Preparation and Characterization of Vitamin D3-Based Binary Amorphous Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of VD3–Co-Former Binary Systems
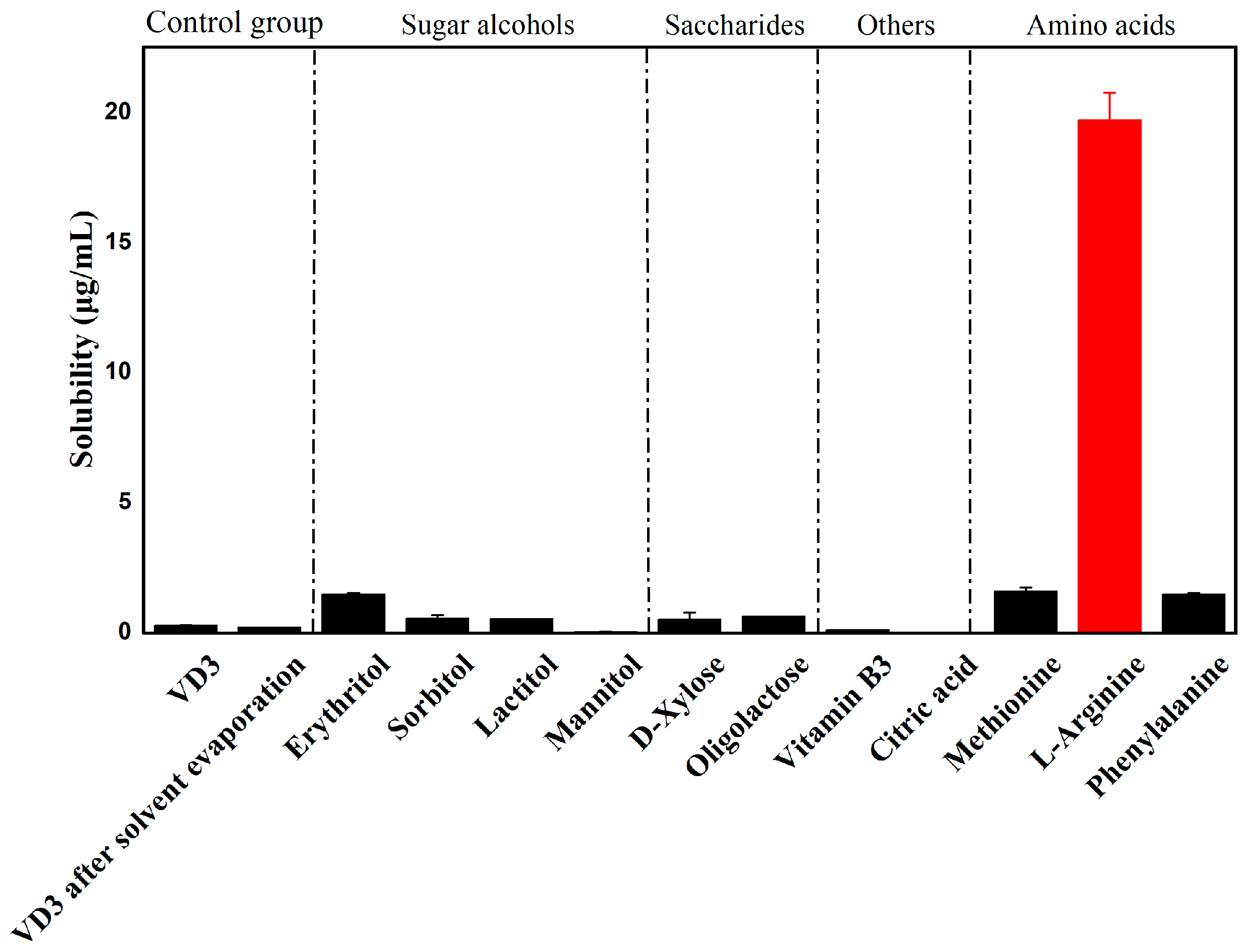
2.3. Co-Former Screening by Water Solubility Measurements
2.4. PXRD Measurements
2.5. DSC Measurements
2.6. SEM Measurements
2.7. FT-IR Analysis
2.8. MD Simulations
2.9. Dissolution Tests
2.10. Data Analysis
3. Results and Discussion
3.1. Co-Former Screening
3.2. Characterization of VD3–ARG Binary System
3.2.1. PXRD of VD3–ARG Binary System
3.2.2. DSC of VD3–ARG Binary System
3.2.3. Morphology of VD3–ARG Binary Amorphous System
3.3. Molecular Interactions of VD3–ARG Binary Amorphous System
3.3.1. FT-IR of VD3–ARG Binary Amorphous System
3.3.2. MD Simulations of VD3–ARG Binary Amorphous System
3.4. Dissolution Performance of the VD3–ARG Binary Amorphous System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armas, L.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]
- Hoyer-Hansen, M.; Nordbrandt, S.; Jäättelä, M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol. Med. 2010, 16, 295–302. [Google Scholar] [CrossRef]
- Picotto, G.; Liaudat, A.C.; Bohl, L.; de Talamoni, N.T. Molecular aspects of vitamin D anticancer activity. Cancer. Investig. 2012, 30, 604–614. [Google Scholar] [CrossRef]
- Stefanowski, B.; Antosik-Wójcinska, A.; Swiecicki, L. The effect of vitamin D3 deficiency on the severity of depressive symptoms. Overview of current research. Psychiatr. Pol. 2017, 51, 437–454. [Google Scholar] [CrossRef]
- Wu, B.X.; Tao, X.R.; Liu, C.L.; Li, H.X.; Jiang, T.; Chen, Z.J.; Wang, Q.; Liu, F.; Mu, M.; Chen, Z.Y. Vitamin D3 reduces hippocampal NR2A and anxiety in nicotine withdrawal mice. Transl. Neurosci. 2021, 12, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Kiely, M.E.; Andersen, R.; Grønborg, I.M.; Madsen, K.H.; Nissen, J.; Tetens, I.; Tripkovic, L.; Lanham-New, S.A.; Toxqui, L.; et al. Individual participant data (IPD)-level meta-analysis of randomized controlled trials with vitamin D-fortified foods to estimate dietary reference values for vitamin D. Eur. J. Nutr. 2021, 60, 939–959. [Google Scholar] [CrossRef]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics. 2019, 11, 347. [Google Scholar] [CrossRef]
- Molaveisi, M.; Zhao, Y.; Shi, Q.L.; Fang, Z.X. Function of vitamin D3-loaded lipid-based nanocarriers in food industry: Principles, applications, and challenges. Trends Food Sci. Technol. 2025, 155, 104798. [Google Scholar] [CrossRef]
- Besada, L.N.; Hermet, M.; Bakas, L.; Cortizo, A.M.; Cortizo, M.S. Polymersomes as nanocarriers of vitamin D3: Morphological and in vitro characterization. J. Nanopart. Res. 2022, 24, 180. [Google Scholar] [CrossRef]
