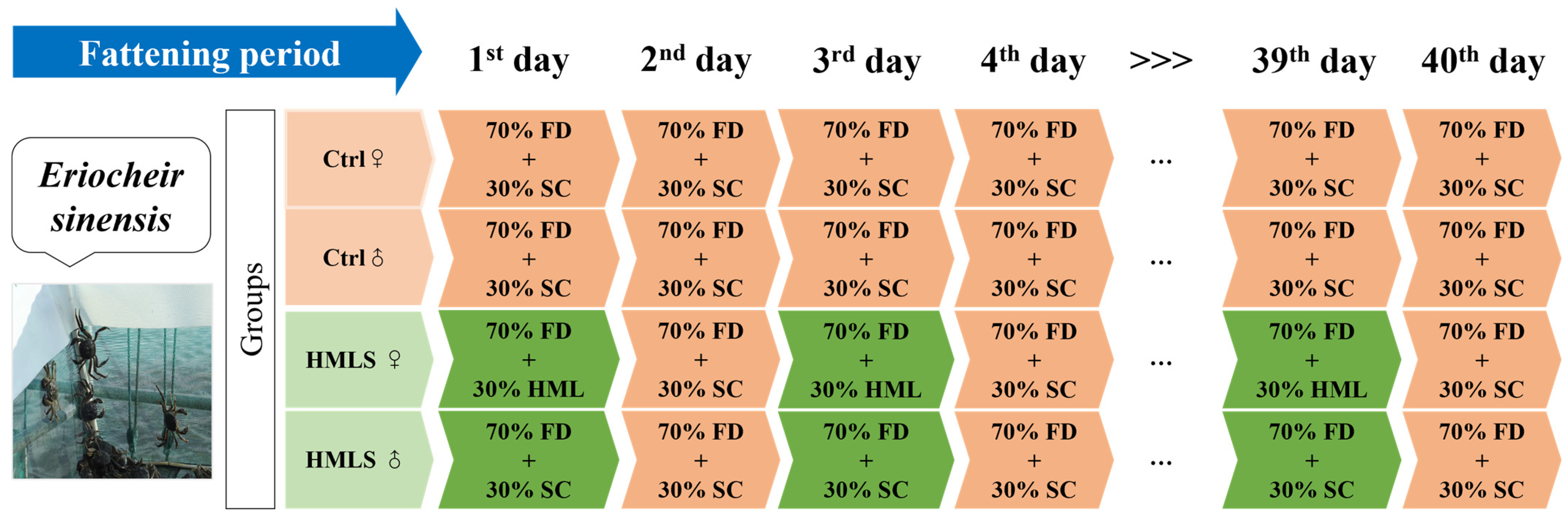
1. Introduction
The Chinese mitten crab (
Eriocheir sinensis) is an important aquaculture species and highly prized by consumers in China due to its high nutritional value and unique flavor [
1,
2]. The total production of
E. sinensis in China ranks first globally, with an annual yield nearing 1 million tons [
3]. Prior to market harvest in September, the fattening of adult
E. sinensis is typically required, as it directly enhances the overall quality and yield of crabs, thus ensuring better market prices [
4,
5]. The duration of the fattening period and efficient dietary feed are crucial factors influencing both nutrition deposition and gonad development in crabs [
5,
6]. Previous research suggests that the optimal fattening period for pond-reared
E. sinensis ranges from 40 to 60 days [
4,
6]. Common dietary options for fattening include iced trash fish (IF), soybean, cornmeal, and formulated diets (FDs) [
5]. IF, once considered the “standard food” for crab fattening, has been associated with several issues, including source instability, unpredictable quality, nutritional imbalances, and water pollution [
7]. As a result, numerous studies have focused on developing high-quality FDs or alternative protein sources to enhance fattening practices [
5,
8].
