Increasing the Bioactive Compound Content of Olive Oil by Acidification of Olive Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Characterization
2.2. Oil Extraction
2.3. Analysis of Olive Oil Quality Parameters
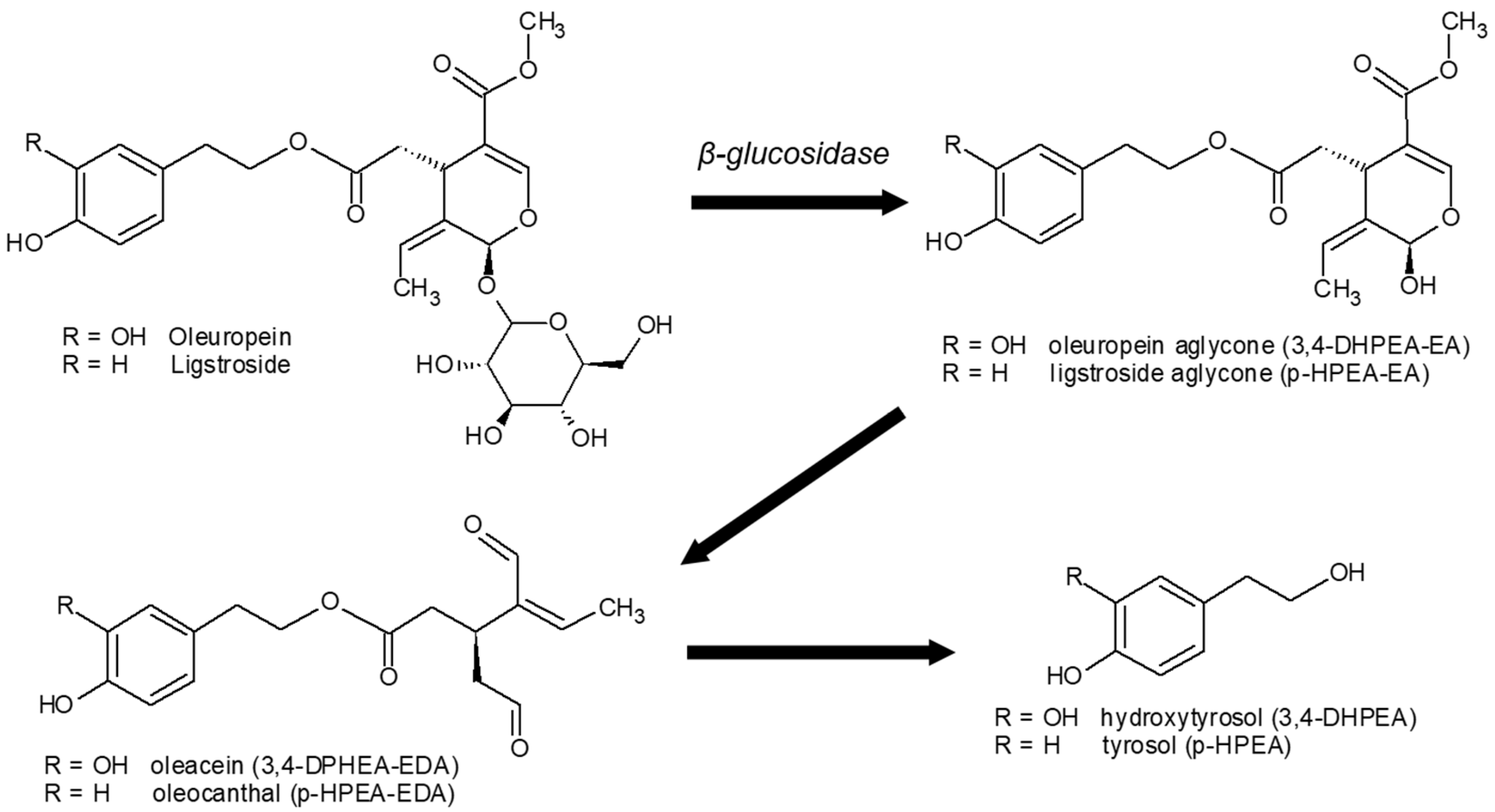
2.4. Phenolic Compounds
2.5. Volatile Compounds
2.6. Antioxidant Capacity
2.7. Statistical Analysis
3. Results
3.1. Characterization of Olive Fruit
3.2. Extraction Efficiency and Water pH
3.3. Oil Quality Parameters
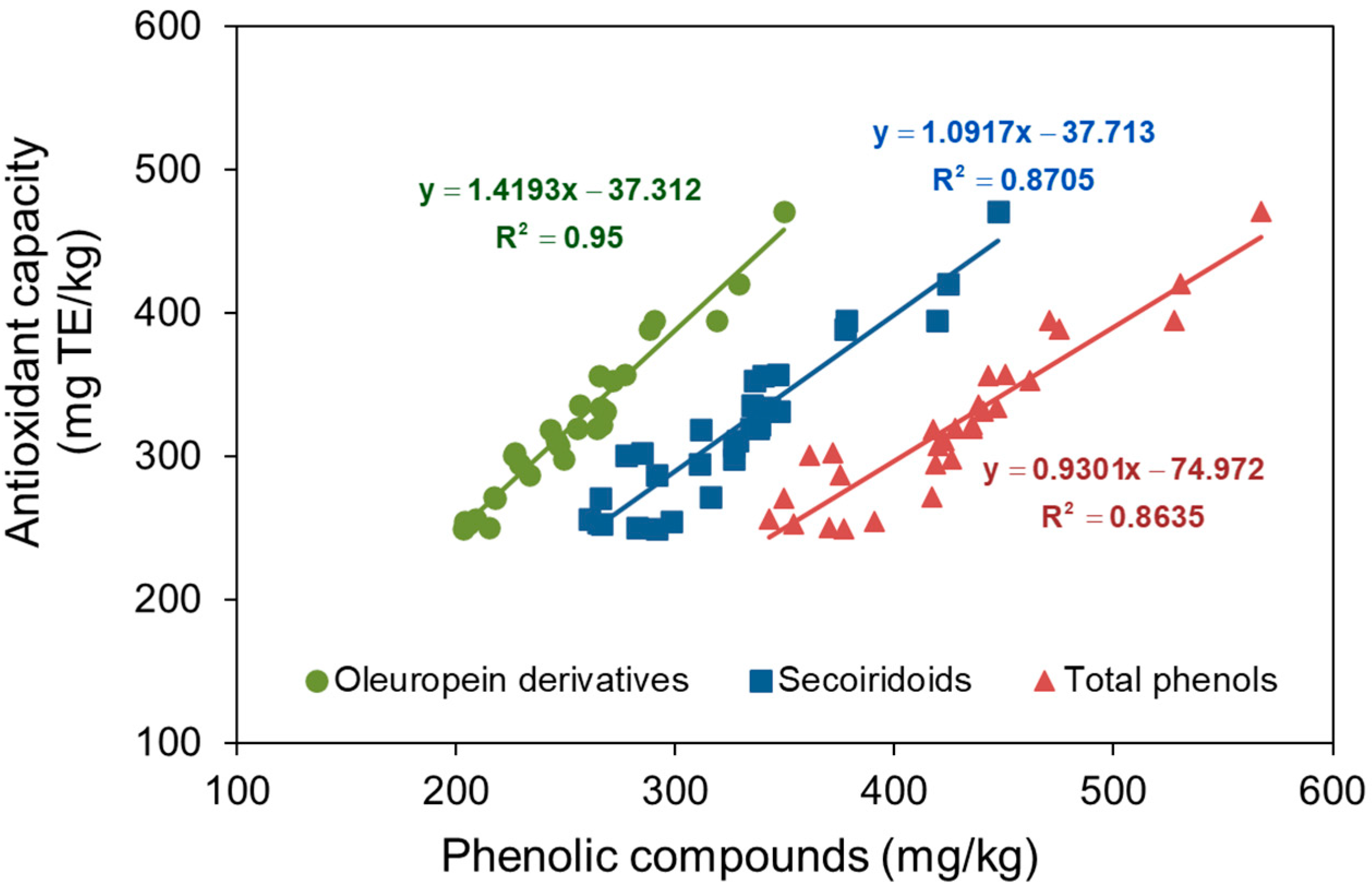
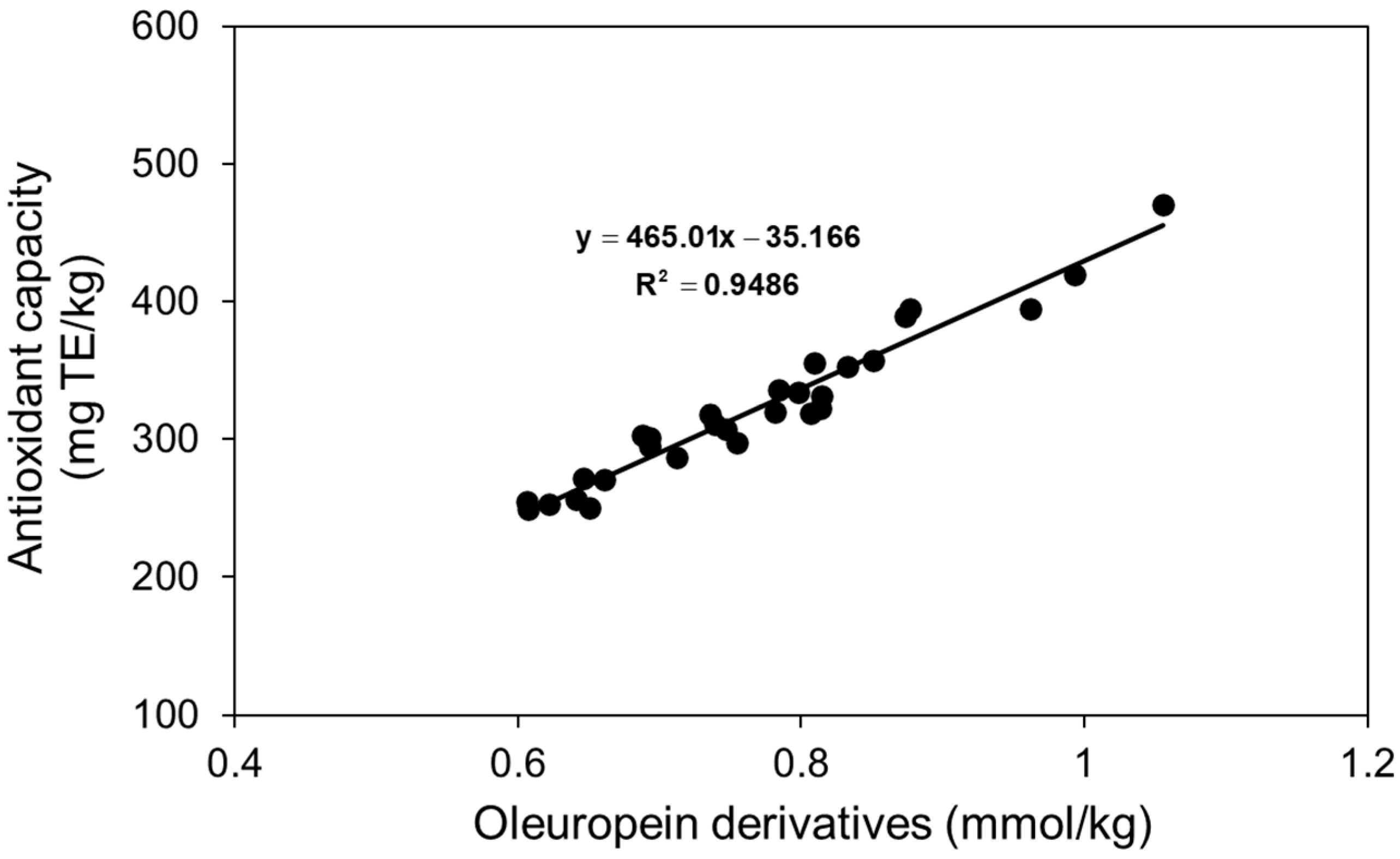
3.4. Phenolic Compounds and Antioxidant Capacity
3.5. Volatile Compounds and Ethanol Content
4. Discussion
4.1. Extraction Efficiency and Water pH
4.2. Phenolic Compounds and Antioxidant Capacity
4.3. Volatile Compound Content in the Oils Obtained with Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neves, B.; Pires, I.M. The Mediterranean Diet and the Increasing Demand of the Olive Oil Sector: Shifts and Environmental Consequences. Region 2018, 5, 101–112. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Mofardin, I.; Butumović, A.; Koprivnjak, O. Filtered vs. naturally sedimented and decanted virgin olive oil during storage: Effect on quality and composition. LWT 2017, 84, 370–377. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.A.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef]
