Mechanism Analysis of Amphotericin B Controlling Postharvest Gray Mold in Table Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Pathogen Culture
2.3. Fruit
2.4. In Vivo Antifungal Efficacy of AMB Towards B. cinerea
2.5. In Vitro Antifungal Efficacy of AMB Towards B. cinerea
2.6. Determination of Cell Viability
2.7. Determination of Malondialdehyde (MDA) Content, Electrical Conductivity, and Cellular Leakage
2.8. RNA-Sequencing
2.9. RT-qPCR Analysis
2.10. Assays of Enzyme Activity
2.11. Statistical Analysis
3. Results
3.1. AMB Demonstrates Efficient Management of Gray Mold in Table Grape Fruit
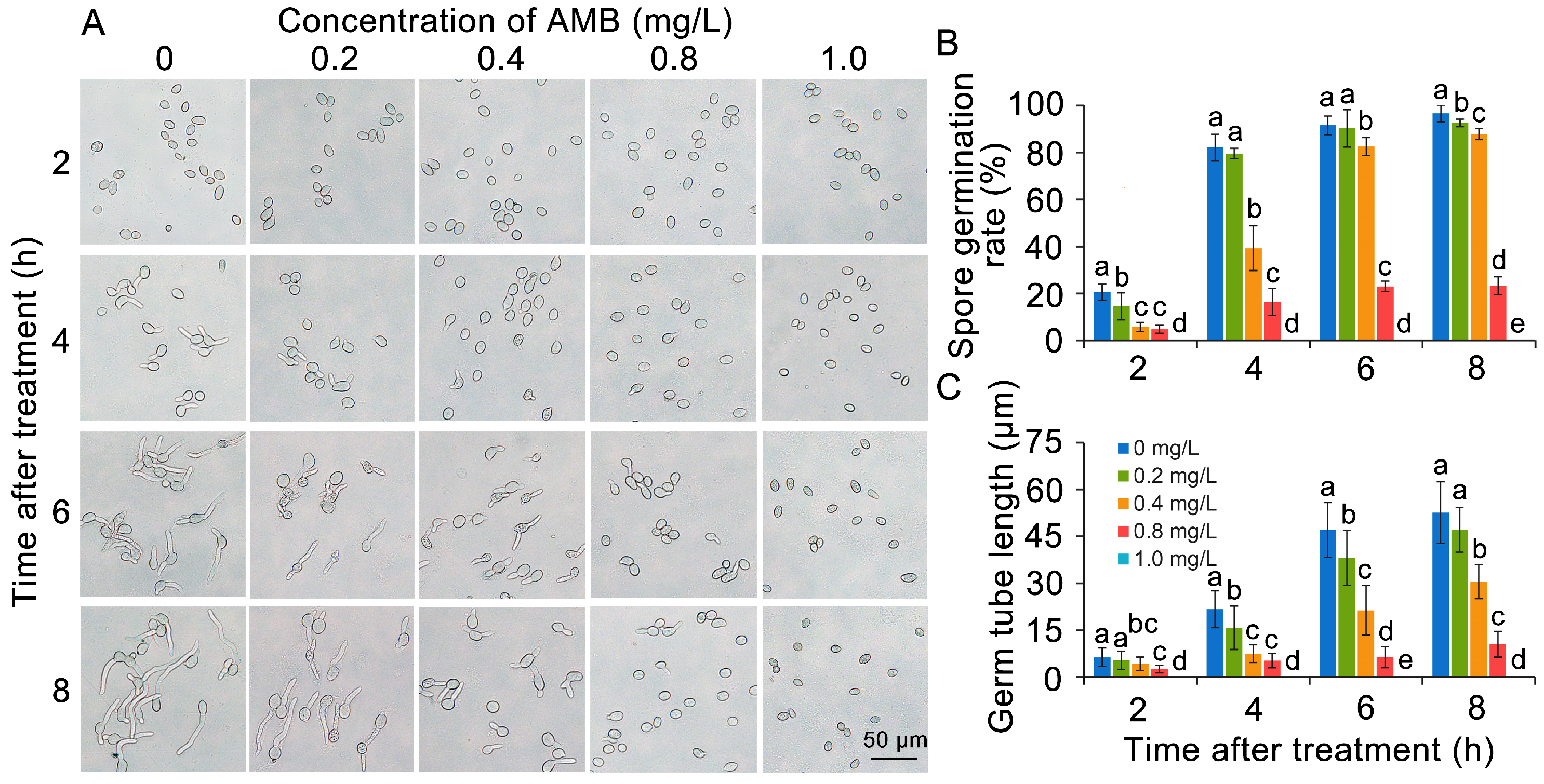
3.2. AMB Impacts the Mycelial Growth and Spore Germination of B. cinerea
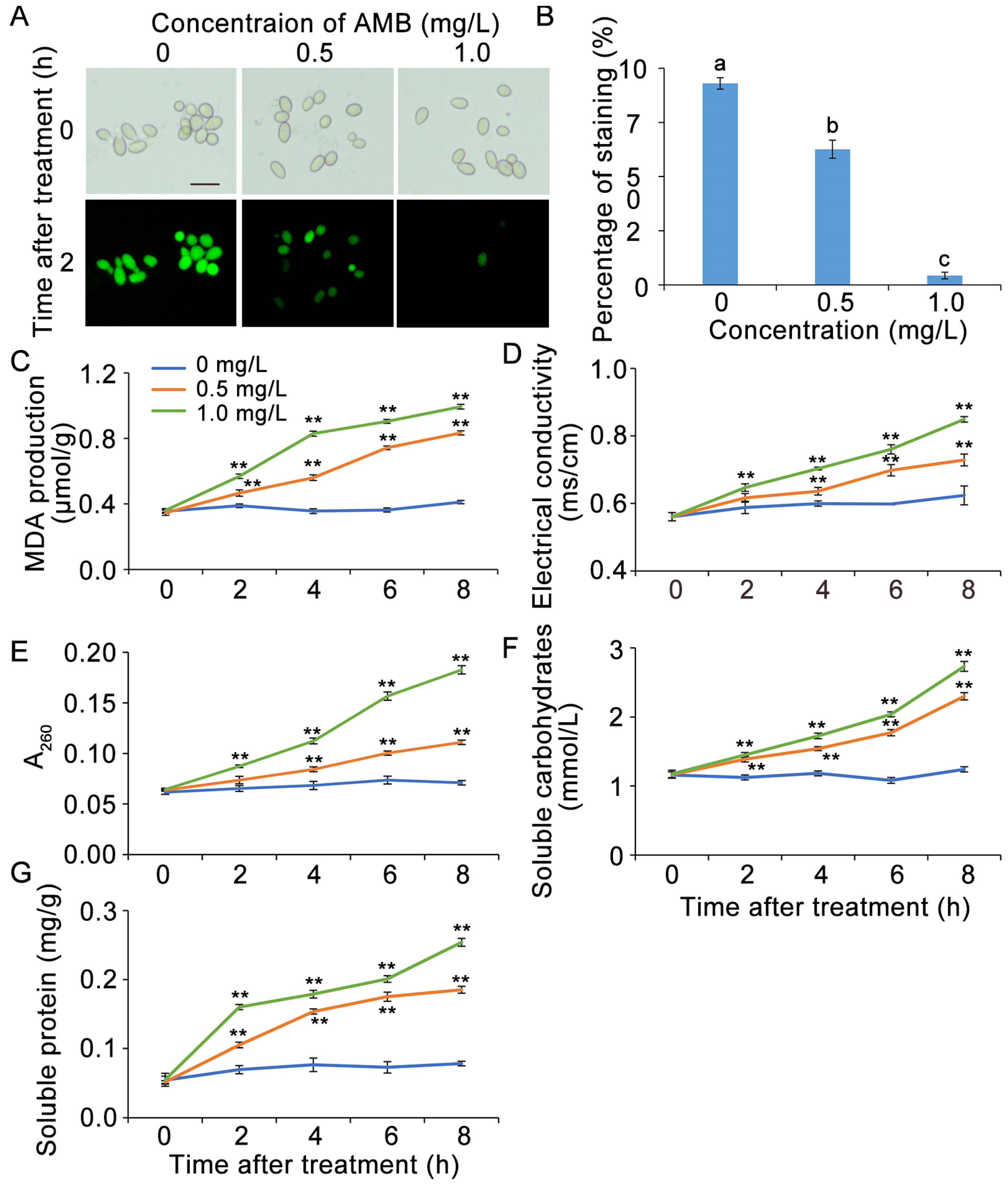
3.3. AMB Treatment Impaired Cell Viability of B. cinerea
3.4. AMB Treatment Influenced the Generation of MDA, Electrical Conductivity, and Cellular Leakage in B. cinerea
3.5. RNA-Seq Analysis of B. cinerea After Treatment with AMB
3.6. Confirmation of Gene Expression via RT-qPCR
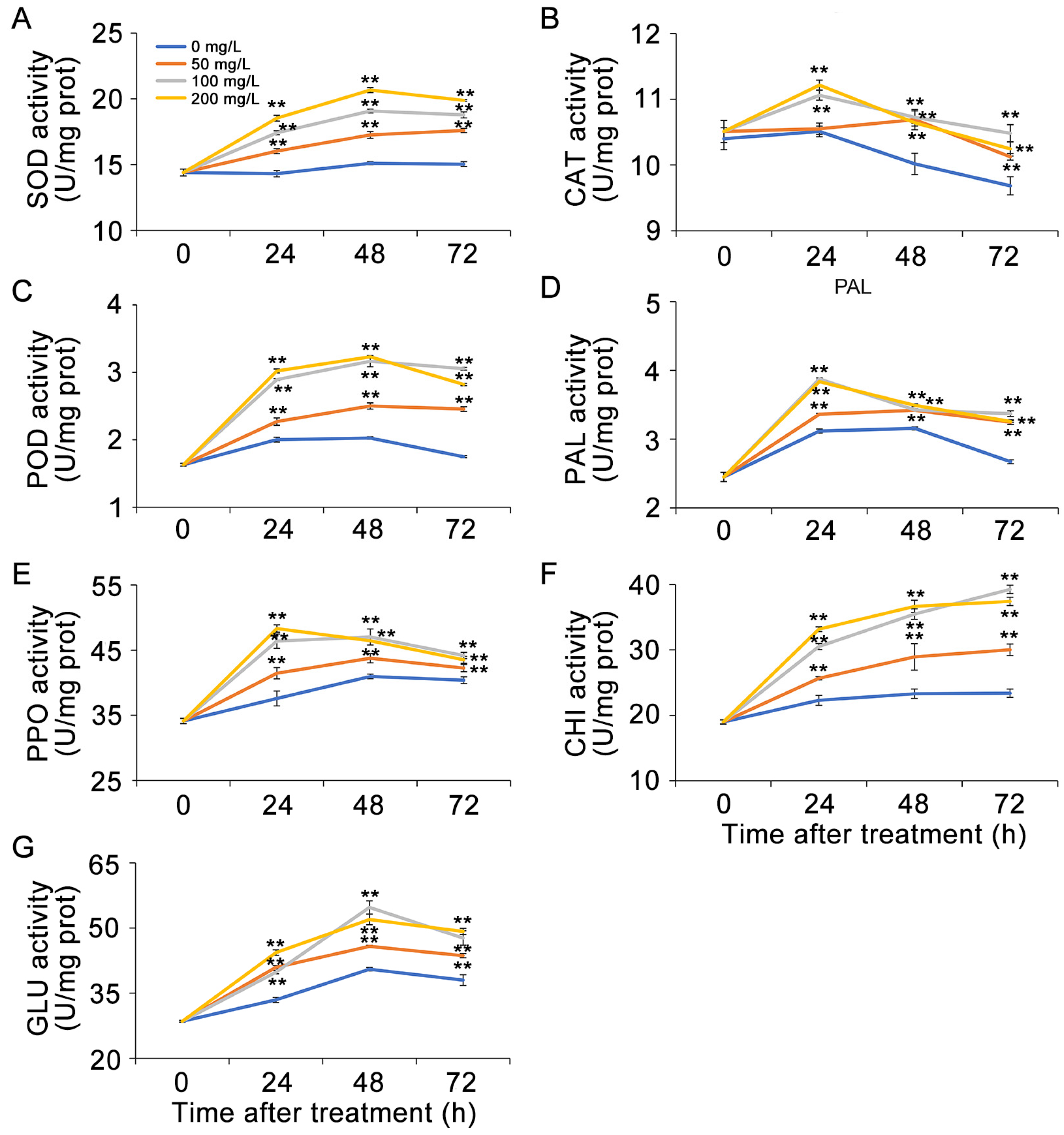
3.7. AMB Treatment Induced the Resistance Response of Table Grapes Against B. cinerea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Jin, Q.; Wang, Z.J.; Tao, X.Y.; Luo, X.D. Quality assurance of postharvest grapes against Botrytis cinerea by terbinafine. Nat. Prod. Bioprospect 2023, 13, 25. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, Y.F.; Wei, H.; Li, X.X.; Zheng, H.T.; Dong, X.Y.; Xu, T.F.; Meng, J.F. Inhibition efficiency of wood vinegar on grey mould of table grapes. Food Biosci. 2020, 38, 100755. [Google Scholar] [CrossRef]
- Tsioka, A.; Psilioti Dourmousi, K.; Poulaki, E.G.; Papoutsis, G.; Tjamos, S.E.; Gkizi, D. Biocontrol strategies against Botrytis cinerea in viticulture: Evaluating the efficacy and mode of action of selected winemaking yeast strains. Lett. Appl. Microbiol. 2024, 77, ovae026. [Google Scholar] [CrossRef]
- Kim, J.D.; Kang, J.E.; Kim, B.S. Postharvest disease control efficacy of the polyene macrolide lucensomycin produced by Streptomyces plumbeus strain CA5 against gray mold on grapes. Postharvest Biol. Technol. 2020, 162, 111115. [Google Scholar] [CrossRef]
