(1→3)-α-d-Glucan from the Pink Oyster Mushroom (Pleurotus djamor): Structural Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material and Fruiting Conditions
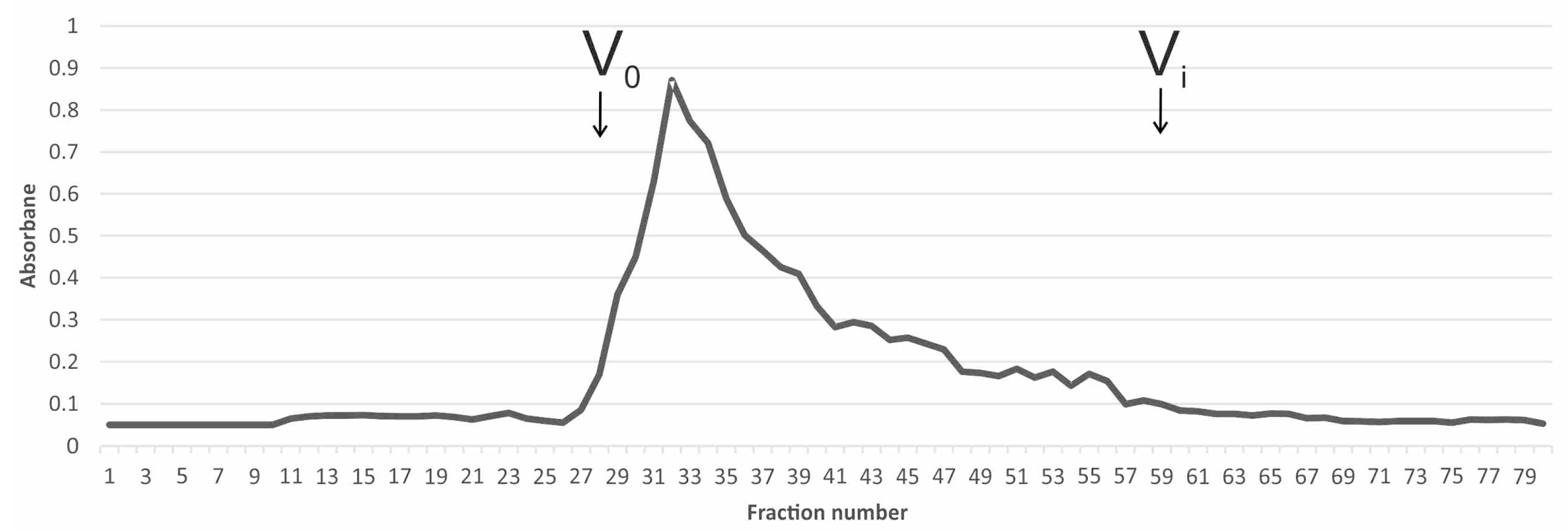
2.2. WI-ASF Isolation
2.3. Sugar Composition and Methylation Analysis of WI-ASF from P. djamor
2.4. Miscellaneous Methods
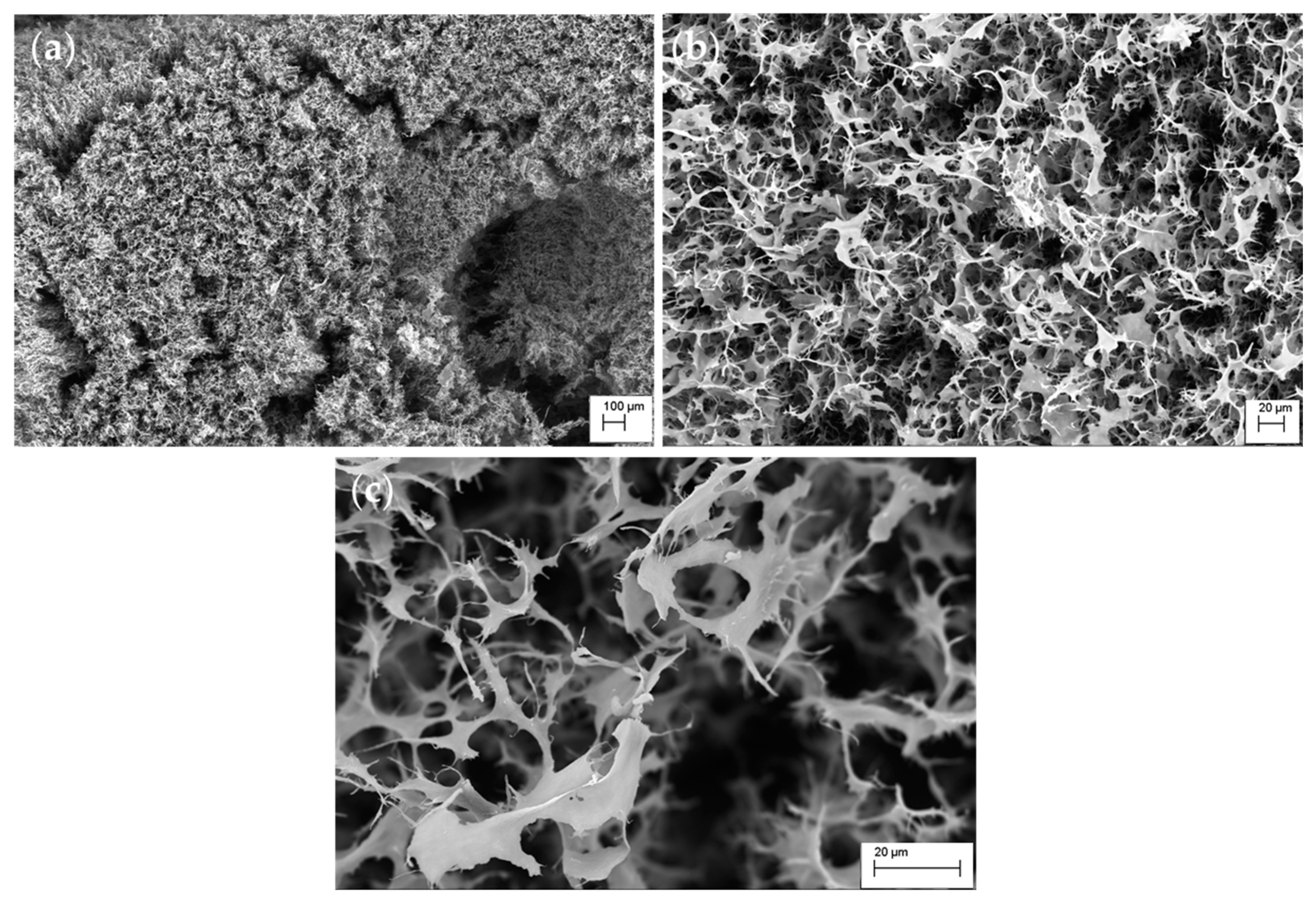
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WI-ASF | Water-insoluble alkali-soluble fraction |
| PBS | Phosphate buffered saline |
| SEM | Scanning electron microscopy |
| TFA | Trifluoroacetic acid |
| GPC | Gel permeation chromatography |
| FT-IR | Fourier transform infrared spectroscopy |
| FT-Raman | Fourier transform Raman |
References
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A report on fungal (1→3)-α-d-glucans: Properties, functions and application. Molecules 2019, 24, 3972. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.R.; Batchu, U.R.; Buddana, S.K.; Rao, K.S.; Penna, S. A comprehensive review on α-d-Glucans: Structural and functional diversity, derivatization and bioapplications. Carbohydr. Res. 2021, 503, 108297. [Google Scholar] [CrossRef]
- Wiater, A.; Pleszczyńska, M.; Szczodrak, J.; Janusz, G. Comparative studies on the induction of Trichoderma harzianum mutanase by α-(1→3)-glucan-rich fruiting bodies and mycelia of Laetiporus sulphureus. Int. J. Mol. Sci. 2012, 13, 9584–9598. [Google Scholar] [CrossRef]
- Wiater, A.; Waśko, A.; Adamczyk, P.; Gustaw, K.; Pleszczyńska, M.; Wlizło, K.; Skowronek, M.; Tomczyk, M.; Szczodrak, J. Prebiotic potential of oligosaccharides obtained by acid hydrolysis of α-(1→3)-glucan from Laetiporus sulphureus: A pilot study. Molecules 2020, 25, 5542. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L. Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1→3)-α-d-glucan from Poria cocos mycelia. Carbohydr. Polym. 2011, 83, 1363–1369. [Google Scholar] [CrossRef]