The crude protein level in most insects ranges from 40% to 63%, which have been recognized as stable sources of dietary proteins for aquaculture [
8,
9,
10,
11]. Housefly maggots (
Musca domestica; HM) are promising and highly nutritious resources, offering significant potential as a protein source for crabs [
11,
12,
13]. The crude protein content of HM ranges from 43% to 62% [
14], and HM are rich in monounsaturated fatty acids, B-complex vitamins, phosphorus, and trace elements [
13,
15]. Previous studies have demonstrated that insect meal can effectively replace fish meal in aquaculture diets without adversely affecting the growth of various fish species, including barramundi (
Lates calcarifera), gilthead seabream (
Sparus aurata), and European sea bass (
Dicentrarchus labrax L.) [
16,
17,
18]. Moreover, incorporating HM larvae (HML) in fish diets has the potential to improve growth rates and feed efficiency without inducing physiological stress [
11,
19]. Dietary HML supplementation has been shown to improve the hardness and moisture in fish muscles, enhancing the flesh quality of Nile tilapia (
Oreochromis niloticus) [
20]. Additionally, HML-supplemented diets promote the growth of swamp eel (
Monopterus albus) and support gonadal development in
Clarias gariepinus (Burchell, 1822) [
21,
22]. In crustaceans, replacing fish meal with defatted insect meal, such as yellow mealworm (
Tenebrio molitor), significantly increased the growth and survival rates of juvenile Pacific white shrimp (
Litopenaeus vannanmei) [
23], while dietary supplementation with black soldier fly (
Hermetia illucens) enhanced the immunity, gut microbiota, and protein content in freshwater crayfish marron (
Cherax cainii) [
24]. Furthermore, the exoskeleton of insects contains chitin, a structural polysaccharide that may contribute to the formation of the exoskeletons in crustaceans [
25]. However, studies on the effects of HML on the growth and health of crustaceans remain limited.
Although preliminary investigations have revealed the regulatory effects of HML on growth and nutrient metabolism in decapod crustaceans [
12,
19], their efficacy in enhancing nutritional quality remains insufficiently explored. Therefore, this study aimed to systematically evaluate the impacts of HML supplementation on the growth indices and nutrient composition of edible tissues in
E. sinensis during the fattening period. Specifically, we assessed various growth parameters, the antioxidant status, amino acid (AA) and nucleotide composition, texture profiles, and flavor characteristics of different edible tissues. The findings will validate its application potential as a functional feed additive and establish a theoretical foundation for precision nutrient regulation in crustacean aquaculture.
4. Discussion
Currently, the demand for high-quality
E. sinensis is increasing, and a key factor in improving quality is optimizing the fattening process [
9]. Diet composition and water environmental conditions have been identified as contributing factors to the final fattening outcomes in various studies [