- Valverde, J.M.; Guillén, F.; Mareínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Improvement of table grapes quality and safety by the combination of modified atmosphere packaging (MAP) and eugenol, menthol, or thymol. J. Agric. Food Chem. 2005, 53, 7458–7464. [Google Scholar] [CrossRef]
- Dwivedi, M.; Singh, P.; Pandey, A.K. Botrytis fruit rot management: What have we achieved so far? Food Microbiol. 2024, 122, 104564. [Google Scholar] [CrossRef]
- Zhou, F.; Hu, H.Y.; Song, Y.L.; Gao, Y.Q.; Liu, Q.L.; Song, P.W.; Chen, E.Y.; Yu, Y.A.; Li, D.X.; Li, C.W. Biological characteristics and molecular mechanism of fludioxonil resistance in Botrytis cinerea from Henan province of China. Plant Dis. 2020, 104, 1041–1047. [Google Scholar] [CrossRef]
- Zhao, J.J.; Bi, Q.Y.; Wu, J.; Lu, F.; Han, X.Y.; Wang, W.Q. Occurrence and management of fungicide resistance in Botrytis cinerea on tomato from greenhouses in Hebei, China. J. Phytopathol. 2019, 167, 413–421. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Cheng, Q.X.; Yao, F.; Wang, X.Z.; Kong, L.X.; Cao, B.; Xu, M.; Lin, S.J.; Deng, Z.X.; Chooi, Y.H.; et al. Bio-synthesis of the pyrrolidine protein synthesis inhibitor anisomycin involves novel gene ensemble and cryptic biosynthetic steps. Proc. Natl. Acad. Sci. USA 2017, 144, 4135–4140. [Google Scholar] [CrossRef]
- Shen, J.F.; Kong, L.X.; Li, Y.; Zheng, X.Q.; Wang, Q.; Yang, W.N.; Deng, Z.X.; You, D.L. A LuxR family transcriptional regulator AniF promotes the production of anisomycin and its derivatives in Streptomyces hygrospinosus var. beijingensis. Syn. Syst. Biotechnol. 2019, 4, 40–48. [Google Scholar] [CrossRef]
- Zhang, K.P.; Moshsin, A.; Dai, Y.C.; Chen, Z.B.; Zhuang, Y.P.; Chu, J.; Guo, M.J. Combinatorial effect of ARTP mutagenesis and ribosome engineering on an industrial strain of Streptomyces albus S12 for enhanced biosynthesis of salinomycin. Front. Bioeng. Biotechnol. 2019, 7, 212. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Lee, F.W.; Tsai, Y.S.; Kuan, Y.C. Tanespimycin and rapamycin exhibit antifungal activity against Colletotrichum gloeosporioides and enhance mango resistance to anthracnose via differentially modulating heat shock response. Postharvest Biol. Technol. 2023, 204, 112474. [Google Scholar] [CrossRef]