- Presti, G.; Guarrasi, V.; Gulotta, E.; Provenzano, F.; Provenzano, A.; Giuliano, S.; Monfreda, M.; Mangione, M.R.; Passantino, R.; San Biagio, P.L.; et al. Bioactive compounds from extra virgin olive oils: Correlation between phenolic content and oxidative stress cell protection. Biophys. Chem. 2017, 230, 109–116. [Google Scholar] [CrossRef]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Petričević, D.; Velimirović, D.; Drlje, T.D. Positive Health Effects of Olive Oil. Eur. J. Nutr. Food Saf. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No 432/2012, Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432 (accessed on 10 February 2025).
- de Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Galardi, C.; Vincieri, F.F.; Liberatore, L.; Cichelli, A. HPLC and HRGC analyses of polyphenols and secoiridoid in olive oil. Chromatographia 2001, 53, 279–284. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1719. [Google Scholar] [CrossRef]
- Oliveras-López, M.J.; Innocenti, M.; Giaccherini, C.; Ieri, F.; Romani, A.; Mulinacci, N. Study of the phenolic composition of spanish and italian monocultivar extra virgin olive oils: Distribution of lignans, secoiridoidic, simple phenols and flavonoids. Talanta 2007, 73, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation Effects on Quality, Phenolic Composition, and Selected Volatiles of Virgin Olive Oils Cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Medina, E.; Brenes, M.; Romero, C. Endogenous enzymes involved in the transformation of oleuropein in Spanish table olive varieties. J. Agric. Food Chem. 2014, 62, 9569–9575. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Modulating oxidoreductase activity modifies the phenolic content of virgin olive oil. Food Chem. 2015, 171, 364–369. [Google Scholar] [CrossRef]