- Araujo, R.V.S.; Melo-Junior, M.R.; Beltrao, E.I.C.; Mello, L.A.; Iacomini, M.; Carneiro-Leao, A.M.A.; CarvalhoJr, L.B.; Satos-Magalhaes, N.S. Evaluation of the antischistosomal activity of sulfated α-d-glucan from the lichen Ramalina celastri free and encapsulate into liposomes. Braz. J. Med. Biol. Res. 2011, 44, 311–318. [Google Scholar] [CrossRef]
- Nowak, K.; Wiater, A.; Choma, A.; Wiacek, D.; Bieganowski, A.; Siwulski, M.; Wasko, A. Fungal (1→3)-α-d-glucans as a new kind of biosorbent for heavy metals. Int. J. Biol. Macromol. 2019, 137, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Puanglek, S.; Kimura, S.; Enomoto-Rogers, Y.; Kabe, T.; Yoshida, M.; Wada, M.; Iwata, T. In vitro synthesis of linear α-1,3-glucan and chemical modification to ester derivatives exhibiting outstanding thermal properties. Sci. Rep. 2016, 6, 30479. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Próchniak, K.; Pleszczynska, M.; Siwulski, M.; Bialas, W.; Szczodrak, J. Ocena aktywności biologicznej karboksymetylowanych pochodnych α-(1→3)-glukanów wyizolowanych z owocników uprawnych gatunków boczniaka (Pleurotus). Zywn. Nauk. Technol. Ja. 2015, 98, 193–206. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Pleszczyńska, M.; Próchniak, K.; Choma, A.; Kandefer-Szerszeń, M.; Szczodrak, J. α-(1→ 3)-d-Glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol. Lett. 2011, 33, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2011; pp. 295–300. [Google Scholar]
- Salmones, D.; Valdez, L.M.; Gatian-Hernande, R. Entecruzamiento y evaluacion de la produccion de las variedades de Pleurotus djamor (Fr.) Boedijn. Rev. Mex. Mic. 2004, 18, 21–26. [Google Scholar] [CrossRef]
- Arbaayah, H.H.; Umi, K.Y. Antioxidant properties in the oyster mushrooms (Pleurotus spp.) and split gill (Scizophyllum commune) ethanolic extracts. Mycosphere 2013, 4, 661–673. [Google Scholar] [CrossRef]