- Saito, S.; Wang, F.; Xiao, C.-L. Natamycin as a postharvest treatment to control gray mold on stored blueberry fruit caused by multi-fungicide resistant Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111862. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.Q.; Li, B.Q.; Xu, Y.; Tian, S.P. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Ma, D.Y.; Ji, D.C.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Xu, Y.; Chen, T.; Tian, S.P. Efficacy of rapamycin in modulating autophagic activity of Botrytis cinerea for controlling gray mold. Postharvest Biol. Technol. 2019, 150, 158–165. [Google Scholar] [CrossRef]
- Byrne, B.; Carmody, M.; Gibson, E.; Rawlings, B.; Caffrey, P. Biosynthesis of deoxyamphotericins and deoxyamphoteronolides by engineered strains of Streptomyces nodosus. Chem. Biol. 2003, 10, 1215–1224. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, X.J.; Wang, Z.J.; Jin, Q.; Wang, X.N.; Chen, S.S.; Luo, X.D. Amphotericin B and 5-flucytosine as fungicides against Penicillium italicum for citrus fruit rot. Postharvest Biol. Technol. 2022, 193, 112058. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Silva, J.A.D.; Santos-Veloso, M.A.O.; Lima, S.G.; Chaves-Markman, A.V.; Juca, M.B. Antifungal efficacy of amphotericin B in Candida albicans endocarditis therapy: Systematic review. Braz. J. Cardiovasc. Surg. 2020, 35, 789–796. [Google Scholar] [CrossRef]
- Johnson, M.D. Antifungals in clinical use and the pipeline. Infect. Dis. Clin. N. Am. 2021, 35, 341–371. [Google Scholar] [CrossRef]
- Gutiérrez, N.U.; Vergara López, M.J.; Bustos, C.A.; Vidal, C.C.; Carvajal, J.A.; Severino, N.; Giordano, A.; Baquedano, S.U.; Feuerhake, T.; Rabagliati, R.; et al. Intra-amniotic Candida albicans infection treated with liposomal amphotericin B with a successful neonatal outcome. Open Forum Infect. Dis. 2024, 11, ofae047. [Google Scholar] [CrossRef]