33]. The total edible parts of
E. sinensis include meat, the hepatopancreas, and gonads [
34]. A previous study reported that black soldier fly larvae can enhance the flavor profile of edible tissues in
E. sinensis when used to replace traditional iced trash fish in the diet [
8]. This suggests that insect-based diets may offer a viable approach to improving the nutritional value of edible parts. The use of HML has gained popularity in fish aquaculture in recent years [
14,
21]; however, limited research has focused on the effects of dietary HML on
E. sinensis culture. In this study, we investigated the effects of a diet supplemented with HML on growth performance, antioxidant activity, and nutritional quality in adult
E. sinensis following 40 days of fattening.
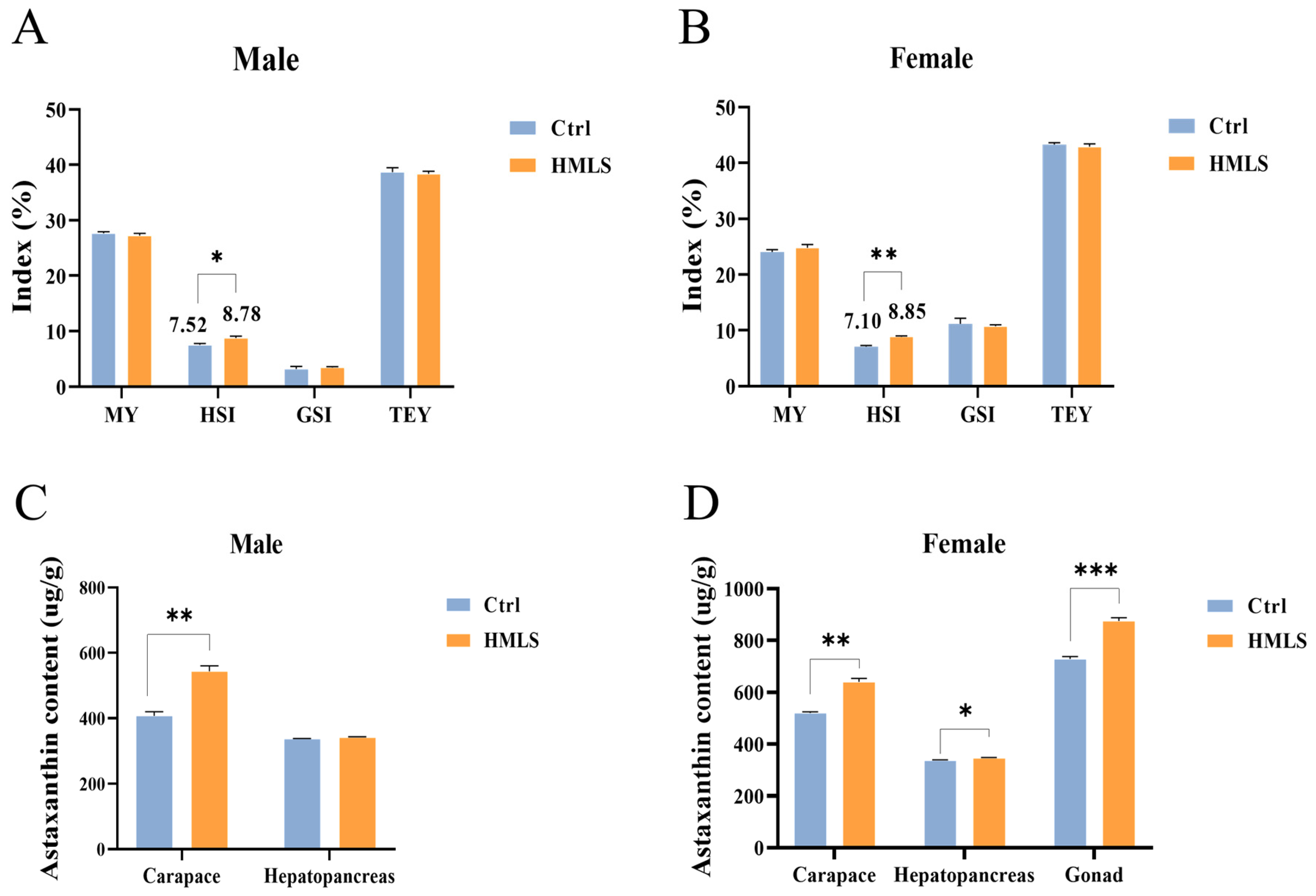
Previous studies have reported on the yield of edible tissues in
E. sinensis during the fattening period [
2,
6,
35]. In this study, the TEY following dietary HML supplementation was composed of MY (male: 27.23%; female: 24.81%), HSI (male: 8.78%; female: 8.85%), and GSI (male: 3.44%; female: 10.77%), which was consistent with previous findings. Efficient nutrient feeding is crucial for achieving optimal fattening effects in
E. sinensis. The existing research has shown no significant differences in the HSI and GSI levels of
E. sinensis fed FDs versus IF [
34]. In comparison with formulated diets, dietary supplementation with astaxanthin or
Haematococcus pluvialis had no significant effects on the HSI and GSI [
35,
36]. Additionally, it was also found that dietary supplementation with 0.4% cholesterol significantly upregulated both the HSI and GSI levels but had no significant effects on the MY in
E. sinensis [
37,
38]. Furthermore, supplementation with 0.33% docosahexaenoic acid (DHA) oil significantly increased the GSI in
E. sinensis [
39]. Our results suggest that HML supplementation in formulated diets effectively improved the HSI of
E. sinensis. Similar to our results, higher HSI values were observed in
O. niloticus fed with diets containing HM [
40]. Studies have shown that HML extract reduces lipid accumulation in the hepatopancreas of rats by modulating the peroxisome proliferator-activated receptor gamma (PPARγ) [
41]; however, the specific effect of HML on higher HSI values of
E. sinensis warrants further investigation.
Astaxanthin, a carotenoid compound, offers various health benefits for crustaceans [
9,
42]. Currently, astaxanthin is widely used in
E. sinensis culture to enhance immune function, antioxidant capacity, and coloration [
43,
44]. Our findings demonstrated that HML feeding significantly increased the astaxanthin content in the carapaces of both male and female
E. sinensis, as well as in the female hepatopancreas and gonad. Previous studies have shown that diets incorporating insect meals (such as cricket, grasshopper, and mealworm) can elevate carotenoid levels in
Xiphophorus maculatus (Gunther, 1866) [
45]. However, there is limited research on the effects of dietary HML on the astaxanthin content in
