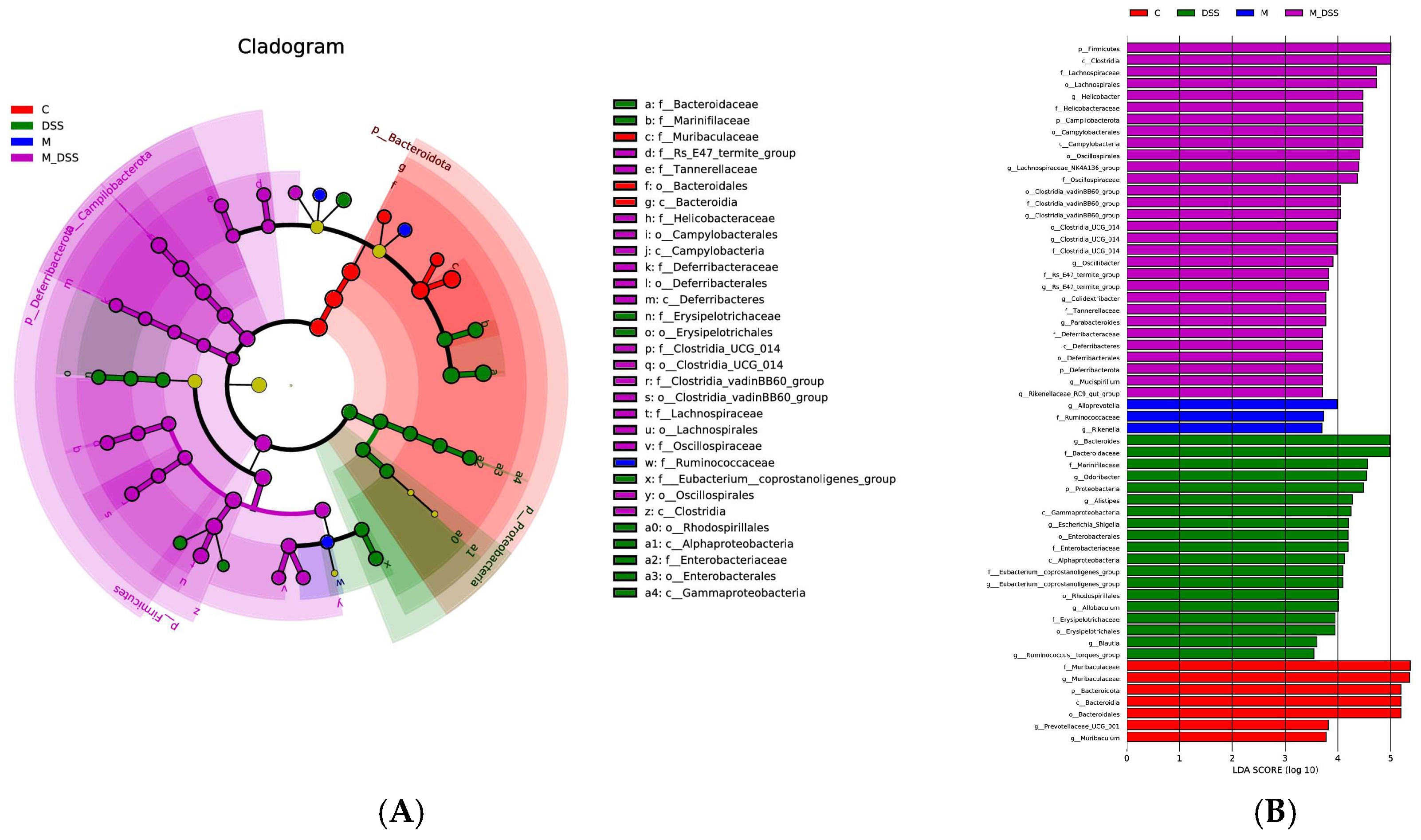
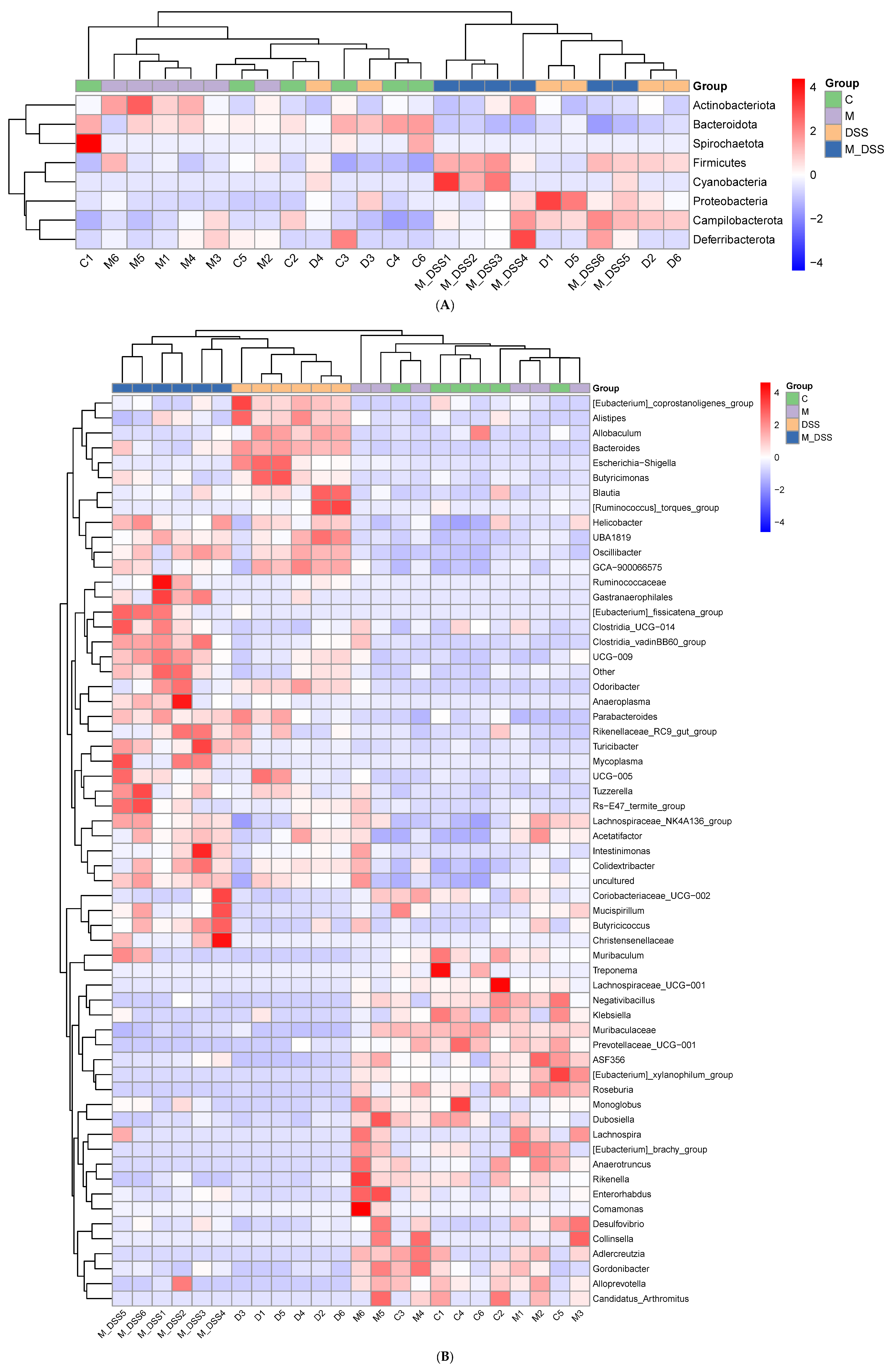
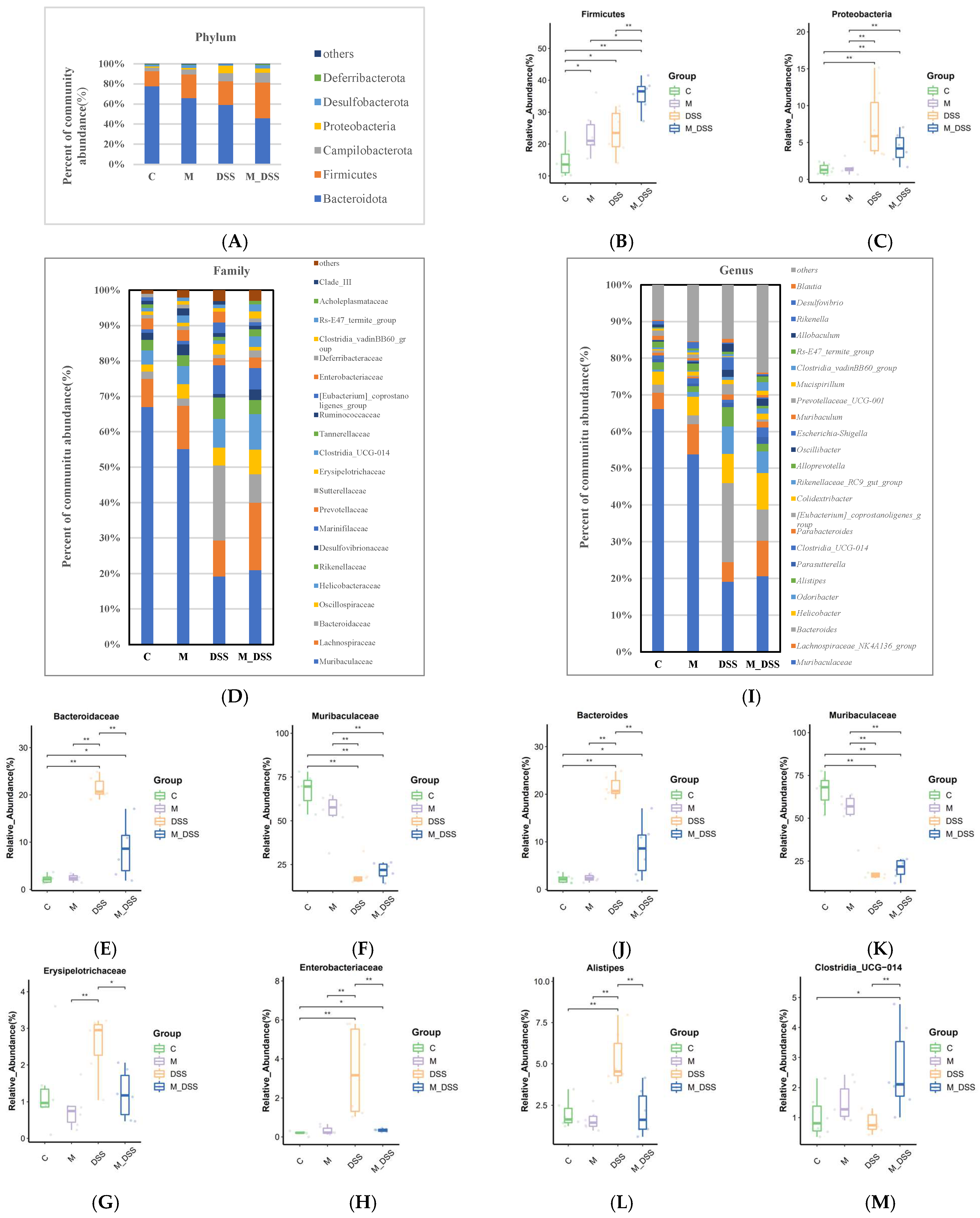
3.11. Effects of MBPs on the Gut Microbiota Structure
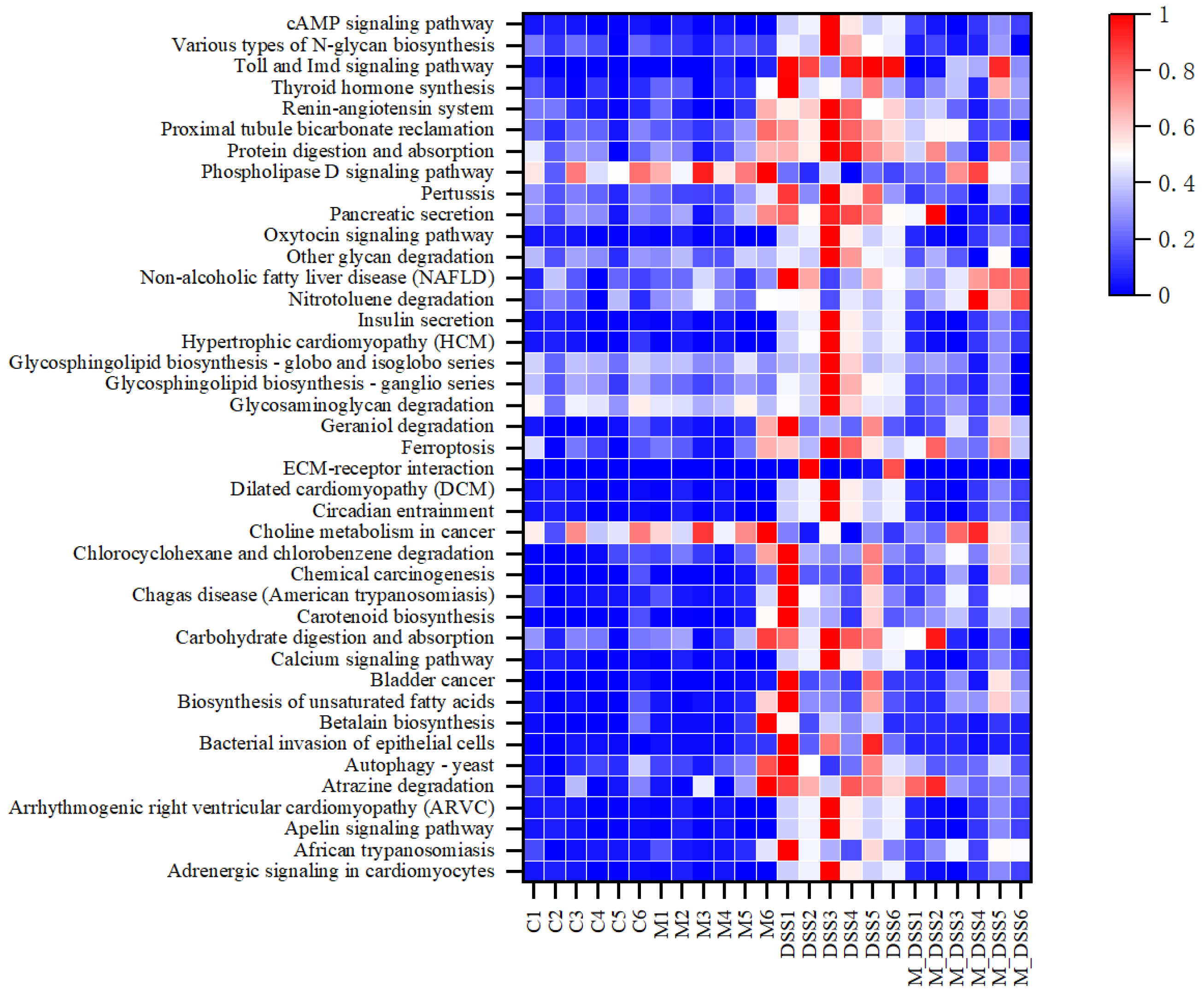
3.12. Effects of MBPs on Enrichment Analysis of Intestinal Microbiota Pathway
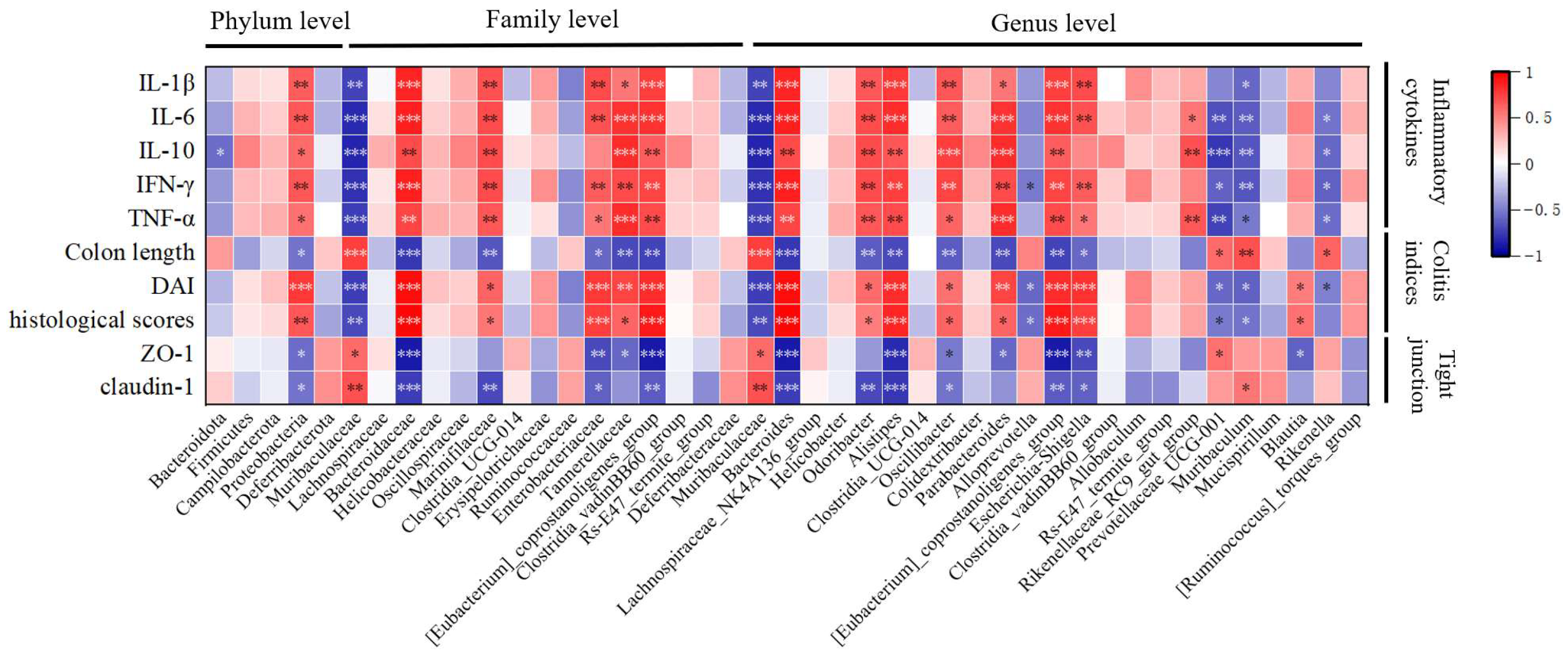
3.13. Correlation Analysis of Colitis Indicators and Gut Microbiota Under the Influence of MBPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, M.T.; Gu, M.L.; Zhou, X.X.; Yu, M.S.; Pan, H.H.; Ji, F.; Ding, C.Y. Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis. World J. Gastroenterol. 2019, 25, 5800–5813. [Google Scholar] [CrossRef] [PubMed]
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, Y.; Huang, G.; Qian, W.; Xiong, J.; Wang, X.; Zheng, W.; Yao, W. Effect of synbiotic containing Bacillus coagulans and lactulose on gut health in mice with DSS-induced ulcerative colitis. Acta Microbiol. Sin. 2022, 62, 869–881. [Google Scholar]
- Shang, K.; Zhao, Z.; Chen, H.; Bian, X.; Zhong, X.; Hu, X.; Lin, X.; Wang, L. Anti-Inflammatory Potential of Wampee (Clausena lansium (Lour.) Skeels) Polyphenol Extract in Ulcerative Colitis: Gut Microbiota and TLR4-p38 MAPK/NF-κB Signaling Axis Regulation. Foods 2025, 14, 619. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Lin, K.; Xu, J.; Zhou, B.; Xie, D.; Ma, J.; Yang, L.; Su, C.; Yang, L. Antimicrobial peptide DP7 alleviates dextran sulfate sodium (DSS)-induced colitis via modifying gut microbiota and regulating intestinal barrier function. MedComm 2025, 6, e70085. [Google Scholar] [CrossRef]
- Wang, Q.; Im, Y.; Park, J.; Lee, H.L.; Ryu, D.G.; Kim, H. Eisenia bicyclis Extract Ameliorates Colitis in In Vitro and In Vivo Models Through Modulation of mTOR Axis and Gut Microbiota Composition. Foods 2025, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Huan, Y.; Chang, Y.; Wang, Y.; Tang, Q. Investigating the Alleviating Effect of Fucoidan from Apostichopus japonicus on Ulcerative Colitis by Mice Experiments and In Vitro Simulation of Human Fecal Fermentation. Foods 2025, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Liu, C.; Zhang, H.; Li, S.; Zhang, T.; Yu, Z.; Chi, X.; Zhang, Z.; Du, Z. Programmable Food-Derived Peptide Coassembly Strategies for Boosting Targeted Colitis Therapy by Enhancing Oral Bioavailability and Restoring Gut Microenvironment Homeostasis. ACS Nano 2025, 19, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, F.; Hodin, R.A. Impact of Modern Drug Therapy on Surgery: Ulcerative Colitis. Visc. Med. 2018, 34, 426–431. [Google Scholar] [CrossRef]
