Preparation, Quality Analysis and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Kombucha Beverage at Different Fermentation Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
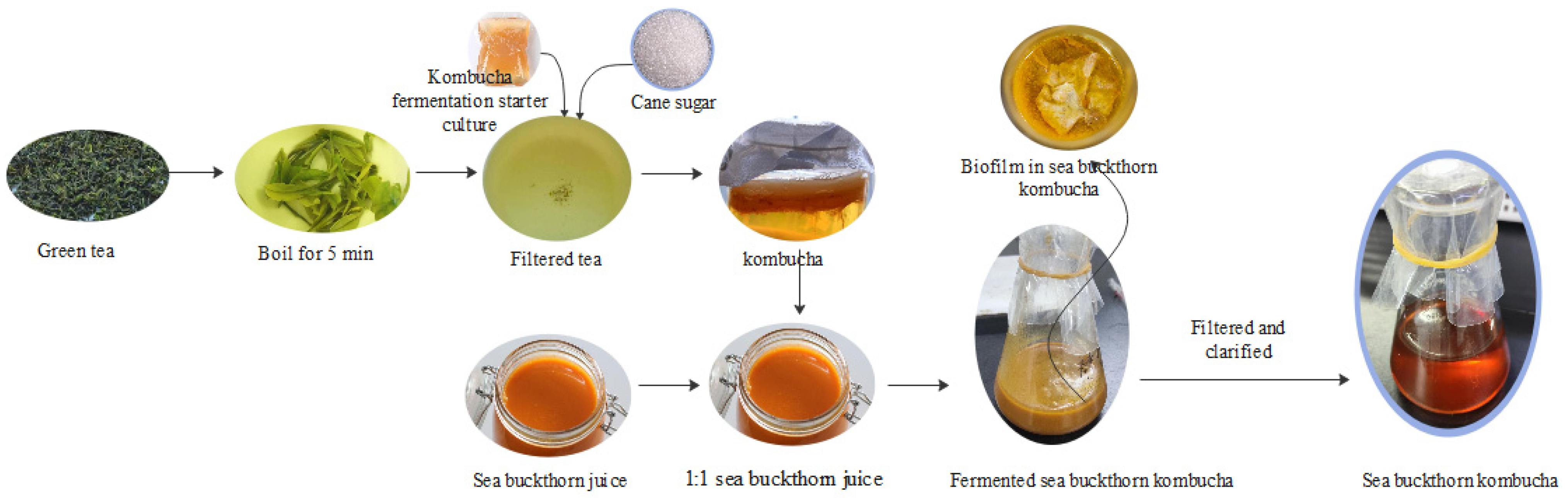
2.2. Kombucha Production
2.3. Sea Buckthorn Kombucha (SBK) Fermentation
2.4. Kombucha Quality Analysis
2.4.1. pH and Titratable Acid Determination
2.4.2. Total Soluble Solids
2.4.3. Color Analysis
2.5. Determination of Total Polyphenols
