Preliminary Study on the Functions of Peptides Obtained from White Mullet (Ophiocephalus argus var. Kimnra) Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Pretreatment
2.3. Preparation of Peptide from White Mullet Meat
2.4. Determination of Galactagogue Activity
2.4.1. Establishment of Animal Model
2.4.2. Lactation Volume of Rats
2.4.3. Net Weight Gain
2.4.4. Determination of Prolactin (PRL) and 5-Hydroxytryptamine (5-HT)
2.4.5. Hematoxylin–Eosin (HE) Staining of Breast Histology
2.5. Determination of Antioxidant Activity
2.5.1. DPPH· Radical Scavenging Rate
2.5.2. ABTS· Radical Scavenging Rate
2.5.3. Superoxygen (O2−) Free Radical Clearance
2.5.4. Hydroxyl (OH−·) Free Radical Clearance
2.6. Metal Ion Chelation
2.7. ACE-Inhibitory Activity Determination
2.8. Data Analysis
3. Results and Analysis
3.1. Galactagogue Activity
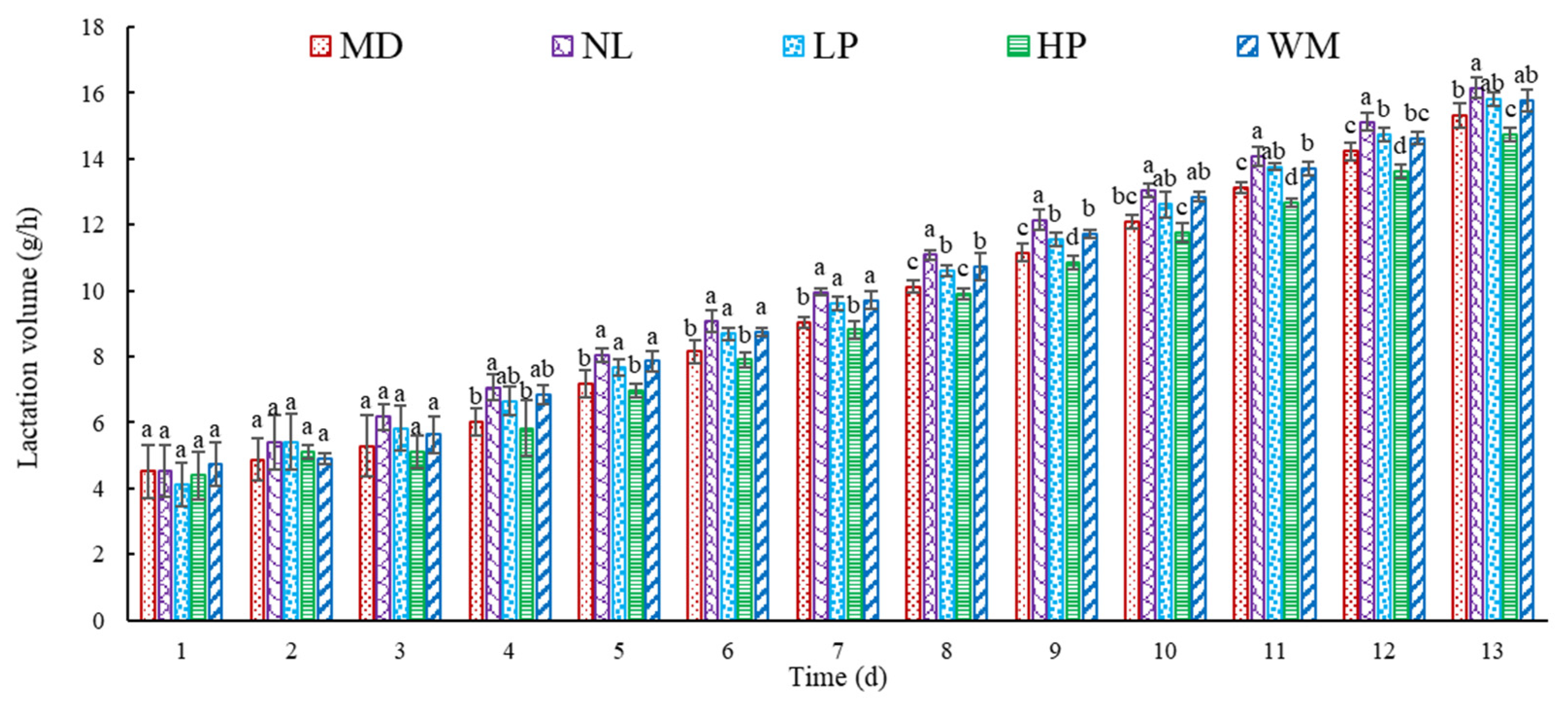
3.1.1. Effect of Peptide on Lactation Volume in Postpartum Rats
3.1.2. Effect of Peptide on NWG of Rat Pups
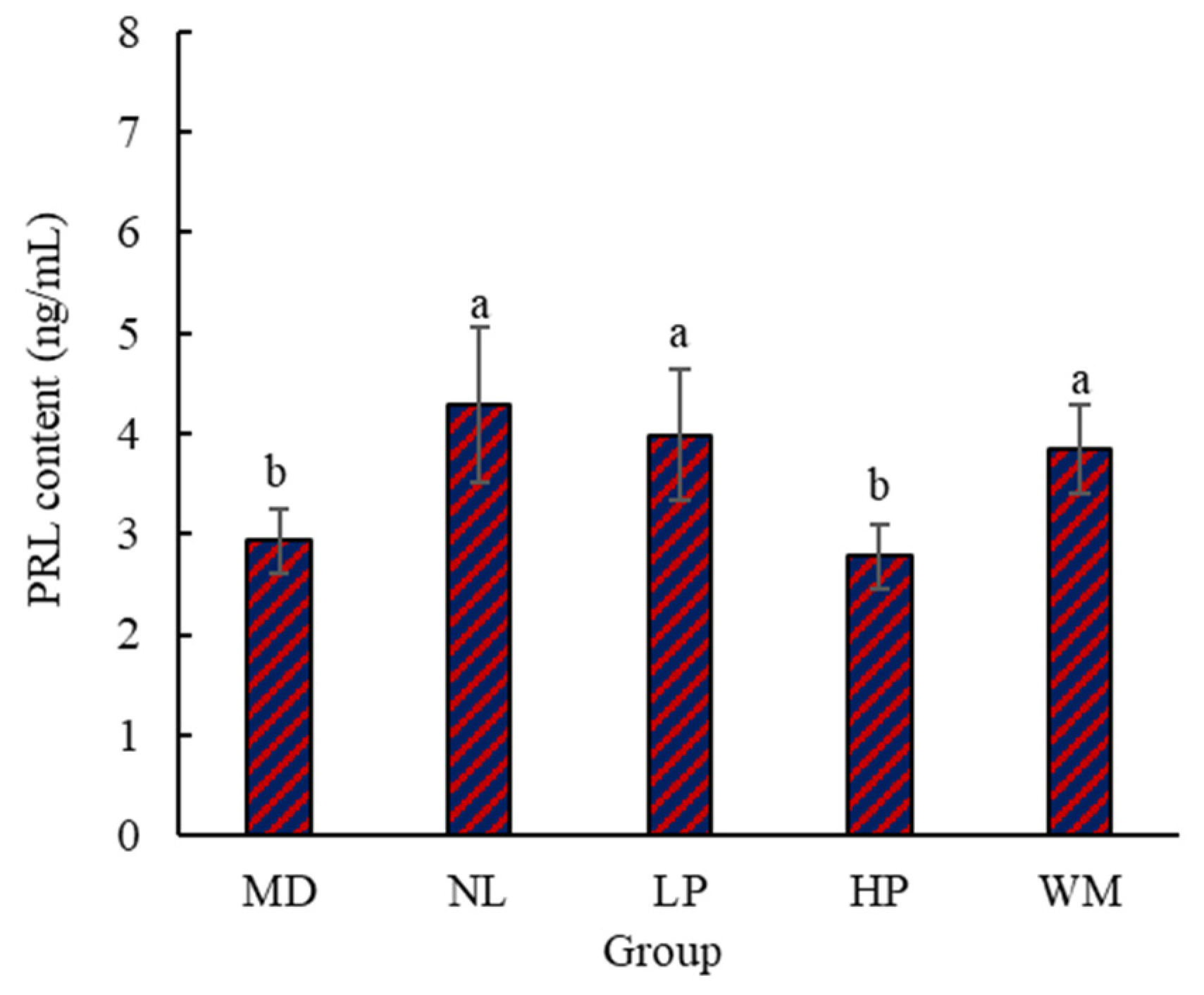
3.1.3. Content of Serum PRL in Rats
3.1.4. Content of Serum 5-HT in Rats
3.1.5. Histopathology of Rat Mammary Glands
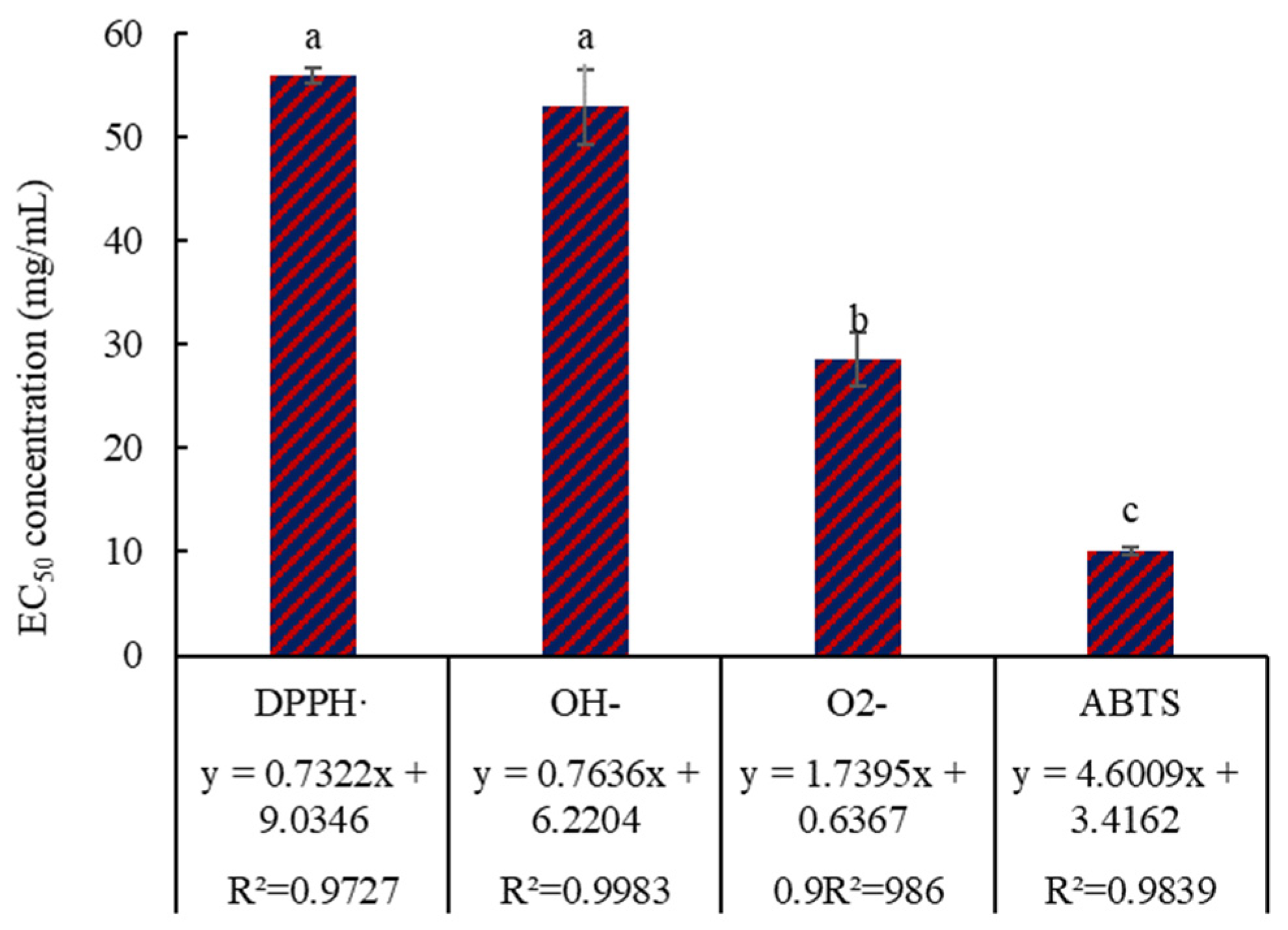
3.2. Antioxidant Activity