- Srivastava, M. A pink coloured Pleurotus djamor (Rumph.) Boedijn from natural habitat of north Bihar, India. Curr. Sci. 2001, 80, 337–338. [Google Scholar]
- İnci, Ş.; Akyüz, M.; Kirbağ, S. Antimicrobial, antioxidant, cytotoxicity and DNA protective properties of the pink oyster mushroom, Pleurotus djamor (Agaricomycetes). Int. J. Med. Mushrooms 2023, 25, 55–66. [Google Scholar] [CrossRef]
- Vega, A.; De León, J.A.; Miranda, S.; Reyes, S.M. Agro-industrial waste improves the nutritional and antioxidant profile of Pleurotus djamor. Clean. Waste Syst. 2022, 2, 100018. [Google Scholar] [CrossRef]
- Maity, G.N.; Maity, P.; Khatua, S.; Acharya, K.; Dalai, S.; Mondal, S. Structural features and antioxidant activity of a new galactoglucan from edible mushroom Pleurotus djamor. Int. J. Biol. Macromol. 2021, 168, 743–749. [Google Scholar] [CrossRef]
- Raman, J.; Sivakumar, A.; Lakshmanan, H.; Raaman, N.; Shin, H.J. Antioxidant activity of partially characterized polysaccharides from the edible mushroom Pleurotus djamor var. roseus. J. Mushroom. 2021, 19, 140–149. [Google Scholar] [CrossRef]
- Shreya, S.; Jain, S.K.; Guru, S.K.; Sahu, A.N. Anti-cancer potential of Pleurotus mushroom: Detailed insight on the potential bioactive molecules, in vitro—in vivo studies, and formulation. Lett. Drug Des. Discov. 2023, 20, 439–456. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Sun, W.; Kong, Y.; Jia, L. Antioxidation, anti-inflammation and anti-fibrosis effect of phosphorylated polysaccharides from Pleurotus djamor mycelia on adenine-induced chronic renal failure mice. Int. J. Biol. Macromol. 2021, 170, 652–663. [Google Scholar] [CrossRef]
- Nayak, H.; Kushwaha, A.; Behera, P.C.; Shahi, N.C.; Kushawaha, K.P.S.; Kumar, A.; Mishra, K.K. The pink oyster mushroom, Pleurotus djamor (Agaricomycetes): A potent antioxidant and hypoglycemic agent. Int. J. Med. Mushrooms 2021, 23, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Afsar, M.; Zia, A.; Salam, M.B.U.; Ahmad, M.N.; Khan, A.A.; Haq, T.; Aziz, T.; Alasmari, A.F. A multifaceted analysis of spent mushroom substrate of selected oyster mushrooms for enzymatic activity, proximate composition, and antimicrobial activity. Ital. J. Food Sci. 2024, 36, 165–174. [Google Scholar] [CrossRef]