2.6. Determination of Total Flavonoids
2.7. Determination of Antioxidant Activity
2.7.1. DPPH Free Radical Scavenging
2.7.2. ABTS Free Radical Scavenging Rate
2.7.3. Total Reducing Power (TRP) Determination
2.8. Electronic Nose Analysis
2.9. Electronic Tongue Analysis
2.10. Sensory Evaluation of SBK
2.11. Statistics and Analyses
3. Results and Discussion
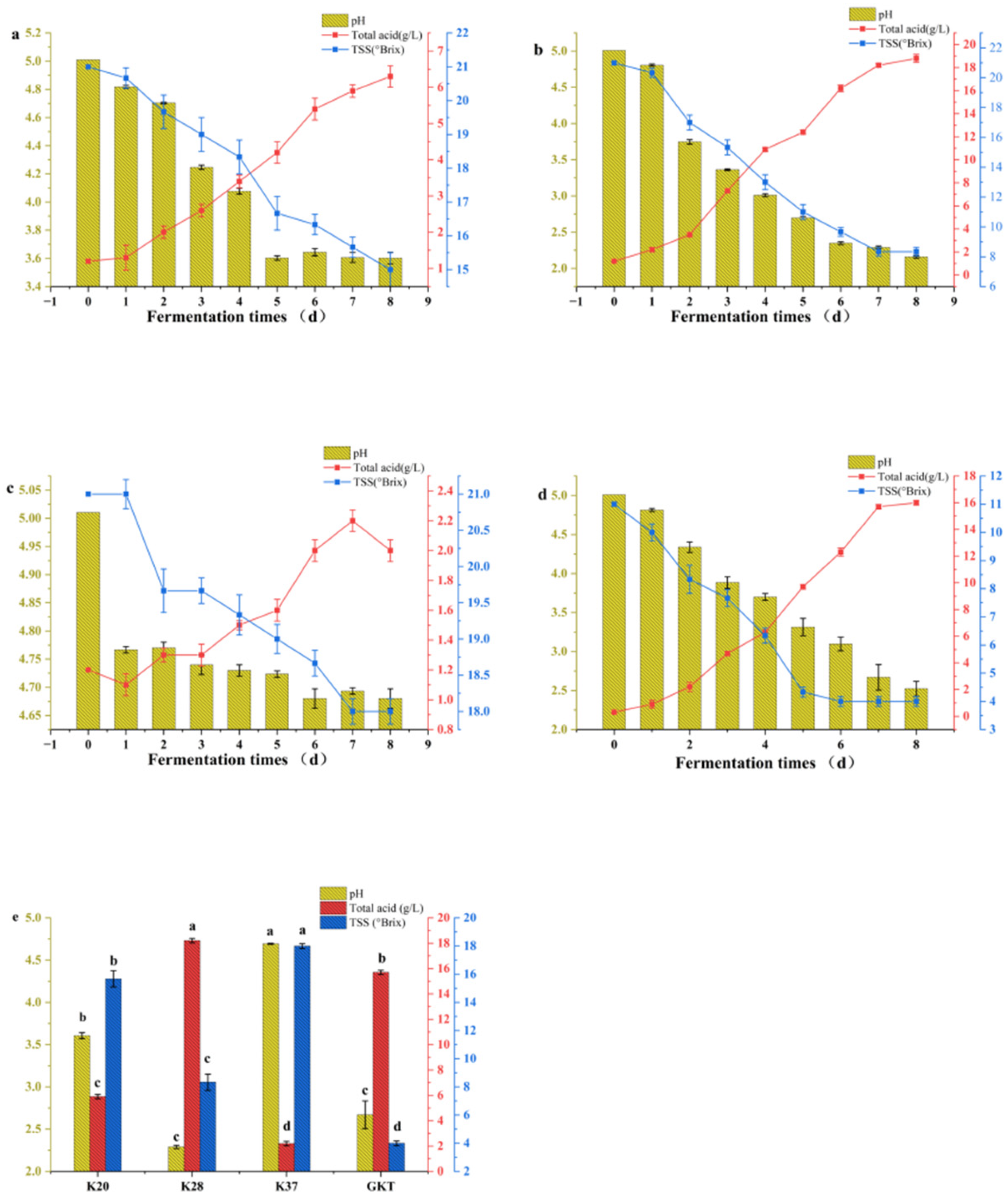
3.1. pH, Total Acid, and TSS