- Sakai, D.; Imai, H.; Nakamura, M. Multiple intravitreal liposomal amphotericin B for a case of Candida glabrata endophthalmitis. Case Rep. Ophthalmol. 2021, 12, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Shi, G.H.; Dai, Y.; Fang, W.X.; Wu, Q. Identifying genetic variants associated with amphotericin B (AMB) resistance in Aspergillus fumigatus via k-mer-based GWAS. Front. Genet. 2023, 14, 1133593. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.Y.; Sun, H.M.; Yang, J.; Zhao, J.J.; Wang, Y. Inhibitory effects of hinokitiol on the development and pathogenicity of Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 2023, 39, 356. [Google Scholar] [CrossRef]
- Cui, X.M.; Ma, D.Y.; Liu, X.Y.; Zhang, Z.Q.; Li, B.Q.; Xu, Y.; Chen, T.; Tian, S.P. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Yuan, S.Z.; Wang, B.G.; Wang, M.; Sun, M.M.; Wang, X.Q.; Li, X.F.; Yang, N.; Xu, X.D.; Zheng, S.F.; Wang, Q. Antifungal mechanism of protocatechuic acid methyl ester against Botrytis cinerea in postharvest strawberry fruit. Postharvest Biol. Technol. 2024, 211, 112787. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ji, D.C.; Cui, X.M.; Zhang, Z.Q.; Li, B.Q.; Xu, Y.; Chen, T.; Tian, S.P. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Liu, T.; Xu, Y.; Chen, Y.; Chen, T.; Li, B.Q.; Tian, S.P. Sodium pyrosulfite inhibits the pathogenicity of Botrytis cinerea by interfering with antioxidant system and sulfur metabolism pathway. Postharvest Biol Technol. 2022, 189, 111936. [Google Scholar] [CrossRef]
- Shi, J.X.; Huang, D.D.; Du, Y.J.; Zhu, S.H.; Hussain, Z.; Haider, M.S.; Anwar, R. Effects of exogenous nitric oxide treatment on grape berries against Botrytis cinerea and Alternaria alternata related enzymes and metabolites. Plant Dis. 2023, 107, 1510–1521. [Google Scholar] [CrossRef]
- Li, Z.Z.; Zhang, S.J.; Xue, J.X.; Mu, B.Y.; Song, H.; Liu, Y.P. Exogenous melatonin treatment induces disease resistance against Botrytis cinerea on post-harvest grapes by activating defence responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Jourdes, M.; Pasquier, G.; Fermaud, M.; Gény, L.; Rey, P.; Doneche, B.; Teissedre, P.L. Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Aust. J. Grape Wine R. 2012, 18, 215–226. [Google Scholar] [CrossRef]