- Maikon, R.E.; Ávila, S.; Lima, J.J.; Silva, R.S.A.; Andrade, L.P.; Bacila, D.M.; Mathias, A.L.; Jorge, R.M.M. Araucaria angustifolia seed coat waste reduction through its utilization in substrate diversification for Pleurotus djamor production. Sci. Hortic. 2024, 330, 113060. [Google Scholar] [CrossRef]
- Otali, C.C.; Otoikhian, C.S.O.; Onuoha, T.; Akpeji, C.S.; Bosah, B.O. Antibacterial activities of Pleurotus ostreatus and Pleurotus djamor against selected bacterial pathogens. Bima J. Sci.Technol. 2024, 8, 397–402. [Google Scholar] [CrossRef]
- Andrade, G.M.; Souza, E.L.; Zárate-Salazar, J.R.; Oliveira, J.N.; Tavares, J.F.; Lima, M.S.; Medeiros, R.L.; Albuquerque, T.M.R.; Pereira, F.O. Unveiling the potential prebiotic effects of edible mushroom Pleurotus djamor during in vitro colonic fermentation. J. Agric. Food Chem. 2024, 72, 26722–26732. [Google Scholar] [CrossRef]
- İnci, Ş.; Kirbağ, S.; Akyüz, M. Valorization of local agro-residues for the cultivation of Pleurotus djamor (Rumph. Ex Fr.) Boedijn and their effects on nutritional value. Biomass Conv. Bioref. 2024, 1–10. [Google Scholar] [CrossRef]
- Nguyen, B.T.T.; Le, V.V.; Nguyen, H.N.; Nguyen, H.T.T.; Nguyen, L.T.; Ngo, N.X. Cotton waste as an optimal substrate for cultivation of the pink oyster mushroom Pleurotus djamor. J. App. Biol. Biotech. 2025, 13, 184–191. [Google Scholar] [CrossRef]
- Cruz-Moreno, B.A.; Pérez, A.A.F.; García-Trejo, J.F.; Pérez-García, S.A.; Gutiérrez-Antonio, C. Identification of secondary metabolites of interest in Pleurotus djamor using agave tequilana bagasse. Molecules 2023, 28, 557. [Google Scholar] [CrossRef]