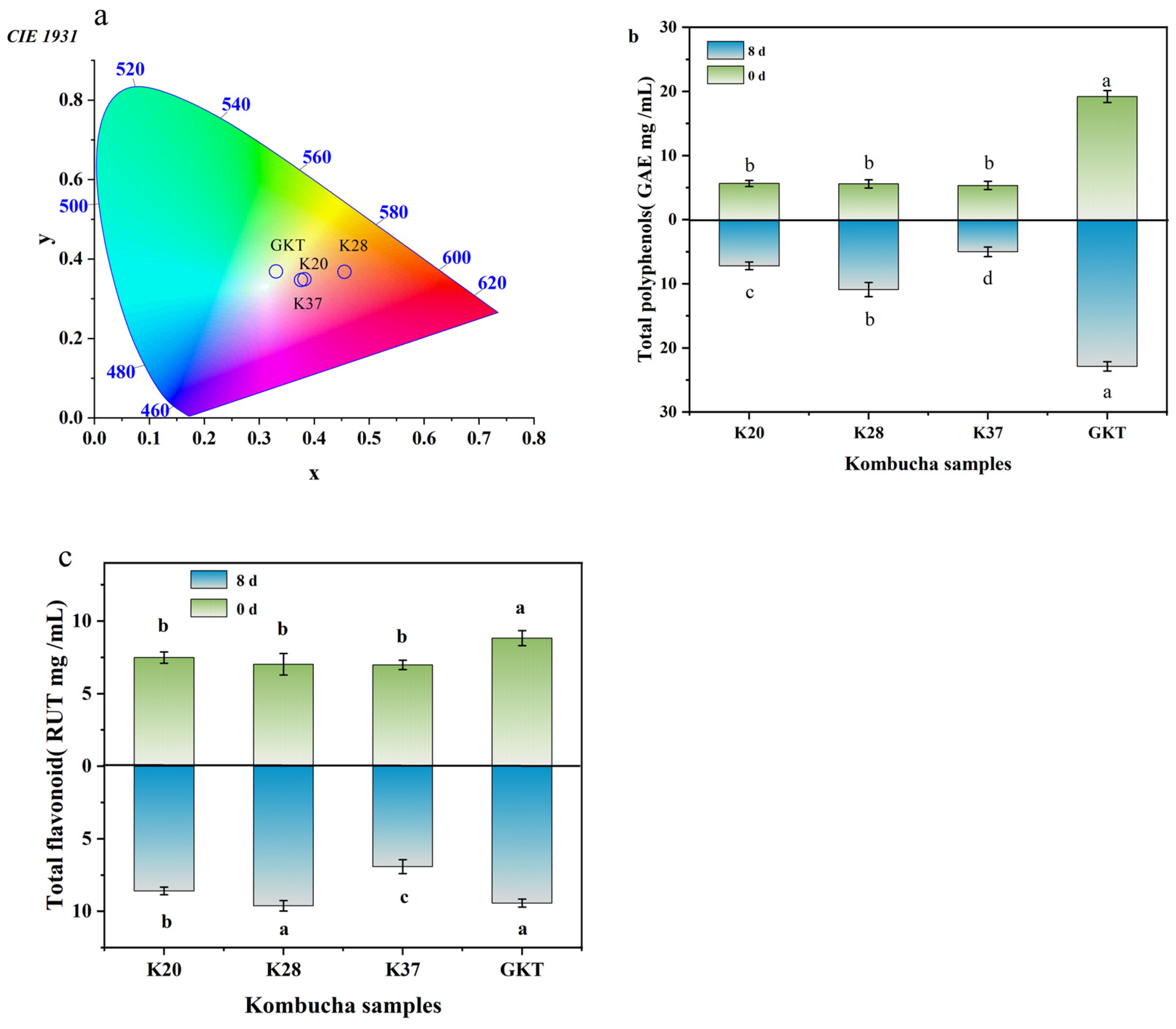
3.2. CIELab Colors
3.3. Bioactive Ingredient
3.3.1. Total Polyphenols
3.3.2. Total Flavonoids
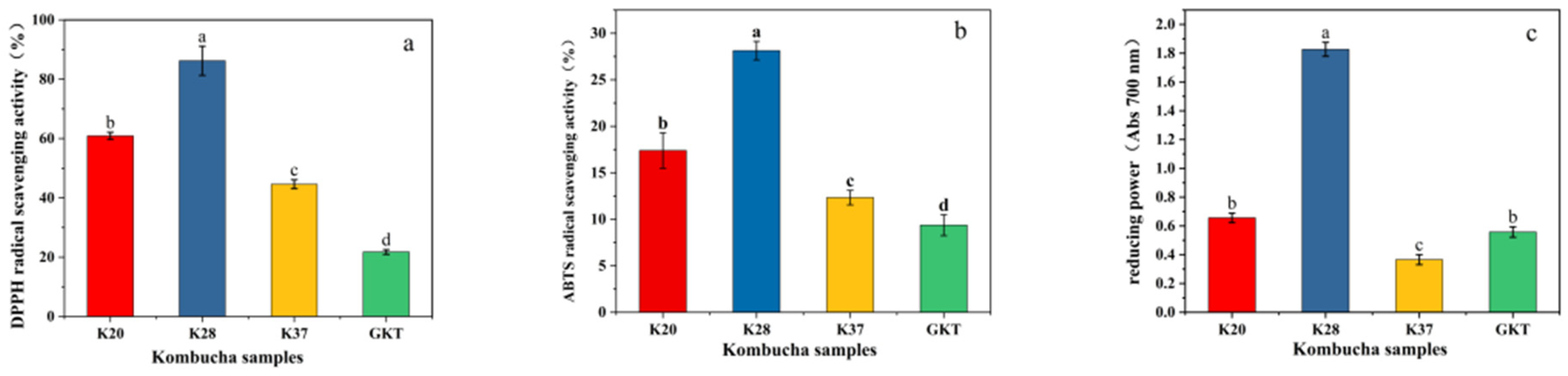
3.3.3. Determination of Antioxidant Activity
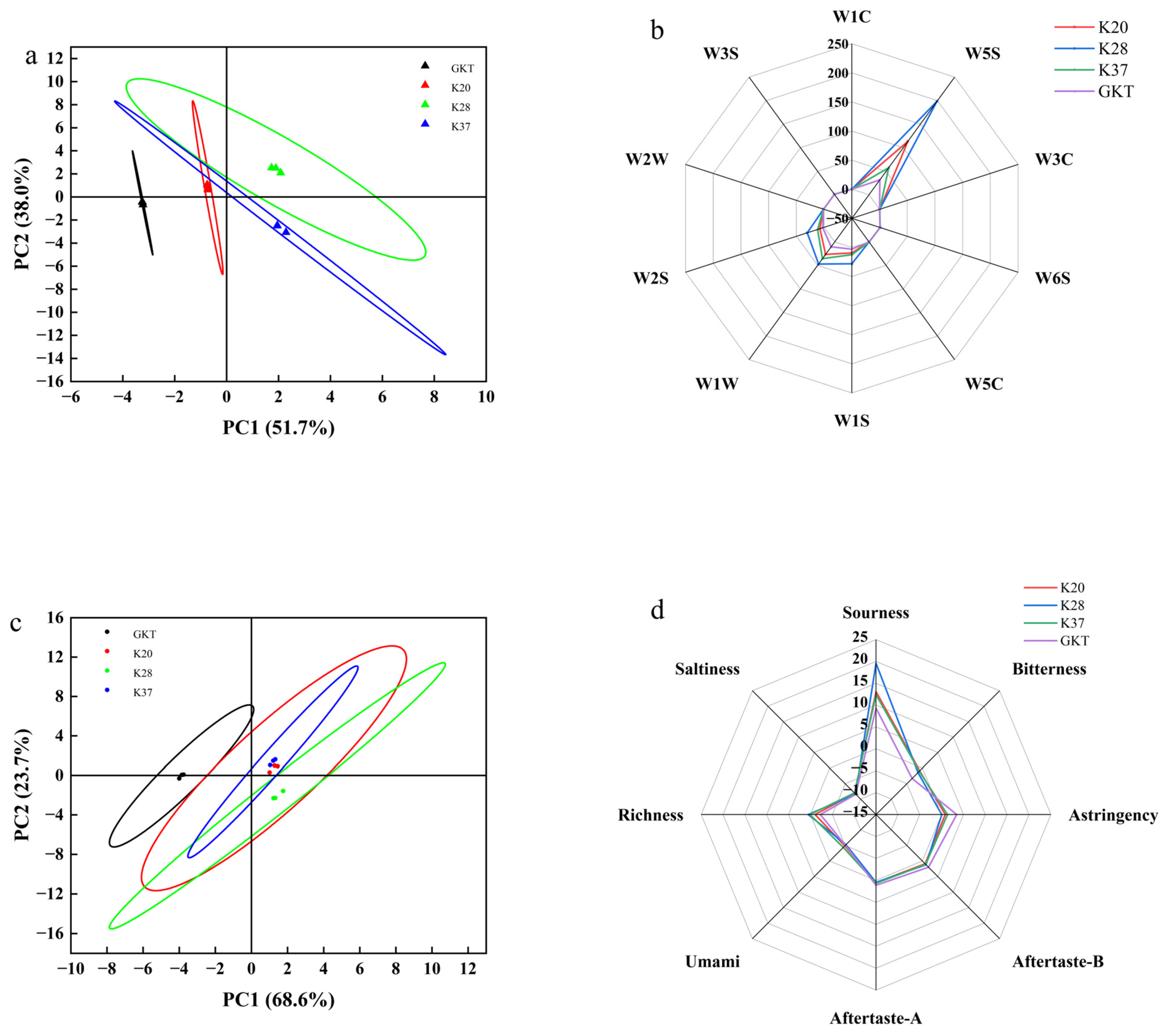
3.4. Analysis of Electronic Nose and Tongue Results
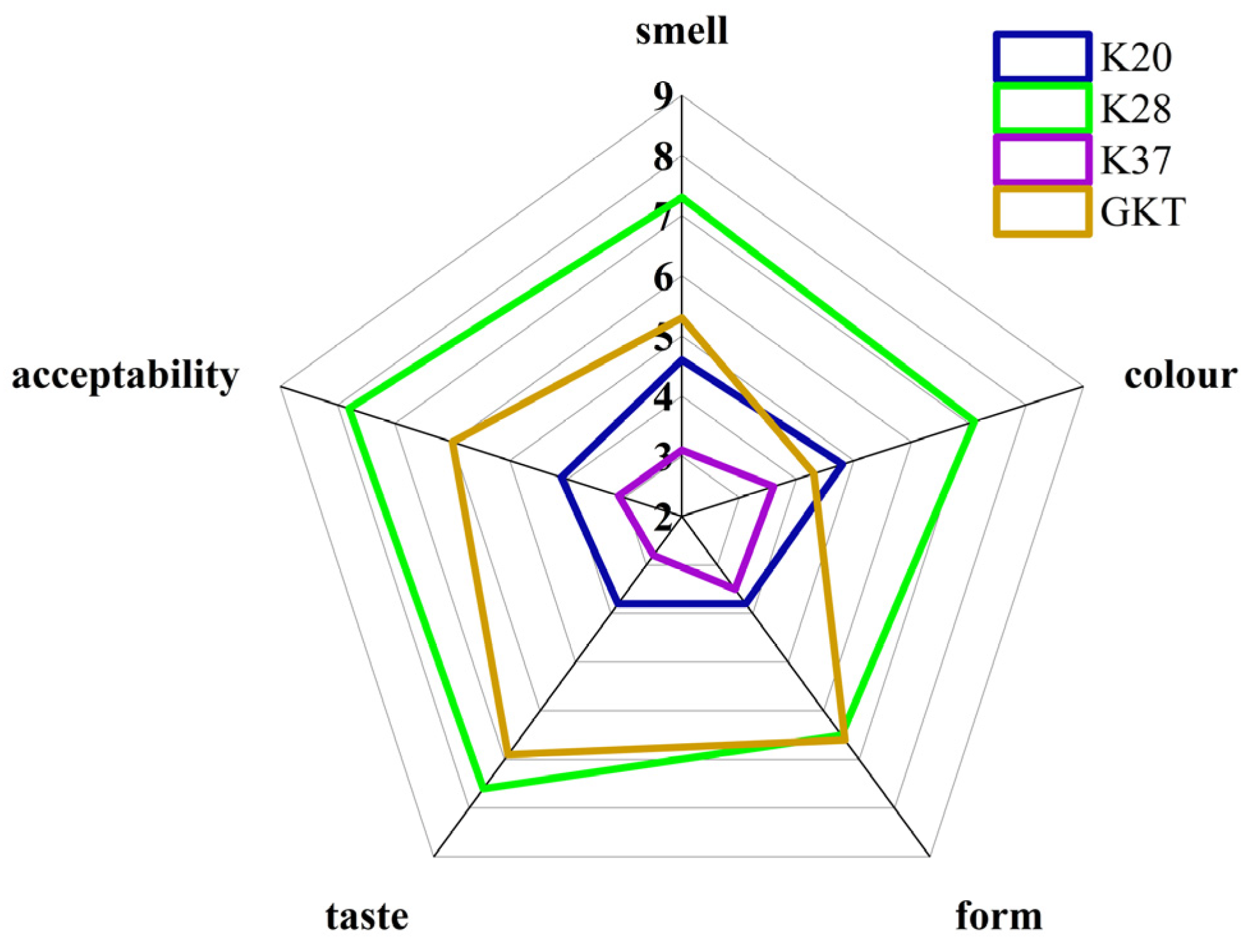
3.5. Sensory Evaluation
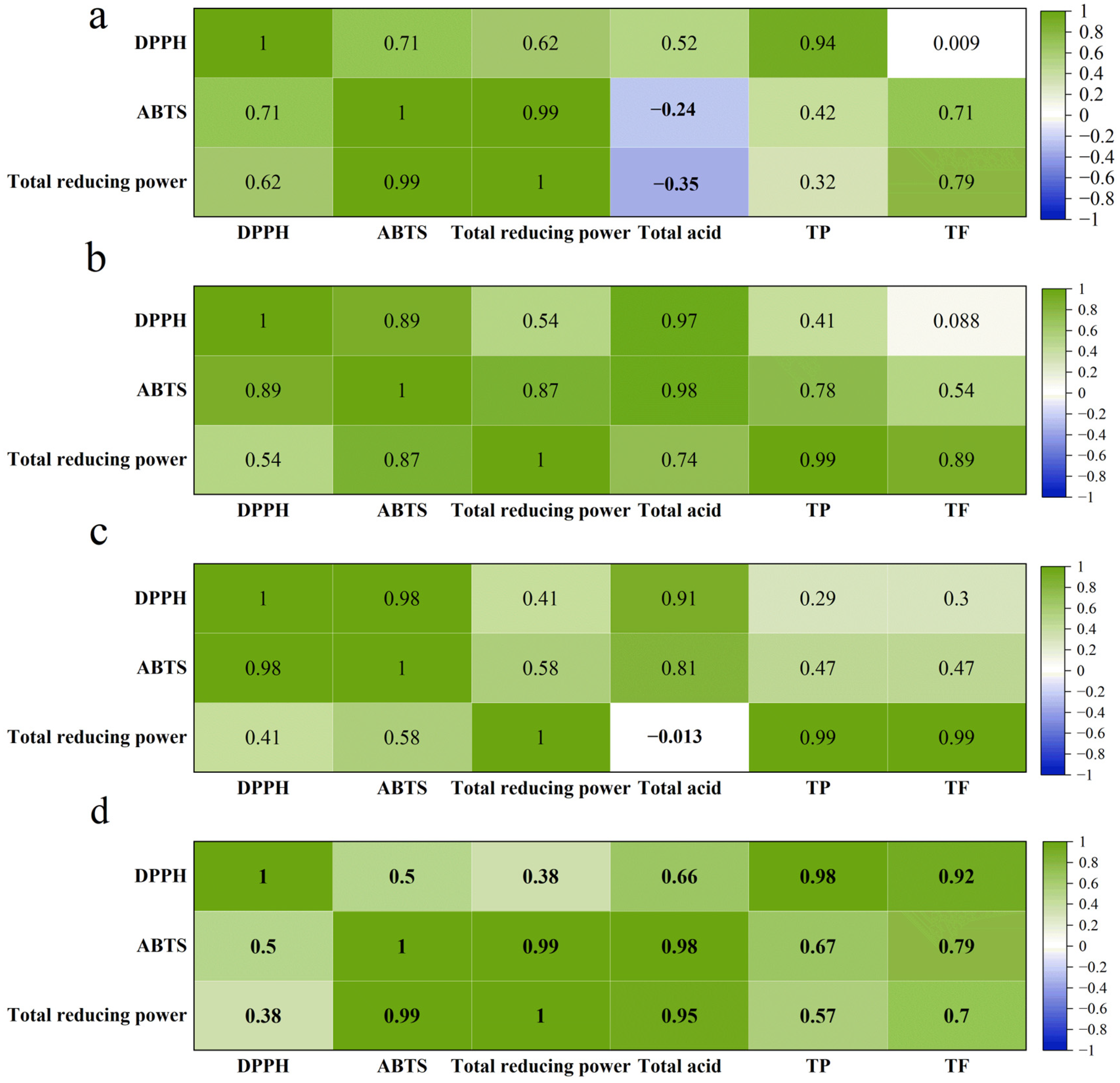
3.6. Correlation Analysis Between Antioxidant Parameters and Bioactivities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods 2023, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Aloulou, A.; Hamden, K.; Elloumi, D.; Ali, M.B.; Hargafi, K.; Jaouadi, B.; Ayadi, F.; Elfeki, A.; Ammar, E. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 63. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; Dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Vitas, J.; Malbaša, R.; Grahovac, J.; Lončar, E. The antioxidant activity of kombucha fermented milk products with stinging nettle and winter savory. Chem. Ind. Chem. Eng. Q. 2013, 19, 129–139. [Google Scholar] [CrossRef]
- Lončar, E.; Djurić, M.; Malbaša, R.; Kolarov, L.J.; Klašnja, M. Influence of Working Conditions Upon Kombucha Conducted Fermentation of Black Tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Du, W.; Ding, J.; Lu, S.; Wen, X.; Hu, J.; Ruan, C. Identification of the key flavonoid and lipid synthesis proteins in the pulp of two sea buckthorn cultivars at different developmental stages. BMC Plant Biol. 2022, 22, 299. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Eun, J.B. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef]
- GB 12456-2021; National Food Safety Standards Determination of Total Acid in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2021.