- Qi, L.; Du, H.F.; Sun, T.T.; Li, L.; Zhang, Y.H.; Liu, Y.F.; Cao, F. Natural products from marine fungi as a source against agricultural pathogenic fungi. Appl. Microbiol. Biotechnol. 2023, 107, 5003–5017. [Google Scholar] [CrossRef]
- Al Jalali, V.; Sauermann, R.; Eberl, S.; Zeitlinger, M. In vitro activity of voriconazole and amphotericin B against Candida albicans, Candida krusei, and Cryptococcus neoformans in human cerebrospinal fluid. Infection 2019, 47, 565–570. [Google Scholar] [CrossRef]
- Bochennek, K.; Tramsen, L.; Schedler, N.; Becker, M.; Klingebiel, T.; Groll, A.H.; Lehrnbecher, T. Liposomal amphotericin B twice weekly as antifungal prophylaxis in paediatric haematological malignancy patients. Clin. Microbiol. Infect. 2011, 17, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, B.; Pakkish, Z.; Najafzadeh, R. Shelf life improvement of grape (Vitis vinifera L. cv. Rish Baba) using nitric oxide (NO) during chilling damage. Int. J. Food Prop. 2018, 20, S2750–S2763. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.Q.; Yao, H.Y.; Deng, S.W.; Gao, T.; Shang, L.; Chen, X.C.; Cui, X.J.; Zeng, J. Peroxisomal b-oxidation stimulates cholesterol biosynthesis in the liver in diabetic mice. J. Biol. Chem. 2022, 298, 101572. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.X.; Guo, J.; Hao, Z.N.; Zhang, Z.; Lu, Z.Q.; Wang, J.Y.; Zhu, X.M.; Wang, Y.L.; Chen, J.; et al. The peroxins BcPex8, BcPex10, and BcPex12 are required for the development and pathogenicity of Botrytis cinerea. Front. Microbiol. 2022, 13, 962500. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, L.; Chai, R.Y.; Qiu, H.P.; Zhang, Z.; Wang, Y.L.; Liu, X.H.; Lin, F.C.; Sun, G.C. Pex13 and Pex14, the key components of the peroxisomal docking complex, are required for peroxisome formation, host infection and pathogenicity-related morphogenesis in Magnaporthe oryzae. Virulence 2019, 10, 292–314. [Google Scholar] [CrossRef]