- Hasanvand, E.; Fathi, M.; Bassiri, A.; Javanmard, M.; Abbaszadeh, R. Novel starch based nanocarrier for vitamin D fortification of milk: Production and characterization. Food Bioprod. Process. 2015, 96, 264–277. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of vitamin D3 in pickering emulsion stabilized by nanofibrillated mangosteen cellulose: Effect of environmental stresses. J. Food Sci. 2019, 84, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duggan, E. Improved stability of vitamin D3 encapsulated in whey protein isolate microgels. Int. Dairy J. 2022, 129, 105351. [Google Scholar] [CrossRef]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D incorporation in foods: Formulation strategies, stability, and bioaccessibility as affected by the food matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef]
- Grohganz, H.; Löbmann, K.; Priemel, P.; Jensen, K.T.; Graeser, K.; Strachan, C.; Rades, T. Amorphous drugs and dosage forms. J. Drug Deliv. Sci. Technol. 2013, 23, 403–408. [Google Scholar] [CrossRef]
- Shelke, R.; Velagacherla, V.; Nayak, U.Y. Recent advances in dual-drug co-amorphous systems. Drug Discov. Today 2024, 29, 103863. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zograf, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Liu, J.W.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.J. Co-amorphous drug formulations in numbers: Recent advances in co-amorphous drug formulations with focus on co-formability, molar ratio, preparation methods, physical stability, in vitro and in vivo performance, and new formulation strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef]
- Wang, R.N.; Han, J.W.; Jiang, A.; Huang, R.; Fu, T.M.; Wang, L.C.; Zheng, Q.; Li, W.; Li, J.S. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Wu, T.; Feng, Y.; Jiang, C.P.; Du, H.Z.; Lu, S. Apigenin-oxymatrine binary co-amorphous mixture: Enhanced solubility, bioavailability, and anti-inflammatory effect. Food Chem. 2022, 373, 131485. [Google Scholar] [CrossRef]
- Song, X.N.; Luo, Y.T.; Zhao, W.D.; Liu, S.M.; Wang, Y.Z.; Zhang, H. Preparation and characterization of lutein co-amorphous formulation with enhanced solubility and dissolution. Foods 2024, 13, 2029. [Google Scholar] [CrossRef]
- Li, Y.X.; Luo, Y.T.; Song, X.N.; Wang, Y.Z.; Liu, S.M.; Ren, F.Z.; Kong, L.Y.; Zhang, H. Enhancing water solubility of phytosterols through co-amorphization with food-grade co-formers. Curr. Res. Food Sci. 2025, 10, 100984. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of low-and non-calorie sweeteners on the gut microbiota: A review of clinical trials and cross-sectional studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- Wu, G.Y. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Su, Y.; Li, S.H.; Man, C.X.; Jiang, Y.J.; Qu, B.; Yang, X.Y.; Guo, L. Advances in oligosaccharides and polysaccharides with different structures as wall materials for probiotics delivery: A review. Int. J. Biol. Macromol. 2024, 277, 134468. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Rekka, E.A. The antioxidant potential of vitamins and their implication in metabolic abnormalities. Nutrients 2024, 16, 2740. [Google Scholar] [CrossRef]
- Danimayostu, A.A.; Lukitaningsih, E.; Martien, R.; Danarti, R. Determination of vitamin D3 loaded selfnanoemulsifying drug delivery systems (SNEDDS) based hydrogel. J. Res. Pharm. 2023, 27, 1213–1219. [Google Scholar] [CrossRef]