3.3. ACE-Inhibitory Activity
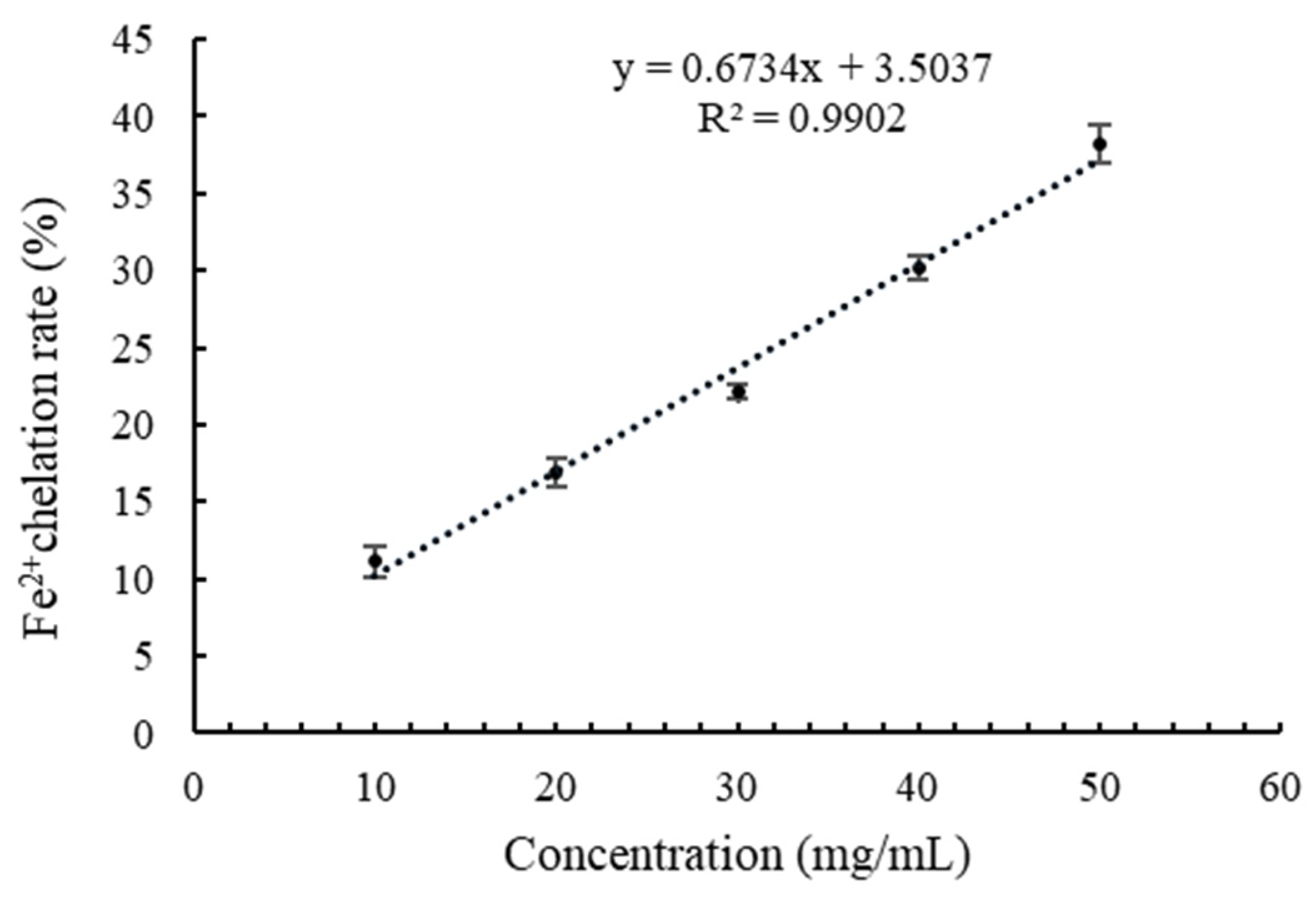
3.4. Fe2+ Chelation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, Y.; Zhang, P.; Li, Y.; Tian, M.; Wang, X.; Ke, H.; Xiong, W. Effects of carbon monoxide on the quality of white mullet meat during cold storage. Mod. Food Technol. 2019, 35, 95–100. [Google Scholar]
- Qin, J.; Cudmore, B.; Schroeder, B. Channa argus (Northern snakehead). In CABI Compendium; CABI International: Wallingford, UK, 2023. [Google Scholar]
- Jiao, X.; Su, J.; Luo, Y.; Fan, W.; Shu, C.; Wu, J.; Lishi, J. Study on the processing technology of white mullet fish cake. Chin. Condiment 2022, 47, 163–165. [Google Scholar]
- Zhang, Y.; Qian, Q.; Guo, S.; Zhang, L.; Ya, B.; Xiong, W. Effects of repeated freeze-thaw treatment on the quality of white mullet fish. Food Ind. 2019, 40, 27–30. [Google Scholar]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Lan, X.; Liao, D.; Wu, S.; Wang, F.; Sun, J.; Tong, Z. Rapid purification and characterization of angiotensin converting enzyme inhibitory peptides from lizard fish protein hydrolysates with magnetic affinity separation. Food Chem. 2015, 182, 136–142. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Hu, S.