- Clodoveo, M.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Olive and Olive Oil Bioactive Constituents; Academic Press: Cambridge, MA, USA; AOCS Press: Champaign, IL, USA, 2015; pp. 179–215. [Google Scholar] [CrossRef]
- Hachicha Hbaieb, R.; Kotti, F.; Valli, E.; Bendini, A.; Toschi, T.G.; Gargouri, M. Effect of Tunisian olive ripeness on endogenous enzymes and virgin olive oil phenolic composition. J. Food Compos. Anal. 2017, 62, 43–50. [Google Scholar] [CrossRef]
- Yousfi, K.; Cert, R.M.; García, J.M. Changes in quality and phenolic compounds of virgin olive oils during objectively de-scribed fruit maturation. Eur. Food Res. Technol. 2006, 223, 117–124. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of Malaxation Conditions on Phenol and Volatile Profiles in Olive Paste and the Corresponding Virgin Olive Oils (Olea europaea L. Cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef]
- García-Vico, L.; Sánchez, R.; Fernández, G.; Sanz, C.; Pérez, A.G. Study of the olive β-glucosidase gene family putatively involved in the synthesis of phenolic compounds of virgin olive oil. J. Sci. Food Agric. 2021, 101, 5409–5418. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2018, 55, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newman, J.W.; Holt, R.R. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Pino, J.; Lago, F.; Smith, A.B.; Gómez-Reino, J.J.; Gualillo, O. New drugs from ancient natural foods. Oleocanthal, the natural occurring spicy compound of olive oil: A brief history. Drug Discov. Today 2015, 20, 406–410. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein enhances anti-inflammatory activity of human macrophages by increasing CD163 receptor expression. Phytomedicine 2015, 22, 1255–1261. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Polański, J.A.; Naruszewicz, M. Oleacein may inhibit destabilization of carotid plaques from hypertensive patients. Impact on high mobility group protein-1. Phytomedicine 2017, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Peviani, E.G.; Porta, A.; D’Alfonso, A.; Zanoni, G.; Vidari, G. A Concise and Efficient Total Synthesis of Oleocanthal. Eur. J. Org. Chem. 2013, 2013, 4332–4336. [Google Scholar] [CrossRef]
- Adhami, H.; Zehl, M.; Dangl, C.; Dorfmeister, D.; Stadler, M.; Urban, E.; Hewitson, P.; Ignatova, S.; Krenn, L. Preparative isolation of oleocanthal, tyrosol, and hydroxytyrosol from olive oil by HPCCC. Food Chem. 2015, 170, 154–159. [Google Scholar] [CrossRef]
- Angelis, A.; Hamzaoui, M.; Aligiannis, N.; Nikou, T.; Michailidis, D.; Gerolimatos, P.; Termentzi, A.; Hubert, J.; Halabalaki, M.; Renault, J.; et al. An integrated process for the recovery of high added-value compounds from olive oil using solid support free liquid-liquid extraction and chromatography techniques. J. Chromatogr. A 2017, 1491, 126–136. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; Ocaña, M.T.; De Torres, A.; Espínola, F.; Moya, M. Elaboration of extra-virgin olive oils rich in oleocanthal and oleacein: Pilot plant’s proposal. Eur. Food Res. Technol. 2020, 246, 1459–1468. [Google Scholar] [CrossRef]