- Harmand, P.O.; Solassol, J. Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT. Genes 2020, 11, 1212. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, Y.; Mei, F.; Dong, X.; Xia, G.; Shen, X. Tilapia head glycolipids protect mice against dextran sulfate sodium-induced colitis by ameliorating the gut barrier and suppressing NF-kappa B signaling pathway. Int. Immunopharmacol. 2021, 96, 107802. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary Tangeretin Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Inhibiting Inflammatory Response, Restoring Intestinal Barrier Function, and Modulating Gut Microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Liu, D. Research progress in intestinal tight junction membrane proteins. Pract. Pharm. Clin. Rem. 2019, 22, 1214–1219. [Google Scholar]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, C.; Wang, X.; Tang, J.; Wu, Z.; Huang, Y.; Shao, W.; Geng, K.; Xie, H.; Pu, Z. Schisandrin protects against ulcerative colitis by inhibiting the SGK1/NLRP3 signaling pathway and reshaping gut microbiota in mice. Chin. Med. 2023, 18, 112. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Zheng, H.; Zhong, J.; Xu, L.; Jiang, X.; Ni, S.; Li, S. Protective Effects of Berberine Hydrochloride on the Intestinal Mucosal Mechanical Barrier in Mice with Ulcerative Colitis. Chin. J. Mod. Appl. Pharm. 2018, 35, 1765–1770. [Google Scholar]
- Li, F.; Zhao, S.; Zhang, Q.; Yin, S. Functions of Dietary Peptides and Its Applications in Food Industry. Food Res. Dev. 2020, 41, 210–217. [Google Scholar]