-Y.; Lu, X.-J.; Hu, J.-R. Identification and characterization of two novel antimicrobial peptides from Japanese sea bass (Lateolabrax japonicus) with antimicrobial activity and MO/MΦ activation capability. Dev. Comp. Immunol. 2023, 145, 104726. [Google Scholar] [CrossRef]
- Hashem, A.M.; Venmarath, A.; Kudre, T.G. Preparation, purification, and identification of novel antioxidant peptides from red-bellied pacu (Piaractus brachypomus) fish meat protein hydrolysate. Food Sci. Biotechnol. 2023, 32, 2057–2068. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wang, X.; Zhang, J. Bone soup: Protein nutrition and enzymatic hydrolysis process optimized by response surface method. J. Food Nutr. Res. 2014, 53, 1–12. [Google Scholar]
- Zhang, Y.; Zhang, L.; Venkitasamy, C.; Guo, S.; Pan, Z.; Ke, H.; Tang, H.; Huang, W.; Zhao, L. Improving the flavor of microbone meal with Flavourzyme by response surface method. J. Food Process Eng. 2019, 42, e13040. [Google Scholar] [CrossRef]
- Zhang, Y.; Ke, H.; Bai, T.; Chen, C.; Guo, T.; Mu, Y.; Li, H.; Liao, W.; Pan, Z.; Zhao, L. Characterization of umami compounds in bone meal hydrolysate. J. Food Sci. 2021, 86, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K.; Yokoyama, I.; Ohata, M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Gallego, M.; Escudero, E.; Reig, M.; Aristoy, M.