- Millao, S.; Quilaqueo, M.; Contardo, I.; Rubilar, M. Enhancing the Oxidative Stability of Beeswax–Canola Oleogels: Effects of Ascorbic Acid and Alpha-Tocopherol on Their Physical and Chemical Properties. Gels 2025, 11, 43. [Google Scholar] [CrossRef]
- Osanloo, M.; Jamali, N.; Nematollahi, A. Improving the oxidative stability of virgin olive oil using microformulated vitamin-C. Food Sci. Nutr. 2021, 9, 3712–3721. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cinelli, G.; Chirascu, C.; Marconi, E.; Lopez, F. Antioxidant Effect of Vitamins in Olive Oil Emulsion. Colloids Interfaces 2020, 4, 23. [Google Scholar] [CrossRef]
- Flavia, P.; Mihalescu, L. Effects of α-Tocopherol and Citric Acid on the Oxidative Stability of Alimentary Poultry Fats During Storage at Low Temperatures. Int. J. Food Prop. 2017, 20, 1085–1096. [Google Scholar] [CrossRef][Green Version]
- Aliakbarian, B.; Dehghani, F.; Perego, P. The effect of citric acid on the phenolic contents of olive oil. Food Chem. 2009, 116, 617–623. [Google Scholar] [CrossRef]
- Vidal Castro, A.; Alcalá Reyes, S.; Torres, A.; Espínola, F.; Moya, M. Modeling of volatile and phenolic compounds and optimization of the process conditions for obtaining balanced extra virgin olive oils. Grasas Aceites 2018, 69, e250. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No 2568/91, on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991R2568 (accessed on 10 February 2025).
- International Olive Council. Determination of Biophenols in Olive Oils by HPLC. COI/T.20/Doc No 29/Rev 2 2022. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/COI-T20-Doc.-29-REV-1-2017-Eng.pdf (accessed on 10 February 2025).