- Molaveisi, M.; Noghabi, M.S.; Tabasi, S.N. Vitamin D3-loaded nanophytosomes for enrichment purposes: Formulation, structure optimization, and controlled release. J. Food Process Eng. 2020, 43, 13560. [Google Scholar] [CrossRef]
- Bakirova, R.; Nukhuly, A.; Iskineyeva, A.; Burkeyev, M.Z.; Mustafayeva, A.; Minayevа, Y.V.; Sarsenbekova, A.Z. Obtaining and investigation of the β-cyclodextrin inclusion complex with vitamin D3 oil solution. Scientifica 2020, 2020, 6148939. [Google Scholar] [CrossRef]
- Sathisaran, I.; Bhatia, D.D.; Dalvi, S.V. New curcumin-trimesic acid cocrystal and anti-invasion activity of curcumin multicomponent solids against 3D tumor models. Int. J. Pharm. 2020, 587, 119667. [Google Scholar] [CrossRef] [PubMed]
- Lenz, E.; Löbmann, K.; Rades, T.; Knop, K.; Kleinebudde, P. Hot melt extrusion and spray drying of co-amorphous indomethacin-arginine with polymers. J. Pharm. Sci. 2017, 106, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Li, L.Y.; Su, M.L.; Heng, W.L.; Wei, Y.F.; Gao, Y.; Qian, S. Deaggregation and crystallization inhibition by small amount of polymer addition for a co-amorphous curcumin-magnolol system. Pharmaceutics 2021, 13, 1725. [Google Scholar] [CrossRef]
- Al Fatease, A.; Shoman, M.E.; Abourehab, M.; Abou-Taleb, H.A.; Abdelkader, H. A novel curcumin arginine salt: A solution for poor solubility and potential anticancer activities. Molecules 2023, 28, 262. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J. Pharm. Pharmacol. 2009, 61, 971–988. [Google Scholar] [CrossRef]
- Fang, X.P.; Hu, Y.; Yang, G.Y.; Shi, W.F.; Lu, S.; Cao, Y. Improving physicochemical properties and pharmacological activities of ternary co-amorphous systems. Eur. J. Pharm. Biopharm. 2022, 181, 22–35. [Google Scholar] [CrossRef]
- Yarlagadda, D.L.; Vullendula, S.; Nair, A.R.; Sree, K.; Dengale, S.J.; Bhat, K. Considerations for the selection of co-formers in the preparation of co-amorphous formulations. Int. J. Pharm. 2021, 602, 120649. [Google Scholar] [CrossRef]
- Shi, Q.; Moinuddin, S.M.; Cai, T. Advances in coamorphous drug delivery systems. Acta Pharm. Sin. B 2019, 9, 19–35. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, X.; Wu, Q.; Cao, Y.; Song, X.; Luo, Y.; Luo, Z.; Liu, J.; Zhang, H. Preparation and Characterization of Vitamin D3-Based Binary Amorphous Systems. Foods 2025, 14, 1321. https://doi.org/10.3390/foods14081321
Zhao X, Wang X, Wu Q, Cao Y, Song X, Luo Y, Luo Z, Liu J, Zhang H. Preparation and Characterization of Vitamin D3-Based Binary Amorphous Systems. Foods. 2025; 14(8):1321. https://doi.org/10.3390/foods14081321
Chicago/Turabian StyleZhao, Xiaoshuo, Xuemei Wang, Qiuyang Wu, Yiyang Cao, Xuening Song, Yingting Luo, Zisheng Luo, Jingwen Liu, and Hao Zhang. 2025. "Preparation and Characterization of Vitamin D3-Based Binary Amorphous Systems" Foods 14, no. 8: 1321. https://doi.org/10.3390/foods14081321
APA StyleZhao, X., Wang, X., Wu, Q., Cao, Y., Song, X., Luo, Y., Luo, Z., Liu, J., & Zhang, H. (2025). Preparation and Characterization of Vitamin D3-Based Binary Amorphous Systems. Foods, 14(8), 1321. https://doi.org/10.3390/foods14081321