- Chen, T.; Hong, L.; Wang, P.; Teng, Q.; Fang, F.; Liu, Q. Protective Effect and Gut Microbiota Modulation of Grifola frondosa Antioxidant Peptides in Sodium Dextran Sulfate-Induced Ulcerative Colitis Mice. Biotechnol. Appl. Biochem. 2025, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Guo, Y.; Zhao, J.; Yan, W. Silkworm pupa peptide ameliorates dextrose sodium sulfate-induced colitis by enhancing gut barriers and modifying gut flora. Food Sci. Hum. Wellness 2025, 14, 9250050. [Google Scholar] [CrossRef]
- Li, M. Research on the Preparation of Mung Bean Polypeptide by Enzyme Technology and Its Effect on the Activity of Alcohol Dehydrogenase. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2015. [Google Scholar]
- Diao, J.; Wang, K.; Zhang, L.; Cao, L. Separation and Purification of ACE-inhibitory Peptides from Mung Bean by Simulated Moving Bed. J. Chin. Inst. Food Sci. Technol. 2017, 17, 142–150. [Google Scholar]
- Li, L.; Tian, Y.; Zhang, S.; Feng, Y.; Wang, H.; Cheng, X.; Ma, Y.; Zhang, R.; Wang, C. Regulatory Effect of Mung Bean Peptide on Prediabetic Mice Induced by High-Fat Diet. Front. Nutr. 2022, 9, 913016. [Google Scholar] [CrossRef]
- Diao, J.; Chi, Z.; Liu, Y.; Qu, L.; Zhang, L. Immunoregulatory Activity of Mung Bean Peptides. Food Sci. 2020, 41, 133–138. [Google Scholar]
