Construction of a Yeast Protein-Chitooligosaccharide W/O/W Emulsion System for Carrying and Stabilization of Betacyanins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of YP-COS Complex
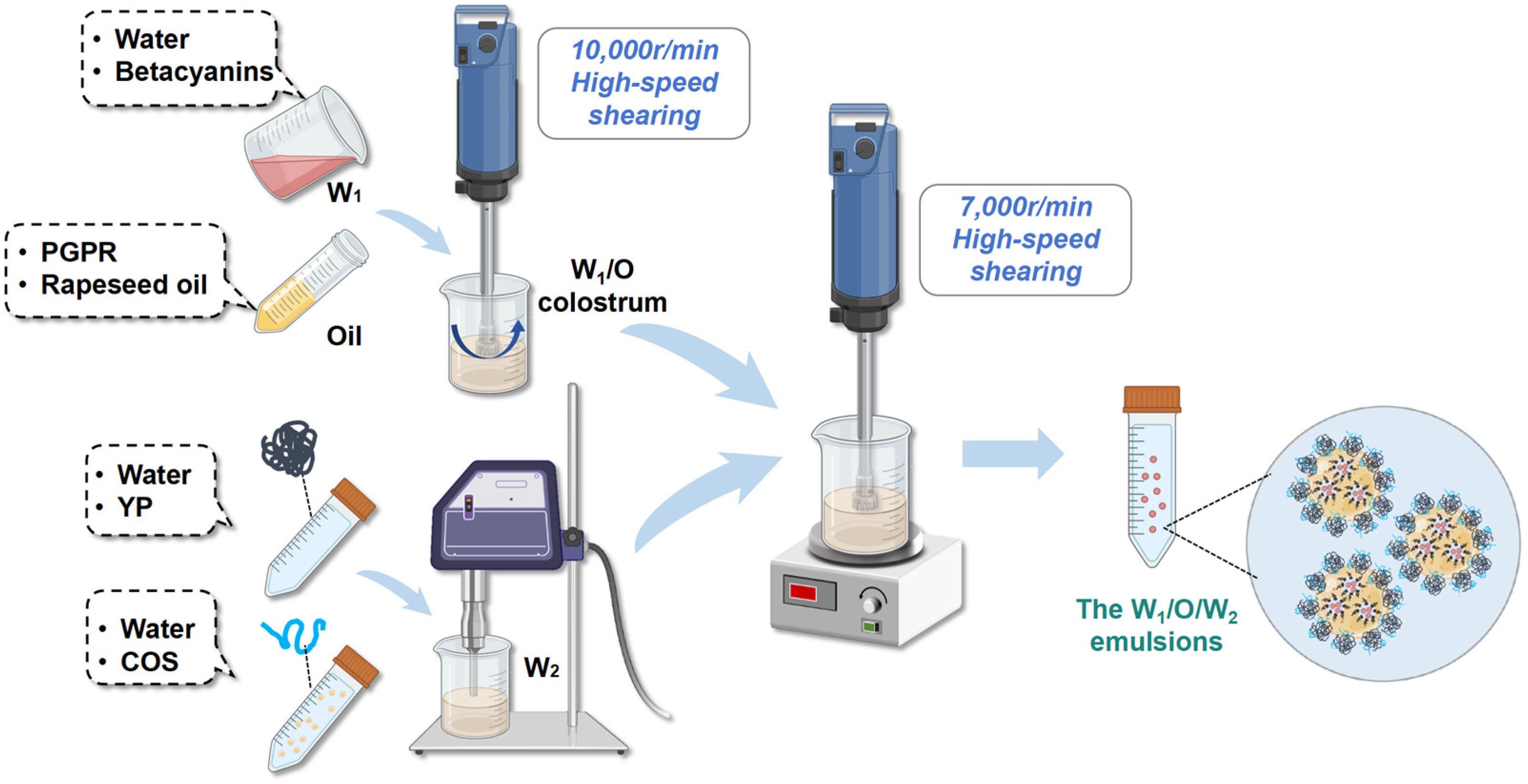
2.3. Preparation of the W1/O Colostrum
2.4. Preparation of YP-COS Stabilized W1/O/W2 Emulsions
2.5. Confocal Laser Scanning Microscopy (CLSM)
2.6. Microstructural Observation
2.7. ζ-Potential and Particle Size Measurement
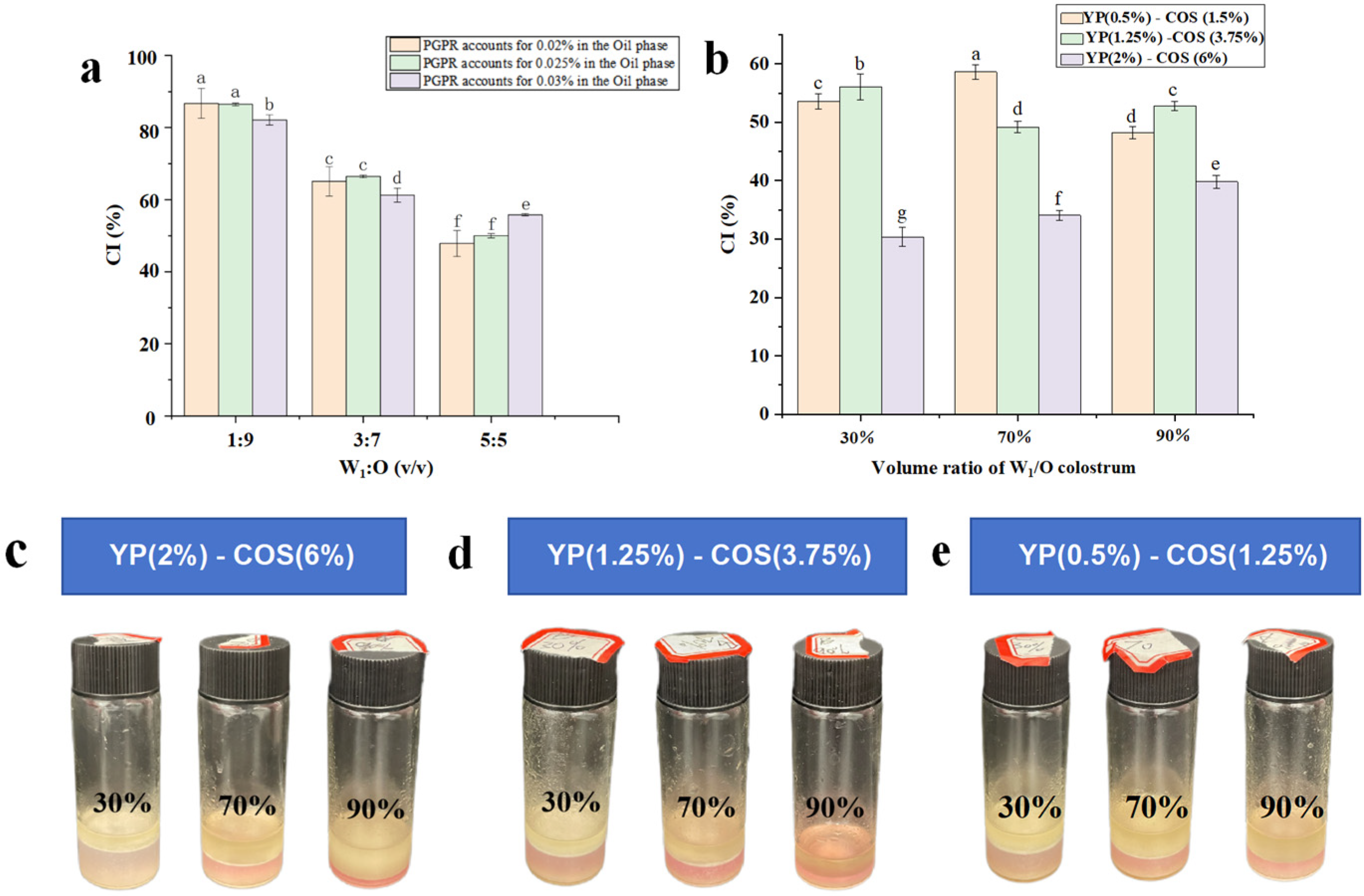
2.8. Determination of the Creaming Index
2.9. Stability Analysis
2.9.1. Thermal Stability Analysis
2.9.2. Photostability Analysis
2.9.3. The pH Stability Analysis
2.9.4. Storage Stability Analysis
2.10. In Vitro Gastrointestinal Digestion
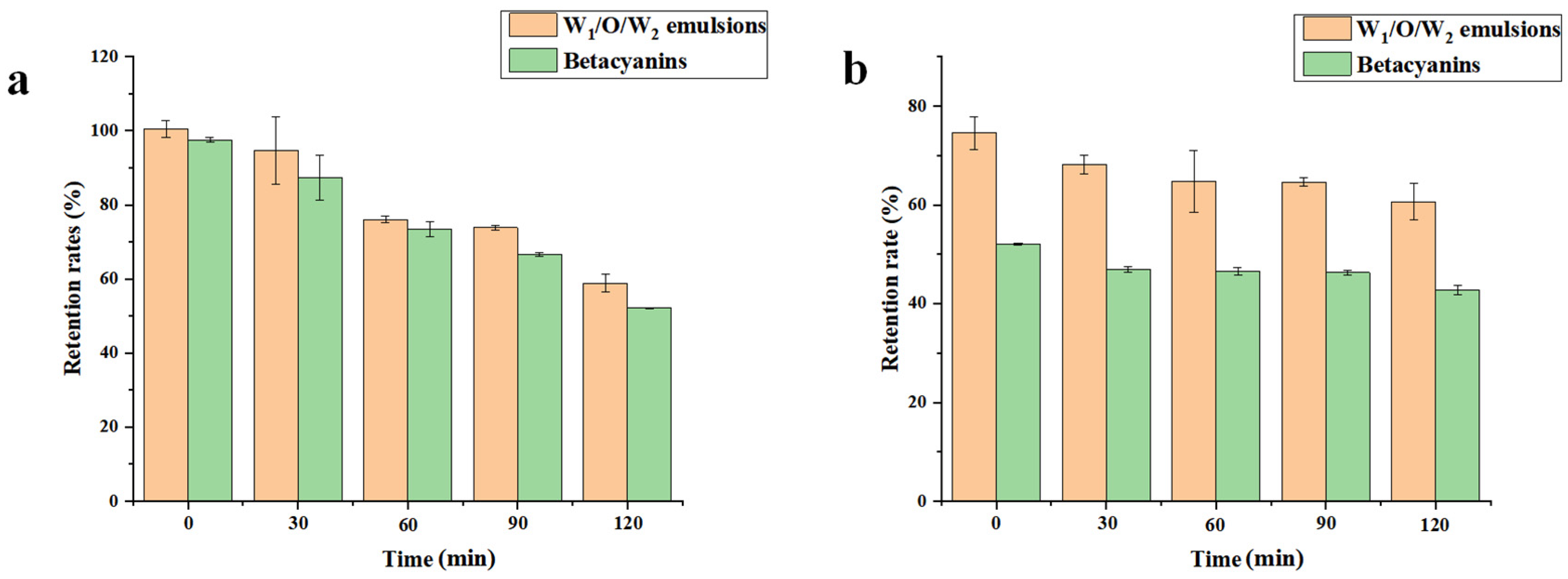
2.10.1. Simulated Gastric Digestion
2.10.2. Simulated Intestinal Digestion
2.11. Experimental Data Analysis and Processing
3. Results and Discussions
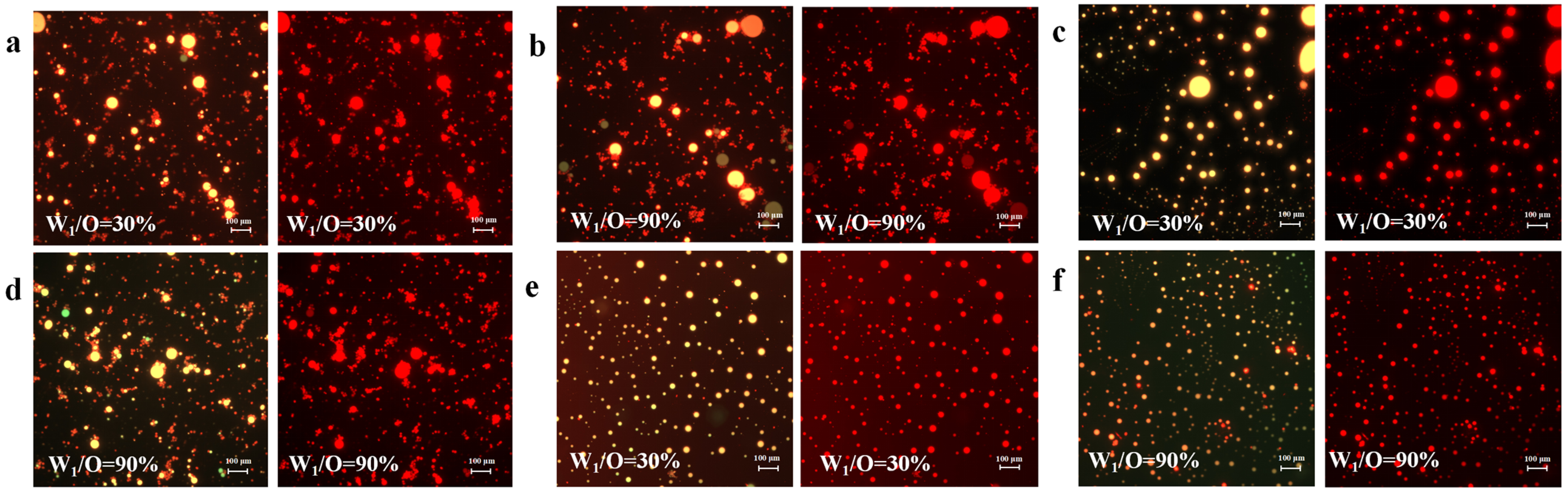
3.1. Confocal Laser Scanning Microscopy (CLSM)
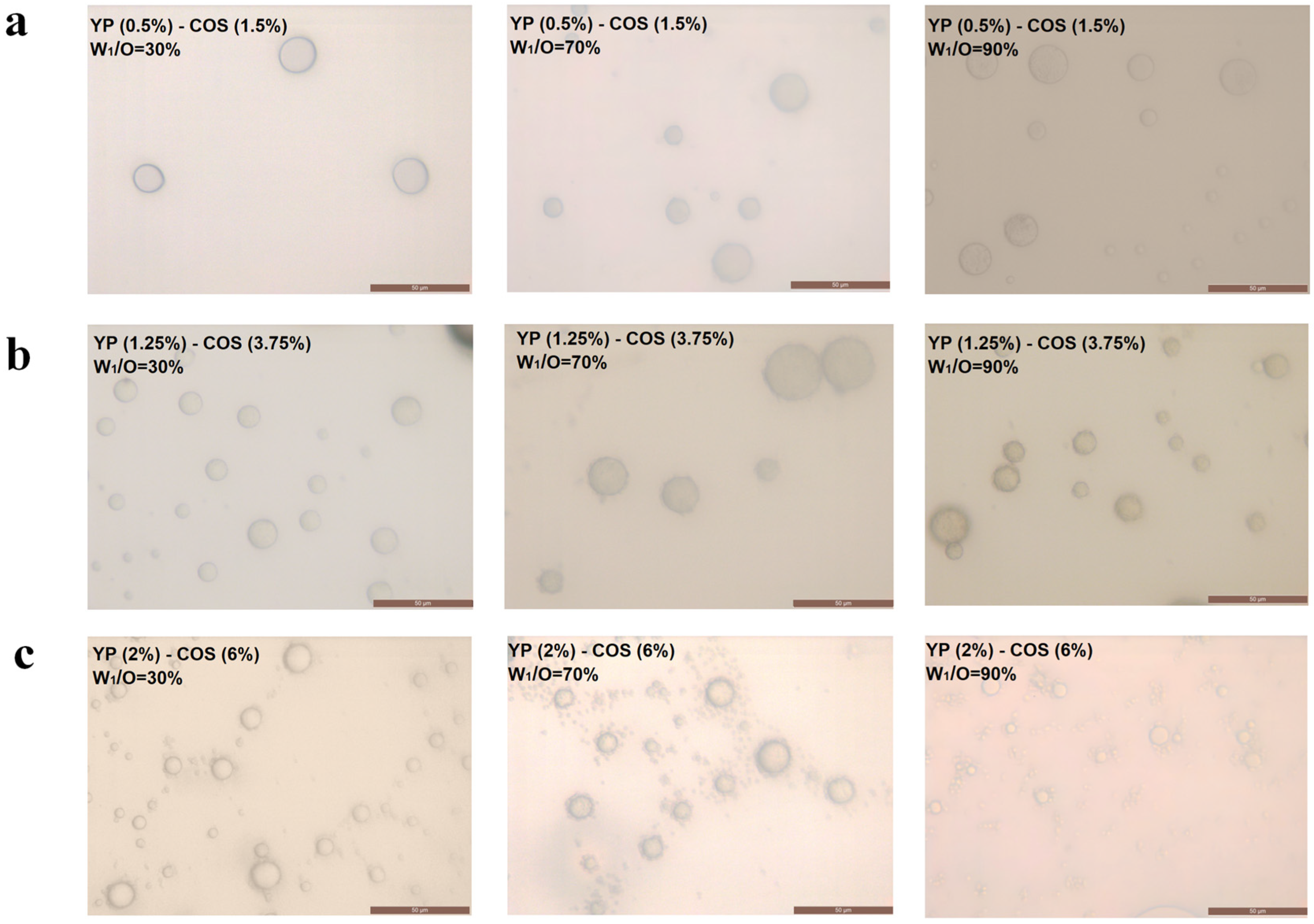
3.2. Microstructure and Appearance
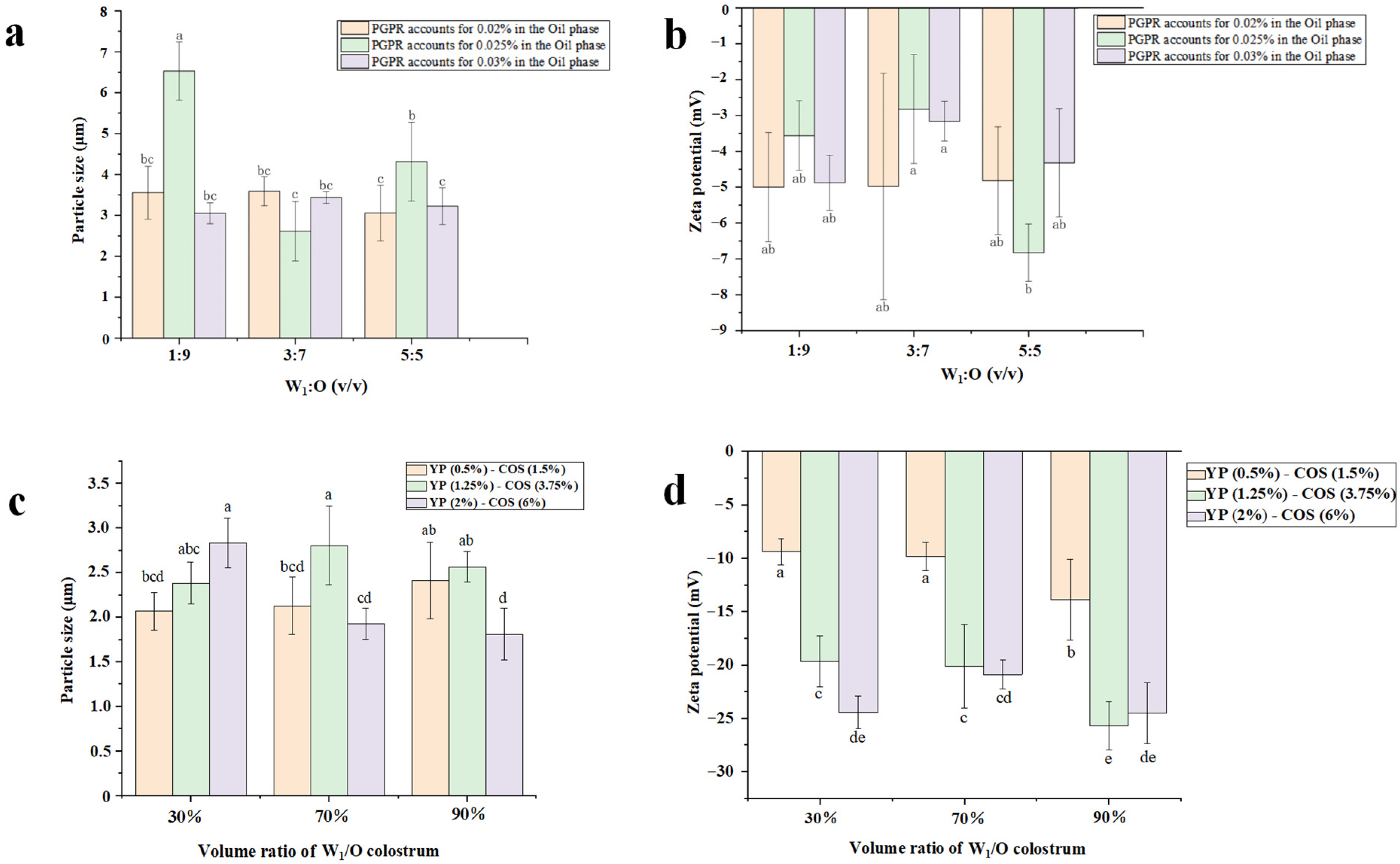
3.3. Average Particle Size
3.4. Zeta-Potential
3.5. Creaming Index
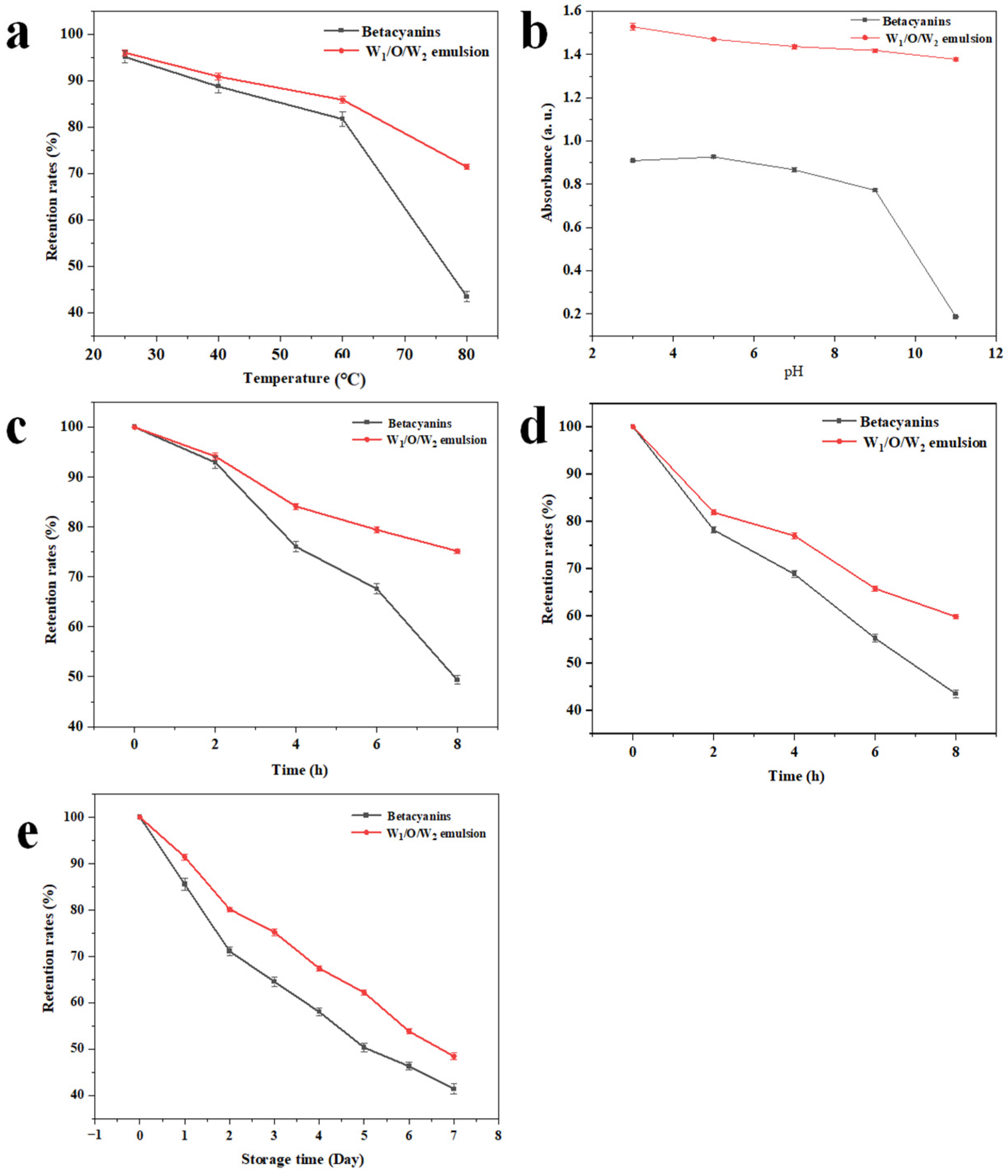
3.6. Stability Analysis
3.6.1. Thermal Stability
3.6.2. The pH Stability
3.6.3. Photostability
3.6.4. Storage Stability
3.7. In Vitro Digestibility of the W1/O/W2 Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amjadi, S.; Almasi, H.; Hamishehkar, H.; Khaledabad, M.A.; Lim, L.-T. Coating of betanin and carvone Co-loaded nanoliposomes with synthesized cationic inulin: A strategy for enhancing the stability and bioavailability. Food Chem. 2021, 373, 131403. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wang, J.; Xu, W.; Wang, G.; Hu, Z.; Hu, C. Fabrication of Sodium Caseinate-Polysaccharide Complexes Sta-bilized Emulsion for Increased Stability and Control Release of Β-Carotene. LWT 2024, 205, 11649. [Google Scholar] [CrossRef]
- Springer, A.; Ziegler, H.; Bach, K. The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions. Cosmetics 2023, 10, 40. [Google Scholar] [CrossRef]
- Cimino, C.; Bonaccorso, A.; Tomasello, B.; Alberghina, G.A.; Musumeci, T.; Puglia, C.; Pignatello, R.; Marrazzo, A.; Carbone, C. W/O/W microemulsions for nasal delivery of hydrophilic compounds: A preliminary study. J. Pharm. Sci. 2024, 113, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moral, N.; Watt, S.; Wilde, P. Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocoll. 2014, 42, 215–222. [Google Scholar] [CrossRef]
- Khan, M.A.; Bao, H.; Cheng, H.; Feng, S.; Wang, Y.; Liang, L. Fabrication of whey-protein-stabilized G/O/W emulsion for the encapsulation and retention of -ascorbic acid and α-tocopherol. J. Food Eng. 2022, 341, 111335. [Google Scholar] [CrossRef]
- Harimurti, N.; Mulia, K.; Nasikin, M. Preparation of W/O/W double nanoemulsions loaded by red dragon fruit extract. AIP Conf. Proc. 2020, 2255, 060030. [Google Scholar] [CrossRef]
- Zhou, Y.; Petrova, S.P.; Edgar, K.J. Chemical synthesis of polysaccharide–protein and polysaccharide–peptide conjugates: A review. Carbohydr. Polym. 2021, 274, 118662. [Google Scholar] [CrossRef]
- Zhuang, H.; Li, X.; Wu, S.; Wang, B.; Yan, H. Fabrication of grape seed proanthocyanidin-loaded W/O/W emulsion gels stabilized by polyglycerol polyricinoleate and whey protein isolate with konjac glucomannan: Structure, stability, and in vitro digestion. Food Chem. 2023, 418, 135975. [Google Scholar] [CrossRef]