-C.; Toldrá, F. Small peptides hydrolysis in dry-cured meats. Int. J. Food Microbiol. 2015, 212, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yin, T. Preparation and In Vivo Metabolism of Antioxidant Peptides from Grass Carp. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Wang, Y. Isolation, Purification and Quantitative Structure-Activity Study of ACE Inhibitory Peptides from Grass Carp. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2012. [Google Scholar]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Xin, M.; Zhao, M.; Tian, J.; Li, B. Guidelines for in vitro simulated digestion and absorption of food. Food Front. 2023, 4, 524–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, W.; Zhang, J. Effect of boiling and frying on nutritional value and in vitro digestibility of rabbit meat. Afr. J. Food Sci. 2014, 8, 92–103. [Google Scholar]
- Zhang, J.; Du, H.; Zhang, G.; Kong, F.; Hu, Y.; Xiong, S.; Zhao, S. Identification and characterization of novel antioxidant peptides from crucian carp (Carassius auratus) cooking juice released in simulated gastrointestinal digestion by UPLC-MS/MS and in silico analysis. J. Chromatogr. B 2020, 1136, 121893. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Mu, Y.; Zeng, Q.; Jia, J.; Zhang, P.; Pan, Z. Effect of deep dormancy temperature cultivation on meat quality of crucian carp (Carassius auratus). Foods 2023, 12, 792. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Q.; Zhang, Y.; Zhang, P.; Li, Q.; Zhou, J.; Dong, L.; Pan, Z. New Method for 5′−Nucleotidase Preparation and Evaluation of Its Catalytic Activity. Foods 2024, 13, 708. [Google Scholar] [CrossRef]
- Guo, S.; Qin, N.; Wang, X.; Zuo, Z.; Li, Q.; Wang, Y. Freeze-dried powder of daylily bud improves bromocriptine-induced lactation disorder in rats via JAK2/STAT5 pathway. J. Ethnopharmacol. 2023, 313, 116536. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wang, N.; Yao, B.; Zhong, Y.; Lin, Y.; You, T. Quercetin improves postpartum hypogalactia in milk-deficient mice via stimulating prolactin production in pituitary gland. Phytother. Res. 2018, 32, 1511–1520. [Google Scholar] [CrossRef]
- GB/T 39100-2020; Determination of Antioxidant Activity for Polypeptides—DPPH and ABTS Methods. China National Standardization Administration: Beijing, China, 2020.