- Vidal, A.M.; Moya, M.; Alcalá, S.; Romero, I.; Espínola, F. Enrichment of Refined Olive Oils with Phenolic Extracts of Olive Leaf and Exhausted Olive Pomace. Antioxidants 2022, 11, 204. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02013R1308-20241108 (accessed on 10 February 2025).
- International Olive Council. Sensory Analysis of Olive Oil. COI/T.20/Doc No 4/Rev 1 2007. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-4-Rev1-2007-Eng.pdf (accessed on 10 February 2025).
- Chabni, A.; Bañares, C.; Vázquez, L.; Torres, C.F. Combination of expeller and supercritical CO2 for the extraction of a phenolic-rich olive oil—A preliminary chemical characterization. J. Ind. Eng. Chem. 2025, in press. [CrossRef]
- Al-Hashmi, Z.H.; Al-Lawati, H.A.; Suliman, F.O.; Hassanzadeh, J.; Aal-Thani, G.S.S.; Forqani, A.S.A.; Fahdi, A.R.A. Quantitative estimation of pharmacologically relevant phenolic compounds in olive oils harvested in Jabal Al Akhdar in Oman. Food Chem. Adv. 2025, 6, 100922. [Google Scholar] [CrossRef]
- Miho, H.; Díez, C.M.; Mena-Bravo, A.; Sánchez de Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Miho, H.; Muñoz, C.; Priego-Capote, F. Deciphering the influence of the cultivar on the phenolic content of virgin olive oil. J. Food Compos. Anal. 2024, 129, 106128. [Google Scholar] [CrossRef]
- López-Huertas, E.; Lozano-Sánchez, J.; Segura-Carretero, A. Olive oil varieties and ripening stages containing the antioxidants hydroxytyrosol and derivatives in compliance with EFSA health claim. Food Chem. 2021, 342, 128291. [Google Scholar] [CrossRef]
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Peres, F.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of fresh olive leaves addition during the extraction of Arbequina virgin olive oils on the phenolic and volatile profiles. Food Chem. 2022, 393, 133327. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Adámez, J.; Baltasar, M.N.; Yuste, M.C.; Martín-Vertedor, D. Oxidative stability, phenolic compounds and antioxidant potential of a virgin olive oil enriched with natural bioactive compounds. J. Oleo Sci. 2014, 63, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Peralta, R.; Espínola, F.; Vidal, A.M.; Moya, M. Olive Oil (Royal Cultivar) from Mill Obtained by Short Time Malaxation and Early Ripening Stage. Foods 2024, 13, 2588. [Google Scholar] [CrossRef]
| Water, % | Oil, % | Solids, % |
|---|---|---|
| 58.65 ± 0.26 | 13.35 ± 0.52 | 27.99 ± 0.72 |