- Diao, J.; Liu, Y.; Li, Z.; Yu, D.; Zuo, F.; Zhang, L. Protective Effect of Mung Bean Hydrolysate on Lipopolysaccharide-induced Acute Lung Injury in Mice. Food Sci. 2020, 41, 176–181. [Google Scholar]
- Yu, D.; Zhou, W.; Guo, Z.; Diao, J.; Chi, Z.; Zhang, L. Anti-inflammatory Effect of Mung Bean Peptide on Lipopolysaccharide-induced Macrophage RAW264.7. J. Chin. Inst. Food Sci. Technol. 2020, 20, 41–48. [Google Scholar]
- Li, L.; Tian, Y.; Feng, Y.; Zhang, S.; Jiang, Y.; Zhang, Y.; Zhan, Y.; Wang, C. Improvement in Mung Bean Peptide on High-Fat Diet-Induced Insulin Resistance Mice Using Untargeted Serum Metabolomics. Front. Nutr. 2022, 9, 893270. [Google Scholar] [CrossRef]
- Diao, J. Immunomodulatory Activity and Mechanism of Mung Bean Peptides on Macrophage. Ph.D. Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, 2019. [Google Scholar]
- Yang, J.; Guo, Z.; Diao, J.; Ma, P.; Li, C.; Yu, D.; Wang, K.; Zhang, L. Effects of Mung Bean Peptides on Cell Proliferation and Immunologically Active Substances of RAW264.7 Macrophages. J. Chin. Inst. Food Sci. Technol. 2019, 19, 22–30. [Google Scholar]
- GB 5009.124-2016; National Standards for Food Safety: Determination of Amino Acids in Foods. National Health and Family Planning Commission: Beijing, China; China Food and Drug Administration: Beijing, China, 2016.