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, T.; Ding, G.-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Gavlighi, H.A.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-product with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. WT-Food Sci. Technol. 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, S. Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability. Food Chem. 2009, 117, 387–392. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.J.; Hernández-Ledesma, B. Gastrointestinal digestion of food proteins under the effects of released bioactive peptides on digestive health. Mol Nutr Food Res. 2020, 64, 2000401. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; Luque, R.M.; Castaño, J.P. Cortistatin: A new link between the growth hormone/prolactin axis, stress, and metabolism. Growth Horm. IGF Res. 2017, 33, 23–27. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, J.; Wang, H.; Gao, R.; Xu, X.; Bai, F.; Zhao, Y.; Liu, K. Effect of Sturgeon Head Decoction on lactation function in rats with Postpartum hypogalactia. Food Sci. 2023, 44, 124–131. [Google Scholar]
- Jin, Y.; Ye, X.; Zhao, Y. The correlation between whole blood 5-hydroxytryptamine and serum prolactin levels in women. J. Fourth Mil. Med. Unv. 1989, 10, 133. [Google Scholar]
- Han, X.; Peng, H.; Bai, T.; Mu, Y.; Qian, Q.; Zhang, Y.; Li, H.; Zhang, Y. Effect of Steaming and Frying on Fish Protein Nutrition and Flavor. J. Chengdu Unv. Nat. Sci. Ed. 2021, 40, 247–255. [Google Scholar]
- Mann, P.C. International Standard for the Terminology and Diagnostic Criteria of Pathological Changes in Rats and Mice (INHAND); China Agriculture Press: Beijing, China, 2019. [Google Scholar]
- Wong, A.; Eloy, J.A.; Couldwell, W.T.; Liu, J.K. Update on prolactinomas. Part 2: Treatment and management strategies. J. Clin. Neurosci. 2015, 22, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Dong, S. Therapeutie Effeets and Mechanism of Traditional Galactopoietic Decoction on Rats with Puerperal Hypogalactia. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Myint, K.Z.; Zhou, Z.; Xia, Y.; Fang, Y.; Wu, M.; Zhu, S.; Shen, J. Stevia polyphenols: A stable antioxidant that presents a synergistic effect with vitamin C. J. Food Process. Preserv. 2021, 45, e15317. [Google Scholar] [CrossRef]
- Wang, R. Study on Antioxidant Activity of Hydrolysates from Egg White Protein by Protease. Master’s Thesis, Dalian Polytechnic University, Dalian, China, 2011. [Google Scholar]
- Qu, W.; Feng, Y.; Xiong, T.; Li, Y.; Wahia, H.; Ma, H. Preparation of corn ACE inhibitory peptide-ferrous chelate by dual-frequency ultrasound and its structure and stability analyses. Ultrason. Sonochem. 2022, 83, 105937. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Zheng, Y.; Shi, P.; Zhang, Y.; Liu, Y.; Long, N. Millet bran globulin hydrolysate derived tetrapeptide-ferrous chelate: Preparation, structural characterization, security prediction in silico, and stability against different food processing conditions. LWT Food Sci. Technol. 2022, 165, 113673. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, P.; Chen, Q.; Wang, A.; Dong, L.; Zhang, L. Preliminary Study on the Functions of Peptides Obtained from White Mullet (Ophiocephalus argus var. Kimnra) Meat. Foods 2025, 14, 1322. https://doi.org/10.3390/foods14081322
Zhang Y, Zhang P, Chen Q, Wang A, Dong L, Zhang L. Preliminary Study on the Functions of Peptides Obtained from White Mullet (Ophiocephalus argus var. Kimnra) Meat. Foods. 2025; 14(8):1322. https://doi.org/10.3390/foods14081322
Chicago/Turabian StyleZhang, Yin, Pengcheng Zhang, Qiuyue Chen, Aodong Wang, Li Dong, and Longyi Zhang. 2025. "Preliminary Study on the Functions of Peptides Obtained from White Mullet (Ophiocephalus argus var. Kimnra) Meat" Foods 14, no. 8: 1322. https://doi.org/10.3390/foods14081322
APA StyleZhang, Y., Zhang, P., Chen, Q., Wang, A., Dong, L., & Zhang, L. (2025). Preliminary Study on the Functions of Peptides Obtained from White Mullet (Ophiocephalus argus var. Kimnra) Meat. Foods, 14(8), 1322. https://doi.org/10.3390/foods14081322