- Kobayashi, Y.; El-Wali, M.; Guðmundsson, H.; Guðmundsdóttir, E.E.; Friðjónsson, Ó.H.; Karlsson, E.N.; Roitto, M.; Tuomisto, H.L. Life-cycle assessment of yeast-based single-cell protein production with oat processing side-stream. Sci. Total Environ. 2023, 873, 162318. [Google Scholar] [CrossRef] [PubMed]
- Narsipur, S.; Kew, B.; Ferreira, C.; El-Gendy, R.; Sarkar, A. Emulsion stabilized by Yeast Proteins and Biomass: A Mini Review. Curr. Opin. Food Sci. 2024, 57, 101167. [Google Scholar] [CrossRef]
- Lin, C.; Luan, F.; Su, S.; Jiang, A.; Tan, W.; Guo, Z. Water-soluble fluorine-functionalized chitooligosaccharide derivatives: Synthesis, characterization and antimicrobial activity. Carbohydr. Res. 2023, 533, 108935. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Liu, Y.; Yang, H.; Yan, X.; Zhang, Y.; Zhong, Z. Preparation, characterization, antioxidant, and antifungal activity of phenyl/indolyl-acyl chitooligosaccharides. Carbohydr. Res. 2024, 538, 109077. [Google Scholar] [CrossRef]
- Han, L.; Zhai, R.; Hu, B.; Yang, J.; Li, Y.; Xu, Z.; Meng, Y.; Li, T. Effects of Octenyl-Succinylated Chitosan—Whey Protein Isolated on Emulsion Properties, Astaxanthin Solubility, Stability, and Bioaccessibility. Foods 2023, 12, 2898. [Google Scholar] [CrossRef]
- Li, X.; Teng, J.; Huang, G.; Xu, Z.; Xiao, J. Preparation and Characterization of Maillard Reaction Products from a Trinary System Composed of the Soy Protein Isolate, Chitosan Oligosaccharide, and Gum Arabic. ACS Food Sci. Technol. 2021, 1, 2107–2116. [Google Scholar] [CrossRef]