- Jegadeesh, R.; Lakshmanan, H.; Kab-Yeul, J.; Sabaratnam, V.; Raaman, N. Cultivation of pink oyster mushroom Pleurotus djamor var. roseus on various agro-residues by low cost technique. J. Mycopathol. Res. 2018, 56, 213–220. [Google Scholar]
- Silva, L.A.; Dulay, R.M.R.; Kalaw, S.P. Mycelial growth of pink oyster mushroom (Pleurotus djamor) on banana sucrose gulaman and fruiting body production on banana-based substrate formulations. CLSU Int. J. Sci. Technol. 2018, 3, 24–32. [Google Scholar] [CrossRef]
- Satpal, S.; Gopal, S.; Kumar, R. Effect of different substrates on the growth and yield of oyster mushroom (Pleurotus djamor). Int. J. Agric. Sci. 2017, 9, 3721–3723. [Google Scholar]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2019, 99, 1691–1699. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Siwulski, M.; Mildner-Szkudlarz, S.; Wąsowicz, E. Studies on the aroma of different species and strains of Pleurotus measured by GC/MS, sensory analysis and electronic nose. Acta Sci. Pol. Technol. Aliment. 2009, 8, 47–61. [Google Scholar]
- Raman, J.; Lakshmanan, H.; Jang, K.Y.; Oh, M.; Oh, Y.L.; Im, J.H. Nutritional composition and antioxidant activity of pink oyster mushrooms (Pleurotus djamor var. roseus) grown on a paddy straw substrate. J. Mushroom 2020, 18, 189–200. [Google Scholar] [CrossRef]
- Madaan, K.; Sharma, S.; Kalia, A. Effect of selenium and zinc biofortification on the biochemical parameters of Pleurotus spp. under submerged and solid-state fermentation. J. Trace Elem. Med. Biol. 2024, 82, 127365. [Google Scholar] [CrossRef]
- Sravani, A.; Sharma, S.; Kalia, A. Effect of selenium enriched wheat substrate on nutritional and antioxidant properties of Pleurotus spp. Acta Aliment. 2021, 50, 358–368. [Google Scholar] [CrossRef]
- Zięba, P.; Sękara, A.; Bernaś, E.; Krakowska, A.; Sułkowska-Ziaja, K.; Kunicki, E.; Suchanek, M.; Muszyńska, B. Supplementation with magnesium salts—A strategy to increase nutarceutical value of Pleurotus djamor fruiting bodies. Molecules 2021, 26, 3273. [Google Scholar] [CrossRef]