| Acid | Extraction Efficiency, % | pH |
|---|---|---|
| Control (0%) | 67.81 ± 1.49 ab | 4.95 ± 0.03 a |
| Acetic (1%) | 63.74 ± 3.96 ab | 4.22 ± 0.04 b |
| Acetic (2%) | 66.27 ± 1.42 ab | 3.99 ± 0.02 c |
| Acetic (4%) | 65.26 ± 2.78 ab | 3.79 ± 0.01 d |
| Ascorbic (1%) | 68.83 ± 1.17 a | 4.33 ± 0.05 b |
| Ascorbic (2%) | 67.53 ± 1.10 ab | 3.96 ± 0.18 c |
| Ascorbic (4%) | 63.70 ± 3.60 ab | 3.77 ± 0.00 d |
| Citric (1%) | 66.24 ± 1.29 ab | 3.89 ± 0.04 cd |
| Citric (2%) | 63.45 ± 4.09 b | 3.48 ± 0.03 e |
| Citric (4%) | 67.63 ± 1.83 ab | 3.01 ± 0.02 f |
| Fisher’s LSD ** | 5.29 | 0.14 |
| Acid | Hydroxytyrosol | Tyrosol | Apigenin | Luteolin | Pinoresinol |
|---|---|---|---|---|---|
| Control (0%) | 2.20 ± 0.21 g | 1.60 ± 0.20 c | 1.64 ± 0.23 f | 4.33 ± 0.30 b | 17.85 ± 0.66 abcd |
| Acetic (1%) | 2.60 ± 0.07 fg | 1.39 ± 0.09 cd | 2.12 ± 0.20 ef | 3.67 ± 0.30 bc | 18.68 ± 0.30 abc |
| Acetic (2%) | 3.46 ± 0.13 cd | 1.62 ± 0.17 c | 2.55 ± 0.33 de | 3.36 ± 0.23 c | 16.43 ± 1.07 cd |
| Acetic (4%) | 5.13 ± 0.40 a | 2.86 ± 0.37 a | 2.59 ± 0.21 cde | 3.86 ± 0.35 bc | 15.85 ± 1.82 d |
| Ascorbic (1%) | 3.03 ± 0.35 def | 0.86 ± 0.10 e | 4.97 ± 0.41 a | 5.21 ± 0.40 a | 19.41 ± 1.22 ab |
| Ascorbic (2%) | 3.18 ± 0.06 de | 0.77 ± 0.06 e | 3.23 ± 0.41 c | 5.28 ± 0.31 a | 17.14 ± 0.41 bcd |
| Ascorbic (4%) | 3.91 ± 0.29 c | 1.00 ± 0.07 de | 2.70 ± 0.35 cde | 5.93 ± 0.73 a | 17.54 ± 0.28 abcd |
| Citric (1%) | 2.83 ± 0.13 ef | 2.19 ± 0.13 b | 2.92 ± 0.16 cd | 3.79 ± 0.28 bc | 18.61 ± 0.87 abc |
| Citric (2%) | 3.00 ± 0.156 def | 2.68 ± 0.26 a | 2.57 ± 0.25 de | 3.76 ± 0.31 bc | 19.40 ± 0.81 ab |
| Citric (4%) | 4.55 ± 0.12 b | 2.56 ± 0.09 ab | 4.28 ± 0.41 b | 5.44 ± 0.64 a | 19.54 ± 1.99 a |
| Fisher’s LSD ** | 0.46 | 0.39 | 0.65 | 0.84 | 2.36 |
| Acid | Oleacein, mg/kg | Oleocanthal, mg/kg | 3,4-DHPEA-EA, mg/kg | p-HPEA-EA, mg/kg | Total Phenols, mg/kg | FRAP, mg TE/kg |
|---|---|---|---|---|---|---|
| Control (0%) | 127.97 ± 2.52 e | 84.16 ± 4.95 ab | 77.92 ± 4.72 b | 11.83 ± 1.06 a | 394.92 ± 16.79 d | 258.38 ± 9.52 e |
| Acetic (1%) | 173.04 ± 5.10 c | 70.61 ± 4.70 cd | 75.80 ± 8.04 bc | 8.15 ± 1.03 cd | 428.75 ± 12.68 c | 321.23 ± 9.79 cd |
| Acetic (2%) | 144.62 ± 6.50 d | 53.73 ± 7.32 e | 64.47 ± 1.45 d | 8.74 ± 1.14 bcd | 357.85 ± 8.80 e | 257.98 ± 8.94 e |
| Acetic (4%) | 150.73 ± 3.88 d | 50.91 ± 2.47 e | 64.41 ± 7.63 d | 8.15 ± 0.47 cd | 358.59 ± 12.15 e | 286.66 ± 21.46 de |
| Ascorbic (1%) | 237.58 ± 9.18 a | 91.39 ± 2.15 a | 91.61 ± 3.65 a | 9.60 ± 0.67 bc | 541.77 ± 18.01 a | 428.63 ± 31.57 a |