- Ma, N.; Liu, L.; Zhang, Y.; Wang, L.; Qian, W. Effect of chitosan and chitosan oligosaccharide on the interaction and in vitro gastric digestive behavior of whey protein isolate. Food Hydrocoll. 2023, 149, 109561. [Google Scholar] [CrossRef]
- Yang, R.; Hu, J.; Ding, J.; Chen, R.; Meng, D.; Li, K.; Guo, H.; Chen, H.; Zhang, Y. Ultrasound assisted fabrication of the yeast protein-chitooligosaccharide-betanin composite for stabilization of betanin. Ultrason. Sonochem. 2024, 104, 106823. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. O/W/O double emulsions stabilized with WPI–polysaccharide conjugates. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 297, 211–220. [Google Scholar] [CrossRef]
- Gao, T.; Zhao, X.; Li, R.; Bassey, A.; Bai, Y.; Ye, K.; Deng, S.; Zhou, G. Synergistic effects of polysaccharide addition-ultrasound treatment on the emulsified properties of low-salt myofibrillar protein. Food Hydrocoll. 2021, 123, 107143. [Google Scholar] [CrossRef]
- Chen, P.; Yang, B.-Q.; Wang, R.-M.; Xu, B.-C.; Zhang, B. Regulate the interfacial characteristic of emulsions by casein/butyrylated dextrin nanoparticles and chitosan based on ultrasound-assisted homogenization: Fabrication and characterization. Food Hydrocoll. 2022, 133, 107983. [Google Scholar] [CrossRef]
- Shi, T.; Liu, H.; Song, T.; Xiong, Z.; Yuan, L.; McClements, D.J.; Jin, W.; Sun, Q.; Gao, R. Use of l-arginine-assisted ultrasonic treatment to change the molecular and interfacial characteristics of fish myosin and enhance the physical stability of the emulsion. Food Chem. 2021, 342, 128314. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Tang, X.; Wang, Z.; Yu, S. Grape seed proanthocyanidin-loaded gel-like W/O/W emulsion stabilized by genipin-crosslinked alkaline soluble polysaccharides-whey protein isolate conjugates: Fabrication, stability, and in vitro digestion. Int. J. Biol. Macromol. 2021, 186, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Cao, Y.; Xu, D. The Improvement of Dispersion Stability and Bioaccessibility of Calcium Carbonate by Solid/Oil/Water (S/O/W) Emulsion. Foods 2022, 11, 4044. [Google Scholar] [CrossRef]
- Sangwan, K.; Garhwal, R.; Mehra, R.; Pal, Y.; Bhardwaj, A.; Nayan, V.; Kumar, N.; Kumar, S.; Goksen, G.; Lorenzo, J.M.; et al. Development and characterization of W/O/W double emulsion of watermelon rind powder. LWT 2023, 182, 114848. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Liang, Q.; Guo, X.; Niu, Z.; Qiu, S.; Xu, W.; Li, R. Effect of konjac glucomannan with different degrees of deacetylation on the gel behavior of transglutaminase induced soybean protein isolate emulsion gels. Food Hydrocoll. 2023, 148, 109493. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.-Q.; Li, L.; Song, H.-L.; Wu, H.-T.; Zhu, B.-W. Fabrication and characterization of salidroside W/O/W emulsion with sodium alginate. Food Chem. X 2024, 22, 101260. [Google Scholar] [CrossRef]
- Paula, D.d.A.; Ramos, A.M.; de Oliveira, E.B.; Martins, E.M.F.; de Barros, F.A.R.; Vidigal, M.C.T.R.; Costa, N.d.A.; da Rocha, C.T. Increased thermal stability of anthocyanins at pH 4.0 by guar gum in aqueous dispersions and in double emulsions W/O/W. Int. J. Biol. Macromol. 2018, 117, 665–672. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.; Dou, Z.; Chen, X.; Chen, X.; Chen, H.; Fu, X. Multiscale combined techniques for evaluating emulsion stability: A critical review. Adv. Colloid Interface Sci. 2022, 311, 102813. [Google Scholar] [CrossRef]