- Min, K.; Son, H.; Lee, J.; Choi, G.J.; Kim, J.C.; and Lee, Y.W. Peroxisome function is required for virulence and survival of Fusarium graminearum. Mol. Plant Microbe Interact. 2012, 25, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Takano, Y.; Furusawa, I.; and Okuno, T. Peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium. Plant Cell 2001, 13, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhao, L.; Wang, K.; Zhang, H. Transcriptome analysis provides insights into potential mechanisms of epsilon-poly-L-lysine inhibiting Penicillium expansum invading apples. Postharvest Biol. Technol. 2024, 207, 112622. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Wang, Y.; Xu, Y.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Application and mechanism of benzyl-isothiocyanate, a natural antimicrobial agent from cruciferous vegetables, in controlling postharvest decay of strawberry. Postharvest Biol. Technol. 2021, 180, 111604. [Google Scholar] [CrossRef]
- Li, H.; Tian, S.P.; Qin, G.Z. NADPH oxidase Is crucial for the cellular redox homeostasis in fungal pathogen Botrytis cinerea. Mol. Plant Microbe Interact. 2019, 32, 1508–1516. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- VanEtten, H.; Temporini, E.; Wasmann, C. Phytoalexin (and phytoanticipin) tolerance as a virulence trait: Why is it not required by all pathogens? Physiol. Mol. Plant Pathol. 2001, 59, 83–93. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Aditya Srivastava, D.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS transporter from Botrytis cinerea provides tolerance to glucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef]
- Stefanato, F.L.; Abou-Mansour, E.; Buchala, A.; Kretschmer, M.; Mosbach, A.; Hahn, M.; Bochet, C.G.; Metraux, J.P.; Schoonbeek, H.J. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J. 2009, 58, 499–510. [Google Scholar] [CrossRef]
- Fan, F.; Zhu, Y.X.; Wu, M.Y.; Yin, W.X.; Li, G.Q.; Hahn, M.; Hamada, M.S.; Luo, C.X. Mitochondrial inner membrane ABC transporter Bcmdl1 is involved in conidial germination, virulence, and resistance to anilinopyrimidine fungicides in Botrytis cinerea. Microbiol. Spectr. 2023, 11, e0010823. [Google Scholar] [CrossRef]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. Penicillium digitatum MFS transporters can display different roles during pathogen-fruit interaction. Int. J. Food Microbiol. 2021, 337, 108918. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Yang, L.F.; Liu, X.L.; Lu, H.Y.; Zhang, C.Z.; Chen, J.; Shi, Z.Q. Cinnamaldehyde inhibits postharvest gray mold on pepper fruits via inhibiting fungal growth and triggering fruit defense. Foods 2023, 12, 3485. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, X.M.; Sun, M.M.; Wang, L.; Zou, Y.Y.; Fu, L.; Han, C.Y.; Li, A.Q.; Li, L.M.; Zhu, C.Y. Impact of vanillin on postharvest disease control of apple. Front. Microbiol. 2022, 13, 979737. [Google Scholar] [CrossRef]
- Vitti, A.; Coviello, L.; Triunfo, M.; Guarnieri, A.; Scieuzo, C.; Salvia, R.; Falabella, P.; Nuzzaci, M. In vitro antifungal activity and in vivo edible coating efficacy of insect-derived chitosan against Botrytis cinerea in strawberry. Int. J. Biol. Macromol. 2024, 279, 135158. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Meng, Z.J.; Li, T.Y.; Zhang, J.J.; Zhang, N.; Luo, G.; Wang, Z.H.; Zhou, Y. Preparation and characterization of a novel green cinnamon essential oil nanoemulsion for the enhancement of safety and shelf-life of strawberries. Int. J. Food Microbiol. 2025, 427, 110935. [Google Scholar] [CrossRef]
- Singh, B.K.; Tiwari, S.; Dubey, N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: A review. J. Sci. Food Agric. 2021, 101, 4879–4890. [Google Scholar] [CrossRef]
- Kalagatur, N.; Nirmal Ghosh, O.S.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, J.; Wang, S.; Ke, Y.; Ren, T.; Wang, Y. Mechanism Analysis of Amphotericin B Controlling Postharvest Gray Mold in Table Grapes. Foods 2025, 14, 1260. https://doi.org/10.3390/foods14071260
Wu Y, Wang J, Wang S, Ke Y, Ren T, Wang Y. Mechanism Analysis of Amphotericin B Controlling Postharvest Gray Mold in Table Grapes. Foods. 2025; 14(7):1260. https://doi.org/10.3390/foods14071260
Chicago/Turabian StyleWu, Yingying, Jingyi Wang, Shenli Wang, Yijie Ke, Tianyi Ren, and Ying Wang. 2025. "Mechanism Analysis of Amphotericin B Controlling Postharvest Gray Mold in Table Grapes" Foods 14, no. 7: 1260. https://doi.org/10.3390/foods14071260
APA StyleWu, Y., Wang, J., Wang, S., Ke, Y., Ren, T., & Wang, Y. (2025). Mechanism Analysis of Amphotericin B Controlling Postharvest Gray Mold in Table Grapes. Foods, 14(7), 1260. https://doi.org/10.3390/foods14071260