| Ascorbic (2%) | 202.80 ± 5.13 b | 78.66 ± 3.75 bc | 76.16 ± 5.79 b | 9.94 ± 0.63 b | 462.12 ± 15.35 b | 371.71 ± 28.81 b |
| Ascorbic (4%) | 195.47 ± 4.91 b | 70.81 ± 3.76 cd | 61.29 ± 0.97 d | 8.85 ± 0.27 bcd | 431.37 ± 3.97 bc | 321.02 ± 1.28 cd |
| Citric (1%) | 172.16 ± 8.00 c | 74.06 ± 2.24 bcd | 66.42 ± 1.20 cd | 8.82 ± 0.54 bcd | 421.42 ± 3.14 cd | 300.17 ± 5.39 d |
| Citric (2%) | 192.81 ± 12.30 b | 64.47 ± 7.52 d | 58.45 ± 2.36 d | 7.35 ± 0.35 d | 417.87 ± 30.50 cd | 320.75 ± 28.09 cd |
| Citric (4%) | 202.57 ± 10.65 b | 67.71 ± 5.51 d | 60.86 ± 1.93 d | 8.10 ± 0.29 cd | 450.18 ± 9.63 bc | 348.67 ± 9.07 bc |
| Fisher’s LSD ** | 15.86 | 10.22 | 9.73 | 1.53 | 32.36 | 39.91 |
| Acid | Total C6 LOX, mg/kg | Total C5 LOX, mg/kg | Ethanol, mg/kg |
|---|---|---|---|
| Control (0%) | 8.69 ± 0.56 e | 2.01 ± 0.05 de | 4.56 ± 0.19 a |
| Acetic (1%) | 10.07 ± 0.62 d | 1.80 ± 0.00 e | 2.52 ± 0.10 c |
| Acetic (2%) | 7.15 ± 0.57 e | 1.43 ± 0.08 f | 2.30 ± 0.16 cd |
| Acetic (4%) | 4.67 ± 0.29 f | 0.49 ± 0.04 g | 1.93 ± 0.17 d |
| Ascorbic (1%) | 12.82 ± 0.61 c | 2.23 ± 0.11 d | 3.74 ± 0.53 b |
| Ascorbic (2%) | 10.92 ± 0.76 d | 3.03 ± 0.15 b | 2.81 ± 0.36 c |
| Ascorbic (4%) | 14.07 ± 1.13 b | 3.27 ± 0.03 ab | 4.33 ± 0.19 ab |
| Citric (1%) | 16.69 ± 0.34 a | 3.33 ± 0.29 a | 4.04 ± 0.48 ab |
| Citric (2%) | 15.09 ± 0.67 b | 2.09 ± 0.12 d | 4.23 ± 0.26 ab |
| Citric (4%) | 11.09 ± 0.64 d | 2.60 ± 0.12 c | 2.74 ± 0.24 c |
| Fisher’s LSD ** | 1.06 | 0.22 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, R.; Vidal, A.M.; Espínola, F.; Ocaña, M.T.; Moya, M. Increasing the Bioactive Compound Content of Olive Oil by Acidification of Olive Paste. Foods 2025, 14, 1336. https://doi.org/10.3390/foods14081336
Peralta R, Vidal AM, Espínola F, Ocaña MT, Moya M. Increasing the Bioactive Compound Content of Olive Oil by Acidification of Olive Paste. Foods. 2025; 14(8):1336. https://doi.org/10.3390/foods14081336
Chicago/Turabian StylePeralta, Raúl, Alfonso M. Vidal, Francisco Espínola, María Teresa Ocaña, and Manuel Moya. 2025. "Increasing the Bioactive Compound Content of Olive Oil by Acidification of Olive Paste" Foods 14, no. 8: 1336. https://doi.org/10.3390/foods14081336
APA StylePeralta, R., Vidal, A. M., Espínola, F., Ocaña, M. T., & Moya, M. (2025). Increasing the Bioactive Compound Content of Olive Oil by Acidification of Olive Paste. Foods, 14(8), 1336. https://doi.org/10.3390/foods14081336