- 3Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Xie, W.; Tang, C.; Zhang, Y.; Fan, W.; Qin, J.; Xiao, H.; Guo, S.; Tang, Z. Effect of Stigmasterol and Polyglycerol Polyricinoleate Concentrations on the Preparation and Properties of Rapeseed Oil-Based Gel Emulsions. Food Chem. X 2024, 23, 101636. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.S.; Stiller, C.; Cavallaro, S.; Karlström, A.E.; Linnros, J.; Dev, A. Influence of molecular size and zeta potential in electrokinetic biosensing. Biosens. Bioelectron. 2020, 152, 112005. [Google Scholar] [CrossRef]
- Shanmugam, A.; Ashokkumar, M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems–Physicochemical characterization. Food Hydrocoll. 2014, 39, 151–162. [Google Scholar] [CrossRef]
- Harimurti, N.; Nasikin, M.; Mulia, K. Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules 2021, 26, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, L.; Liu, Y.; Li, J. Relationship between protein native conformation and ultrasound efficiency: For improving the physicochemical stability of water–in–oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129737. [Google Scholar] [CrossRef]
- Jiménez-González, O.; López-Malo, A.; González-Pérez, J.E.; Ramírez-Corona, N.; Guerrero-Beltrán, J.Á. Thermal and pH stability of Justicia spicigera (Mexican honeysuckle) pigments: Application of mathematical probabilistic models to predict pigments stability. Food Chem. Mol. Sci. 2022, 6, 100158. [Google Scholar] [CrossRef]