- Gu, Z.; Zhu, Y.; Jiang, S.; Xia, G.; Li, C.; Zhang, X.; Zhang, J.; Shen, X. Tilapia head glycolipids reduce inflammation by regulating the gut microbiota in dextran sulphate sodium-induced colitis mice. Food Funct. 2020, 11, 3245–3255. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Zhu, Y.; An, Y.; Huang, Y.; Sun, B.; Xia, X. Structural and functional properties of hemp protein isolate hydrolysates obtained with different proteases. Food Ferment. Ind. 2023, 49, 111–117. [Google Scholar]
- Liu, L.; Wang, H.; Li, D.; Yin, G.; Kang, H. Enzymatic hydrolysis and structural properties of egg white ovalbumin. Food Sci. 2016, 37, 54–61. [Google Scholar]
- Liu, W.; Chen, X.; Li, H.; Zhang, J.; An, J.; Liu, X. Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods 2022, 11, 2361. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- de Assis, P.O.A.; Guerra, G.C.B.; Araujo, D.F.S.; de Andrade, L.; de Araujo, A.A.; de Araujo, R.F.J.; de Carvalho, T.G.; de Souza, M.F.V.; Borges, G.; Lima, M.D.S.; et al. Intestinal anti-inflammatory activity of xique-xique (Pilosocereus gounellei A. Weber ex K. Schum. Bly. Ex Rowl) juice on acetic acid-induced colitis in rats. Food Funct. 2019, 10, 7275–7290. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular mechanisms of action of anti-TNF-α agents–Comparison among therapeutic TNF-α antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef]
- Li, S.; Tao, L.; Peng, S.; Yu, X.; Ma, X.; Hu, F. Structural and antioxidative properties of royal jelly protein by partial enzymatic hydrolysis. Food Sci. Hum. Wellness 2023, 12, 1820–1827. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Li, H.; Liao, T.; Zu, X. Preparation, in vitro anti-inflammatory activity evaluation and structural characterization of Acipenser schrenckii milt peptide. Food Sci. 2024, 45, 112–120. Available online: https://link.cnki.net/urlid/11.2206.TS.20231225.1115.012 (accessed on 2 January 2025).
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Liu, B.-Q.; Cui, B.; Wen, L.; Xu, Z.; Chen, M.-L.; Wu, H. Alanine Substitution to Determine the Effect of LR5 and YR6 Rice Peptide Structure on Antioxidant and Anti-Inflammatory Activity. Nutrients 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, X.; Huang, X. Bletilla striata polysaccharide up-regulates the expression of tight junction protein occludin in intestinal mucosa of mice with ulcerative colitis. Basic Clin. Med. 2021, 41, 941–945. [Google Scholar]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Han, J.; Lu, C.; Zhou, J.; Li, Y.; Su, X.; Chen, Y.; Wang, Q. Anti-fatigue and gut microbiota modulation effects of tuna dark meat hydrolysate in mice. Sci. Technol. Food Ind. 2019, 40, 314–320, 326. [Google Scholar]
- Lim, S.-M.; Kim, D.-H. Bifidobacterium adolescentis IM38 ameliorates high-fat diet–induced colitis in mice by inhibiting NF-κB activation and lipopolysaccharide production by gut microbiota. Nutr. Res. 2017, 41, 86–96. [Google Scholar] [CrossRef]