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea-based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cabral, D.; Valdez-Fragoso, A.; Rocha-Guzman, N.E.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.F.; Morales-Martinez, P.S.; Rojas-Contreras, J.A.; Mujica-Paz, H.; Gallegos-Infante, J.A. Effect of pulsed electric field (PEF)-treated kombucha analogues from Quercus obtusata infusions on bioactives and microorganisms. Innov. Food Sci. Emerg. Technol. 2016, 34, 747–759. [Google Scholar] [CrossRef]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crops Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Ying, Z.; Yuyang, H.; Meiying, L.; Bingyu, S.; Linlin, L.; Mingshou, L.; Min, Q.; Huanan, G.; Xiuqing, Z. High Fischer ratio peptide of hemp seed: Preparation and anti-fatigue evaluation in vivo and in vitro. Food Res. Int. 2023, 165, 112534. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Nyiew, K.-Y.; Kwong, P.J.; Yow, Y.-Y. An overview of antimicrobial properties of kombucha. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1024–1053. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CyTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Sharifudin, S.A.; Ho, W.Y.; Yeap, S.K.; Abdullah, R.; Koh, S.P. Fermentation and characterisation of potential kombucha cultures on papaya-based substrates. LWT 2021, 151, 112060. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.F.; Rosa, M.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha Healthy Drink—Recent Advances in Production. Chem. Compos. Health Benefits 2023, 9, 48. [Google Scholar]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Ferreira, T.V.; Castro, J.C.; Jansen da Silva, C.A.; Pilau, E.J.; Machinski Junior, M.; de Abreu Filho, B.A. Evaluation of antimicrobial activity of green tea kombucha at two fermentation time points against Alicyclobacillus spp. LWT Food Sci. Technol. 2020, 130, 109641. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Chen, Q.; Mei, C.; Liu, G.H. Recent advances in electronic nose techniques for monitoring of fermentation process. World J. Microbiol. Biotechnol. 2015, 31, 1845–1852. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Ren, G.; Li, T.; Wei, Y.; Ning, J.; Zhang, Z. Estimation of Congou black tea quality by an electronic tongue technology combined with multivariate analysis. Microchem. J. 2021, 163, 105899. [Google Scholar] [CrossRef]
- Singh, B.; Oberoi, S.; Kaur, A. Phenolic compounds in sea buckthorn (Hippophae rhamnoides L.) and their health-promoting activities: A review. Int. J. Food Sci. Technol. 2024, 59, 4229–4240. [Google Scholar] [CrossRef]
- Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Canadanovic-Brunet, J.M. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Zheng, Y.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Preparation, Quality Analysis and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Kombucha Beverage at Different Fermentation Temperatures. Foods 2025, 14, 1325. https://doi.org/10.3390/foods14081325
Pei Y, Zheng Y, Yuen M, Yuen T, Yuen H, Peng Q. Preparation, Quality Analysis and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Kombucha Beverage at Different Fermentation Temperatures. Foods. 2025; 14(8):1325. https://doi.org/10.3390/foods14081325
Chicago/Turabian StylePei, Yichao, Yuanju Zheng, Michael Yuen, Tina Yuen, Hywel Yuen, and Qiang Peng. 2025. "Preparation, Quality Analysis and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Kombucha Beverage at Different Fermentation Temperatures" Foods 14, no. 8: 1325. https://doi.org/10.3390/foods14081325
APA StylePei, Y., Zheng, Y., Yuen, M., Yuen, T., Yuen, H., & Peng, Q. (2025). Preparation, Quality Analysis and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Kombucha Beverage at Different Fermentation Temperatures. Foods, 14(8), 1325. https://doi.org/10.3390/foods14081325