- Sivabalan, S.; Ross, C.F.; Tang, J.; Sablani, S.S. Physical, thermal, and storage stability of multilayered emulsion loaded with β-carotene. Food Innov. Adv. 2024, 3, 244–255. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Dey, D.; Hemachandran, H.; D, T.K.; Doss, G.P.; Priyadarshini, R.; Siva, R. Accumulation of betacyanin in Hylocereus undatus rind: Pigment stability analysis and its role in xanthine oxidase inhibition. Phytomed. Plus 2021, 2, 100197. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 106211. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Yan, W.; Wang, P.; Li, J.; Lu, Y. Light Stability and Mechanism of Monascus Pigment Under Different Lights. LWT 2024, 191, 115666. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 16, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.P.E.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A.; Bastos, E.L. Microencapsulation of betanin in monodisperse W/O/W emulsions. Food Res. Int. 2018, 109, 489–496. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Z.; Cheng, Y.; Zhu, J.; Yang, Y.; Ma, L.; Dai, H.; Chen, H.; Yu, J.; Qiao, S.; et al. Structure maintainability of safflomin/betanin incorporated gelatin-chitooligosaccharide complexes based high internal phase emulsions and its combinational 3D printing. Food Hydrocoll. 2022, 138, 108393. [Google Scholar] [CrossRef]
- Liu, J.; Guo, J.; Zhang, H.; Liao, Y.; Liu, S.; Cheng, D.; Zhang, T.; Xiao, H.; Du, Z. The fabrication, characterization, and application of chitosan–NaOH modified casein nanoparticles and their stabilized long-term stable high internal phase Pickering emulsions. Food Funct. 2022, 13, 1408–1420. [Google Scholar] [CrossRef]