- Kiho, T.; Yoshida, I.; Katsuragawa, M.; Sakushima, M.; Usui, S.; Ukai, S. Polysaccharides in fungi XXXIV: A polysaccharide from fruiting bodies of Amanita muscaria and the antitumor activities of its carboxymethylated product. Biol. Pharm. Bull. 1994, 17, 1460–1462. [Google Scholar] [CrossRef][Green Version]
- Sawardeker, J.S.; Sloneker, J.H.; Jeanes, A.R. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 1965, 37, 1602–1604. [Google Scholar] [CrossRef]
- Hakomori, S. Rapid permethylation of glycolipids and polysaccharides catalyzed by methyl carbon. J. Biochem. 1964, 55, 205–208. [Google Scholar]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the d and l configuration of neutral monosaccharides by high-resolution capillary g.l.c. Carbohydr. Res. 1978, 62, 349–357. [Google Scholar] [CrossRef]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol. Microbiol. 2009, 73, 553–570. [Google Scholar] [CrossRef]
- Choma, A.; Wiater, A.; Komaniecka, I.; Paduch, R.; Pleszczyńska, M.; Szczodrak, J. Chemical characterization of a water insoluble (1→3)-α-d-glucan from an alkaline extract of Aspergillus wentii. Carbohydr. Polym. 2013, 91, 603–608. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Grün, C.H. Structure and Bosynthesis of Fungal α-Glucans. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2003. [Google Scholar]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Fontaine, T.; Aimanianda, V.; Latge, J.P. Aspergillus cell wall and biofilm. Mycopathologia 2014, 178, 371–377. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, Y.; Feng, Y.; Zhou, S.; Wang, F. Structural characterisation and antimutagenic activity of a novel polysaccharide isolated from Sepiella maindroni ink. Food Chem. 2008, 110, 807–813. [Google Scholar] [CrossRef]