- Wang, P.-P.; Cheng, X.-Q.; Dou, Z.-J.; Fan, Y.-Q.; Chen, J.; Zhao, L.; Han, J.-X.; Lin, X.-W.; Wang, B. Inhibiting the CB1 receptor in CIH-induced animal model alleviates colon injury. Appl. Microbiol. Biotechnol. 2024, 108, 380. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Sheng, K.; Xu, Y.; Kong, X.; Wang, J.; Zha, X.; Wang, Y. Probiotic Bacillus cereus Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice through Improvement of the Intestinal Barrier Function, Anti-Inflammation, and Gut Microbiota Modulation. J. Agric. Food Chem. 2021, 69, 14810–14823. [Google Scholar] [CrossRef]
- Harrison, C.A.; Laubitz, D.; Ohland, C.L.; Midura-Kiela, M.T.; Patil, K.; Besselsen, D.G.; Jamwal, D.R.; Jobin, C.; Ghishan, F.K.; Kiela, P.R. Microbial dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. 2018, 11, 1329–1341. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2021, 42, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-kappaB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef]
- Jacobson, A.N.; Choudhury, B.P.; Fischbach, M.A. The Biosynthesis of Lipooligosaccharide from Bacteroides thetaiotaomicron. mBio 2018, 9, e02289-17. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Wu, W.; Lv, L.; Bian, X.; Yang, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; et al. Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice. Appl. Microbiol. Biotechnol. 2020, 104, 5915–5928. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Wei, G.Y.; Zhang, R.P.; Zhu, Y.; Wang, Z.; Wang, S.M.; Du, G.H. Cryptotanshinone alleviates chemotherapy-induced colitis in mice with colon cancer via regulating fecal-bacteria-related lipid metabolism. Pharmacol. Res. 2021, 163, 105232. [Google Scholar] [CrossRef]
- Valentini, F.; Evangelisti, M.; Arpinelli, M.; Di Nardo, G.; Borro, M.; Simmaco, M.; Villa, M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: A pilot study. Sleep Med. 2020, 76, 140–147. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, P.Y.; Chen, W.J.; Wu, S.H.; Pan, M.H. Lactobacillus fermentum V3 ameliorates colitis-associated tumorigenesis by modulating the gut microbiome. Am. J. Cancer Res. 2020, 10, 1170–1181. [Google Scholar] [PubMed]
- Huang, A.; Cai, R.; Wang, Q.; Shi, L.; Li, C.; Yan, H. Dynamic Change of Gut Microbiota During Porcine Epidemic Diarrhea Virus Infection in Suckling Piglets. Front. Microbiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef]
- Liu, M.; Du, K. Experimental study on the effect of phospholipase D2 on the growth of colorectal cancer cells in vivo. J. Mod. Med. Health 2022, 38, 2345–2349. [Google Scholar]
- Romano, K.A.; Martinez-Del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef]
- Guo, D.; Liu, D.; Zhang, G. Research progress on the relationship between DNA methylation and atherosclerosis. Shandong Med. J. 2018, 58, 107–110. [Google Scholar]
- Li, X.; Zhang, C. The role of DNA methylation in common autoimmune diseases. J. Med. Res. 2018, 47, 180–182. [Google Scholar]
- Liu, G. Relationship Between Cognitive Impairment and Genome-Wide Methylation in Alzheimer’s Disease. Master’s Thesis, Shanxi Medical University, Jinzhong, China, 2020. [Google Scholar]
- Xu, Y.; Gui, S.; Yang, H.; Xu, W.; Chen, Y.; Pan, F. Research progress of DNA methylation in the pathogenesis of ankylosing spondylitis. Acta Univ. Med. Anhui 2023, 58, 170–175. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Che, Y.; Chen, M.; Zhang, Y.; Zhang, W. Identification of target genes and prognostic biomarkers in colorectal olorectal cancer via bioinformatics. Chin. J. Bioinf. 2021, 19, 195–204. [Google Scholar]