- Wei, W.; Chen, F.; Qiu, Y.; Zhang, L.; Gao, J.; Wu, T.; Wang, P.; Zhang, M.; Zhu, Q. Co-encapsulation of collagen peptide and astaxanthin in WG/OG/W double emulsions-filled alginate hydrogel beads: Fabrication, characterization and digestion behaviors. J. Colloid Interface Sci. 2023, 651, 159–171. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wen, C.; Wang, N.; Yan, C.; Shen, C.; Song, S. Chitosan and chitosan oligosaccharide influence digestibility of whey protein isolate through electrostatic interaction. Int. J. Biol. Macromol. 2022, 222, 1443–1452. [Google Scholar] [CrossRef]
- Wei, L.; Baodong, Z.; Song, M. Improved emulsion stability and modified nutrient release by structuring O/W emulsions using konjac glucomannan. Food Hydrocoll. 2018, 81, 120–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ding, J.; Wu, Y.; Sun, S.; Meng, D.; Gu, C.; Yang, R. Construction of a Yeast Protein-Chitooligosaccharide W/O/W Emulsion System for Carrying and Stabilization of Betacyanins. Foods 2025, 14, 1337. https://doi.org/10.3390/foods14081337
Li Y, Ding J, Wu Y, Sun S, Meng D, Gu C, Yang R. Construction of a Yeast Protein-Chitooligosaccharide W/O/W Emulsion System for Carrying and Stabilization of Betacyanins. Foods. 2025; 14(8):1337. https://doi.org/10.3390/foods14081337
Chicago/Turabian StyleLi, Yichen, Jiaqi Ding, Yaxin Wu, Shihao Sun, Demei Meng, Chunkai Gu, and Rui Yang. 2025. "Construction of a Yeast Protein-Chitooligosaccharide W/O/W Emulsion System for Carrying and Stabilization of Betacyanins" Foods 14, no. 8: 1337. https://doi.org/10.3390/foods14081337
APA StyleLi, Y., Ding, J., Wu, Y., Sun, S., Meng, D., Gu, C., & Yang, R. (2025). Construction of a Yeast Protein-Chitooligosaccharide W/O/W Emulsion System for Carrying and Stabilization of Betacyanins. Foods, 14(8), 1337. https://doi.org/10.3390/foods14081337