- Gieroba, B.; Kalisz, G.; Krysa, M.; Khalavka, M.; Przekora, A. Application of vibrational spectroscopic techniques in the study of the natural polysaccharides and their cross-linking process. Int. J. Mol. Sci. 2023, 24, 2630. [Google Scholar] [CrossRef]
- Seymour, F.R.; Julian, R.L.; Jeanes, A.; Lamberts, B.L. Structural analysis of insoluble d-glucans by Fourier-transform, infrared difference-spectrometry: Correlation between structures of dextrans from strains of Leuconostoc mesenteroides and of d-glucans from strains of Streptococcus mutans. Carbohydr. Res. 1980, 86, 227–246. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Cheng, S. Chemical structure and molecular weights of α-(1→3)-d-glucan from Lentinus edodes. Biosci. Biotechnol. Biochem. 1999, 63, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Russell, N.C.; Weinstein, R.; Wynn-Williams, D.D. Fourier transform Raman spectroscopic study of fungi. J. Raman Spectrosc. 1995, 26, 911–916. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováríková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water-insoluble (1→3)-α-d-glucan isolated from the Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Choma, A.; Nowak, K.; Komaniecka, I.; Waśko, A.; Pleszczyńska, M.; Siwulski, M.; Wiater, A. Chemical characterization of alkali-soluble polysaccharides isolated from a Boletus edulis (Bull.) fruiting body and their potential for heavy metal biosorption. Food Chem. 2018, 266, 329–334. [Google Scholar] [CrossRef]
- Kiho, T.; Yoshida, I.; Nagai, K.; Ukai, S.; Hara, C. (1→3)-α-d-glucan from an alkaline extract of Agrocybe cylindracea, and antitumor activity of its O-(carboxymethyl)ated derivatives. Carbohydr. Res. 1989, 189, 273–279. [Google Scholar] [CrossRef]
- Lin, H.; Han, R.; Wu, W. Glucans and applications in drug delivery. Carbohydr. Polym. 2024, 332, 121904. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.; Malfatti, C.R.M. Biological activities of derivatized d-glucans: A review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Industrial production and applications of α/β linear and branched glucans. Indian Chem. Eng. 2021, 63, 533–547. [Google Scholar] [CrossRef]