- Liu, Y.; Yang, M.; Tang, L.; Wang, F.; Huang, S.; Liu, S.; Lei, Y.; Wang, S.; Xie, Z.; Wang, W.; et al. TLR4 regulates RORgammat(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
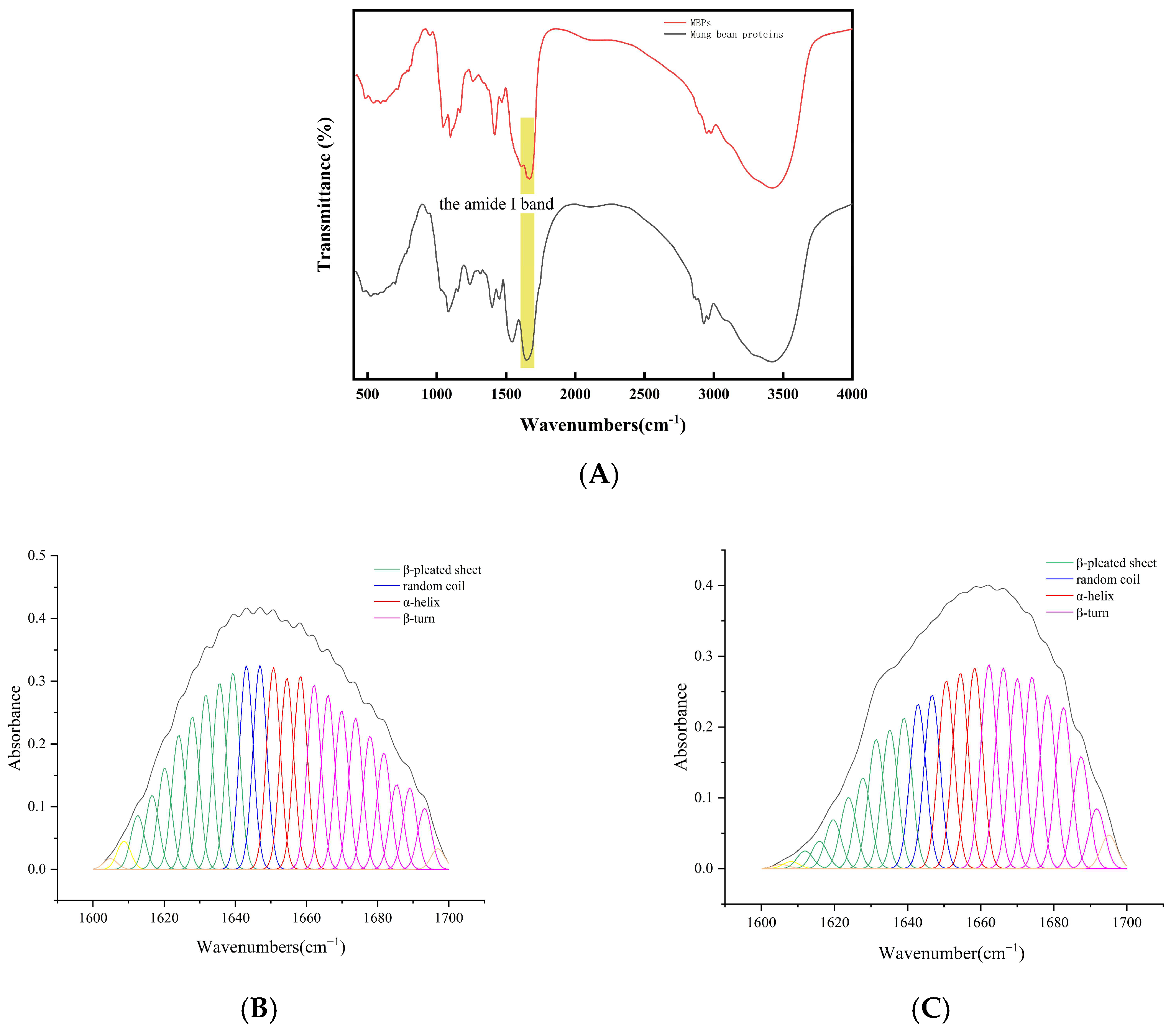

| Scheme 1 | α-Helix | β-Pleated Sheet | Β-Turn | Random Coil |
|---|---|---|---|---|
| the peak position of the amide I band (cm−1) | 1650–1658 | 1610–1640 | 1660–1695 | 1640–1650 |
| mung bean protein (%) | 18.25 | 33.43 | 35.65 | 12.67 |
| MBPs (%) | 20.22 | 23.35 | 44.73 | 11.70 |
| Amino Acid Name | Amino Acid Content (g/100g) | Amino Acid Name | Amino Acid Content (g/100g) |
|---|---|---|---|
| Asp | 8.568 ± 0.324 | Ile | 2.513 ± 0.103 |
| Thr | 2.263 ± 0.096 | Leu | 5.131 ± 0.231 |
| Ser | 4.142 ± 0.188 | Tyr | 2.288 ± 0.087 |
| Glu | 13.644 ± 0.599 | Phe | 3.995 ± 0.201 |
| Gly | 2.832 ± 0.133 | Lys | 4.728 ± 0.223 |
| Ala | 2.711 ± 0.141 | His | 1.632 ± 0.0735 |
| Val | 3.323 ± 0.158 | Arg | 4.826 ± 0.256 |
| Pro | 2.973 ± 0.132 |
| Relative Molecular Mass | Retention Time (min) | Molecular Weight at Peak | Peak Area PERCENTAGE (%) |
|---|---|---|---|
| >10,000 | 11.566 ± 0.043 | 16.239 | 1.66 ± 0.08 |
| 10,000~5000 | 13.677 ± 0.034 | 5001 | 0.92 ± 0.04 |
| 5000~3000 | 14.592 ± 0.026 | 3001 | 1.80 ± 0.07 |
| 3000~2000 | 15.320 ± 0.044 | 1999 | 3.15 ± 0.11 |
| 2000~1000 | 16.292 ± 0.039 | 1162 | 15.32 ± 0.66 |
| 1000~500 | 17.437 ± 0.028 | 614 | 30.68 ± 1.23 |
| 500~180 | 18.833 ± 0.033 | 282 | 35.72 ± 1.50 |
| <180 | 20.517 ± 0.040 | 110 | 10.72 ± 0.06 |
| Sequences | Peptide Ranker Score | Molecular Weight | Protein IDs |
|---|---|---|---|
| FPGAF | 0.98 | 537 | A0A1S3U4I8 |
| NFFAF | 0.98 | 644 | A0A3P9QP39 |
| NPFYF | 0.98 | 686 | A0A3P9QP39 |
| SDRWF | 0.95 | 709 | A0A3P9QP39 |
| GGGFR | 0.93 | 492 | A0A1S3UBT1 |
| NPHRFQDFFL | 0.91 | 1320 | A0A3P9QP39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Diao, J.; Feng, Y.; Zhang, S.; Sheng, Y.; Wang, C. Mung Bean Peptides Alleviate Dextran-Sulfate-Sodium-Induced Colitis Symptoms in Mice by Protecting the Intestinal Mechanical Barrier and Regulating Gut Microbiota. Foods 2025, 14, 1363. https://doi.org/10.3390/foods14081363
Xu C, Diao J, Feng Y, Zhang S, Sheng Y, Wang C. Mung Bean Peptides Alleviate Dextran-Sulfate-Sodium-Induced Colitis Symptoms in Mice by Protecting the Intestinal Mechanical Barrier and Regulating Gut Microbiota. Foods. 2025; 14(8):1363. https://doi.org/10.3390/foods14081363
Chicago/Turabian StyleXu, Chong, Jingjing Diao, Yuchao Feng, Shu Zhang, Yanan Sheng, and Changyuan Wang. 2025. "Mung Bean Peptides Alleviate Dextran-Sulfate-Sodium-Induced Colitis Symptoms in Mice by Protecting the Intestinal Mechanical Barrier and Regulating Gut Microbiota" Foods 14, no. 8: 1363. https://doi.org/10.3390/foods14081363
APA StyleXu, C., Diao, J., Feng, Y., Zhang, S., Sheng, Y., & Wang, C. (2025). Mung Bean Peptides Alleviate Dextran-Sulfate-Sodium-Induced Colitis Symptoms in Mice by Protecting the Intestinal Mechanical Barrier and Regulating Gut Microbiota. Foods, 14(8), 1363. https://doi.org/10.3390/foods14081363