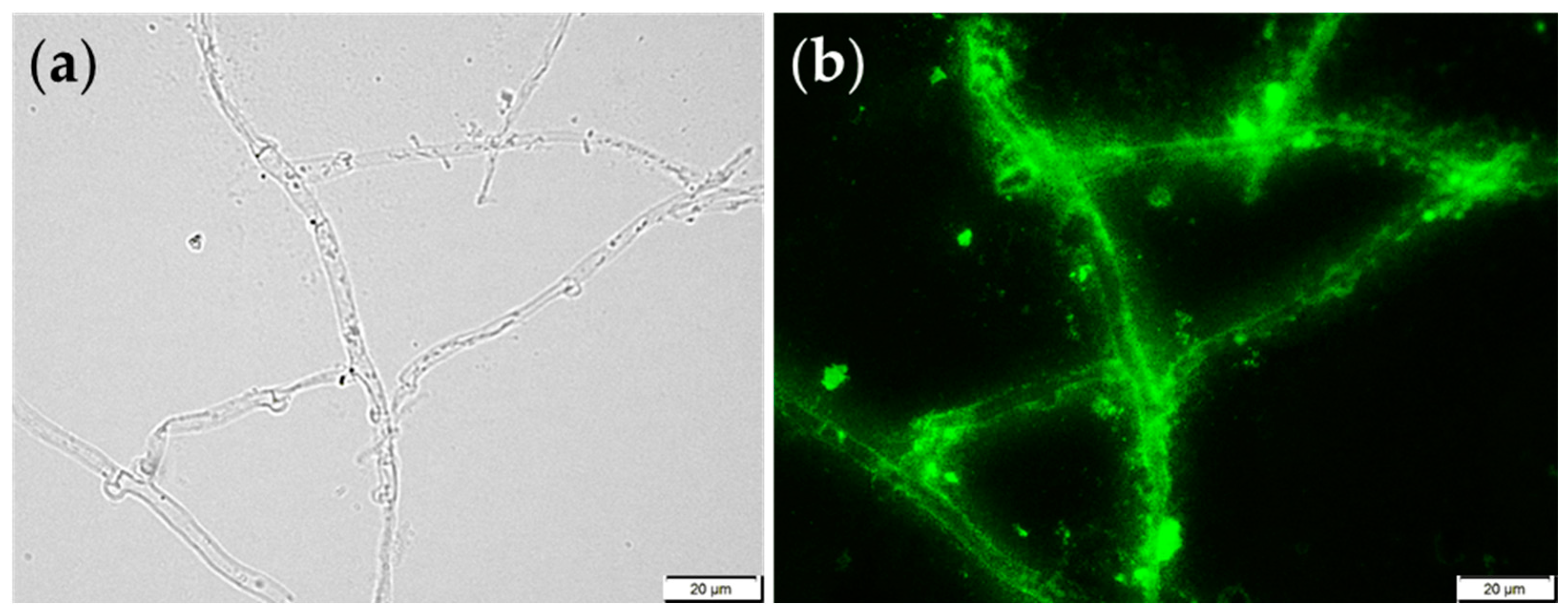
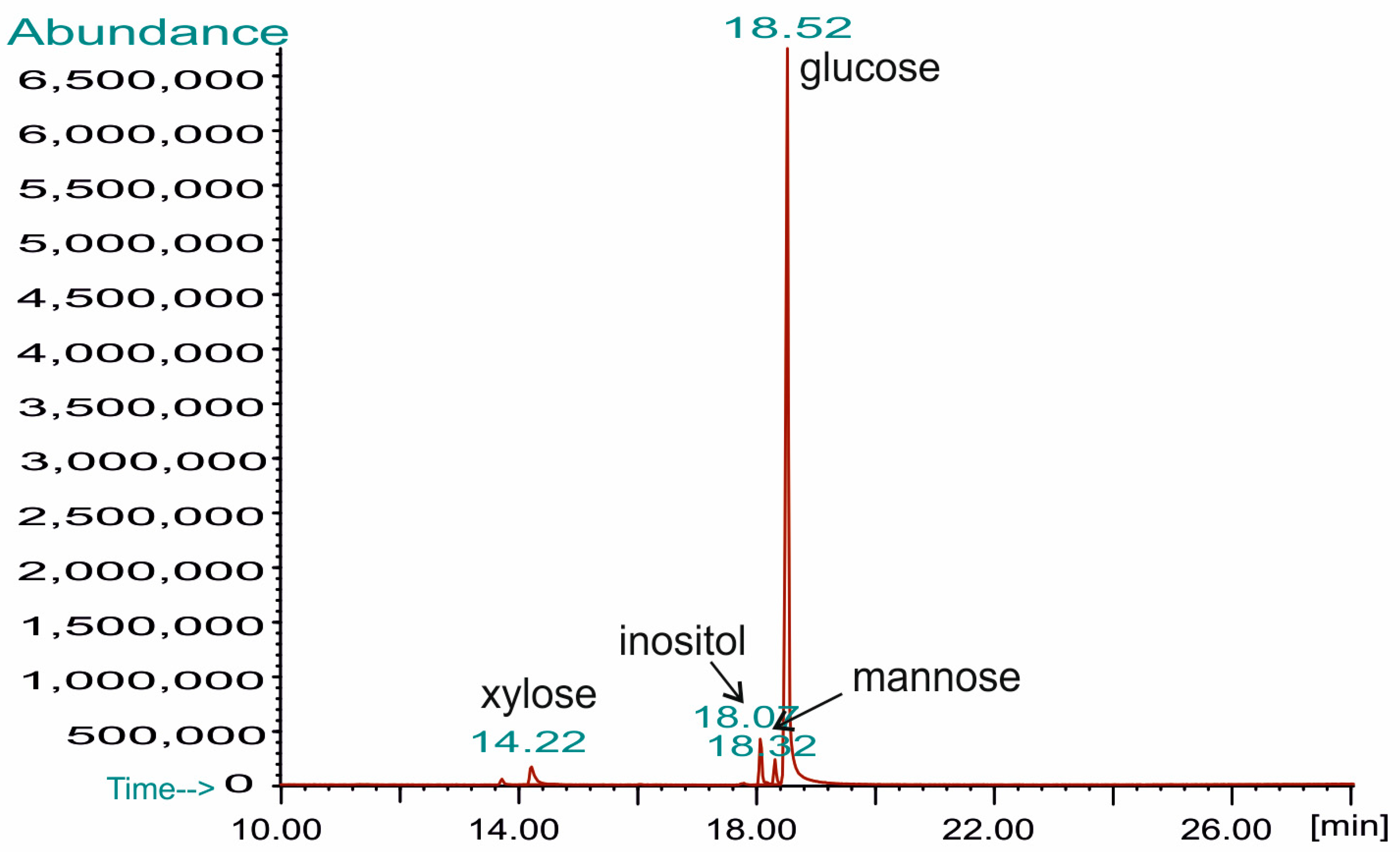
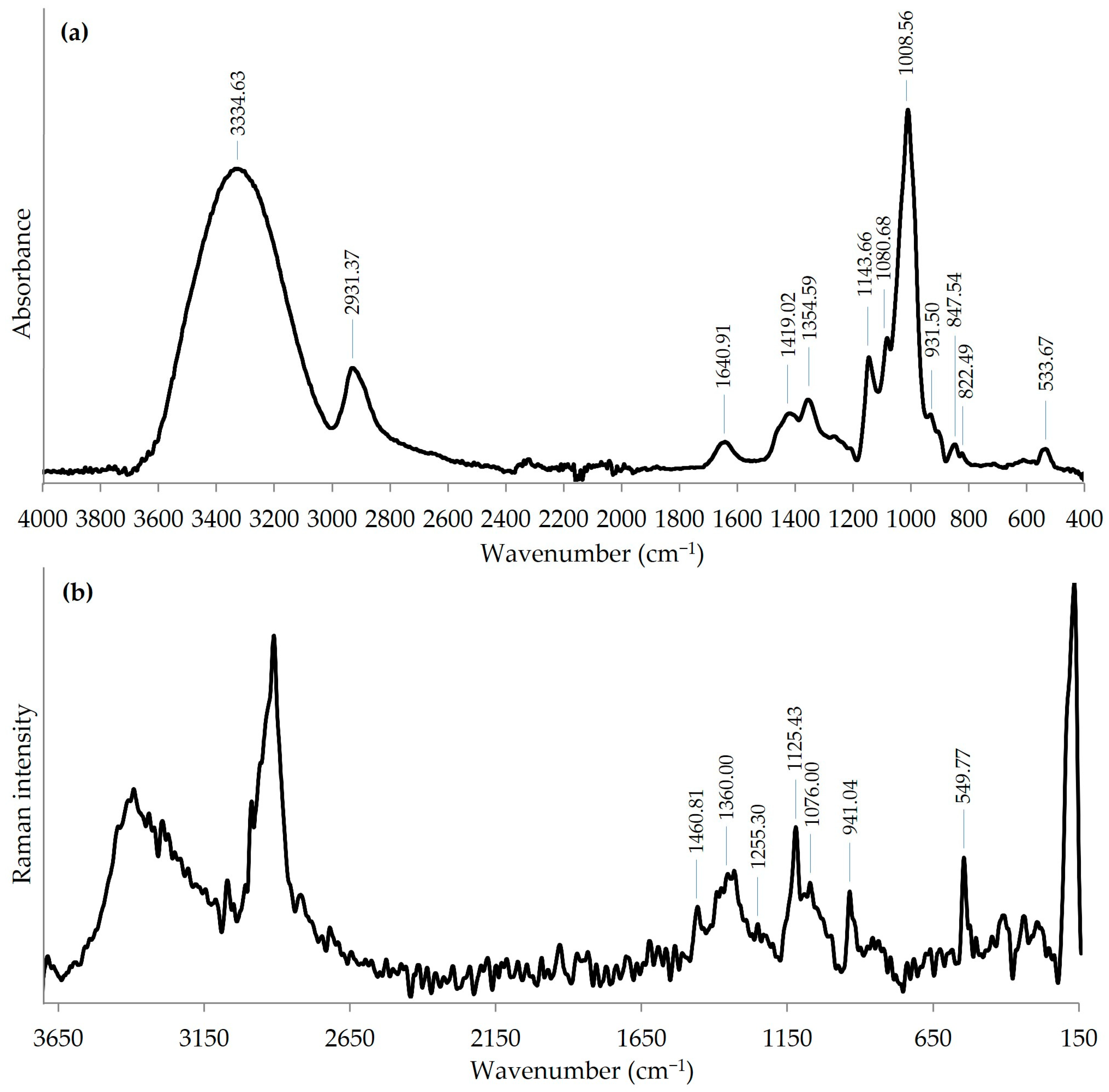
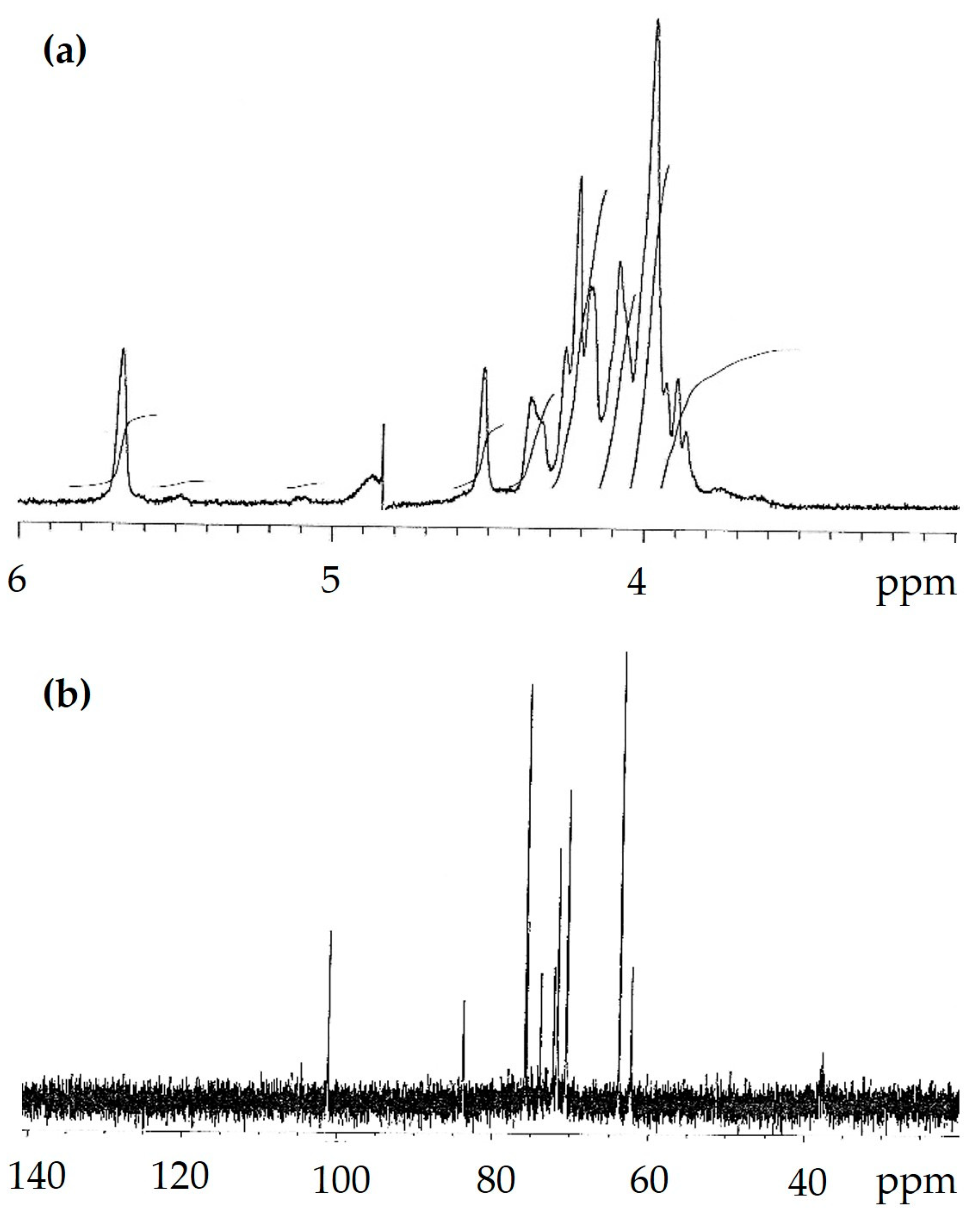
| Methylated Sugar | Linkage Type | Mol (%) |
|---|---|---|
| 2,3,4-O-Me3-pentose | tPenp-(1→ | 2.7 |
| 3,4-O-Me2-pentose | →2)-Penp-(1→ | 1.3 |
| 2,3,4,6-O-Me4-hexose | tHexp-(1→ | 2.1 |
| 2,4,6-O-Me3-hexose | →3)-Hexp-(1→ | 86.4 |
| 2,3,6-O-Me3-hexose | →4)-Hexp-(1→ | 4.5 |
| 2,6-O-Me2- hexose | →3,4)-Hexp-(1→ | 1.2 |
| 2,4-O-Me2- hexose | →3,6)-Hexp-(1→ | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, P.; Komaniecka, I.; Siwulski, M.; Wlizło, K.; Junka, A.; Nowak, A.; Kowalczyk, D.; Waśko, A.; Lisiecka, J.; Grzymajło, M.; et al. (1→3)-α-d-Glucan from the Pink Oyster Mushroom (Pleurotus djamor): Structural Features. Foods 2025, 14, 1272. https://doi.org/10.3390/foods14071272
Adamczyk P, Komaniecka I, Siwulski M, Wlizło K, Junka A, Nowak A, Kowalczyk D, Waśko A, Lisiecka J, Grzymajło M, et al. (1→3)-α-d-Glucan from the Pink Oyster Mushroom (Pleurotus djamor): Structural Features. Foods. 2025; 14(7):1272. https://doi.org/10.3390/foods14071272
Chicago/Turabian StyleAdamczyk, Paulina, Iwona Komaniecka, Marek Siwulski, Kamila Wlizło, Adam Junka, Artur Nowak, Dariusz Kowalczyk, Adam Waśko, Jolanta Lisiecka, Michał Grzymajło, and et al. 2025. "(1→3)-α-d-Glucan from the Pink Oyster Mushroom (Pleurotus djamor): Structural Features" Foods 14, no. 7: 1272. https://doi.org/10.3390/foods14071272
APA StyleAdamczyk, P., Komaniecka, I., Siwulski, M., Wlizło, K., Junka, A., Nowak, A., Kowalczyk, D., Waśko, A., Lisiecka, J., Grzymajło, M., & Wiater, A. (2025). (1→3)-α-d-Glucan from the Pink Oyster Mushroom (Pleurotus djamor): Structural Features. Foods, 14(7), 1272. https://doi.org/10.3390/foods14071272