E. sinensis, and the underlying mechanisms warrant further investigation.
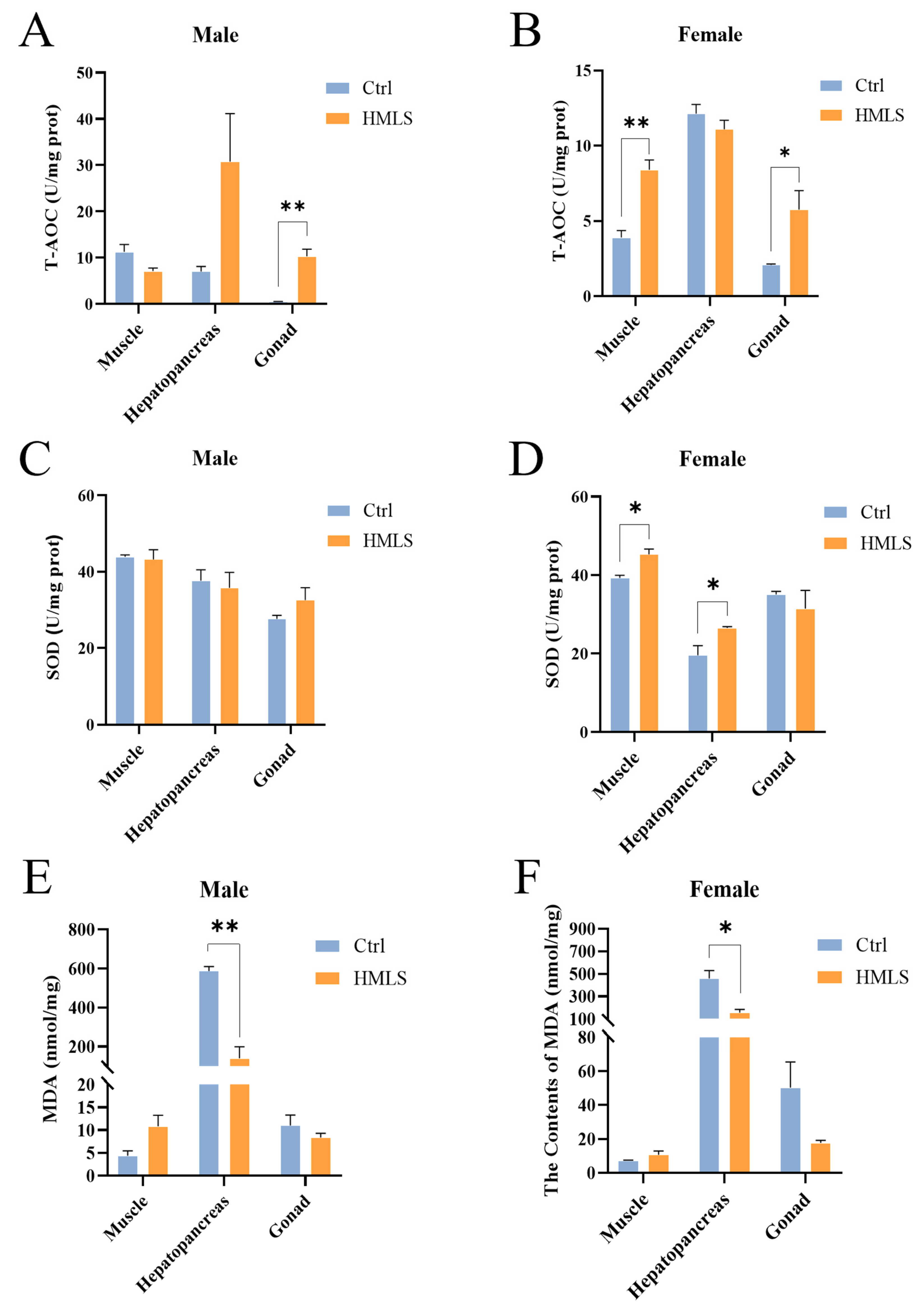
It has been demonstrated that HML exhibit significant antioxidant potential when incorporated into the diets of hybrid catfish (
Clarias gariepinus ♀ x
Heterobranchus longifilis ♂) and (
Lithobates catesbeiana) [
14,
46]. In contrast, the antioxidant capacity of prawn
Palaemon adspersus was unaffected by the inclusion of HML in the diets (Rathke, 1837) [
47]. HM have shown considerable promise in enhancing the antioxidant capacity of crustaceans by providing essential nutrients such as AAs, microelements, and chitin [
11,
12,
13]. However, the effects of dietary HML on the antioxidant capacity of different edible tissues in
E. sinensis remain underexplored. In the present study, we found that dietary HML enhanced the antioxidant capacity of the hepatopancreas and gonad in both male and female
E. sinensis, as well as the female muscle.
Previous studies have demonstrated that a dietary insect meal has the potential to affect textural characteristics by altering the muscle fiber diameter and density [
48]. Research has indicated that the functional components of black soldier fly (
Hermetia illucens) larvae can improve the chewiness, cohesiveness, gumminess, and hardness of barramundi (
Lates calcarifer) muscle [
49]. Additionally, the inclusion of yellow mealworm (
Tenebrio molitor) in the diet has been shown to affect muscle fiber density, which is positively correlated with muscle hardness, adhesiveness, springiness, chewiness, and gumminess [
48]. Here, we observed that HML feeding enhanced the adhesiveness in the male muscle, while improving the cohesiveness, chewiness, and resilience in the female muscle. These changes can be attributed to alterations in muscle cellularity. However, the precise mechanisms by which HML-supplemented formula diets regulate muscle structure warrant further investigation.
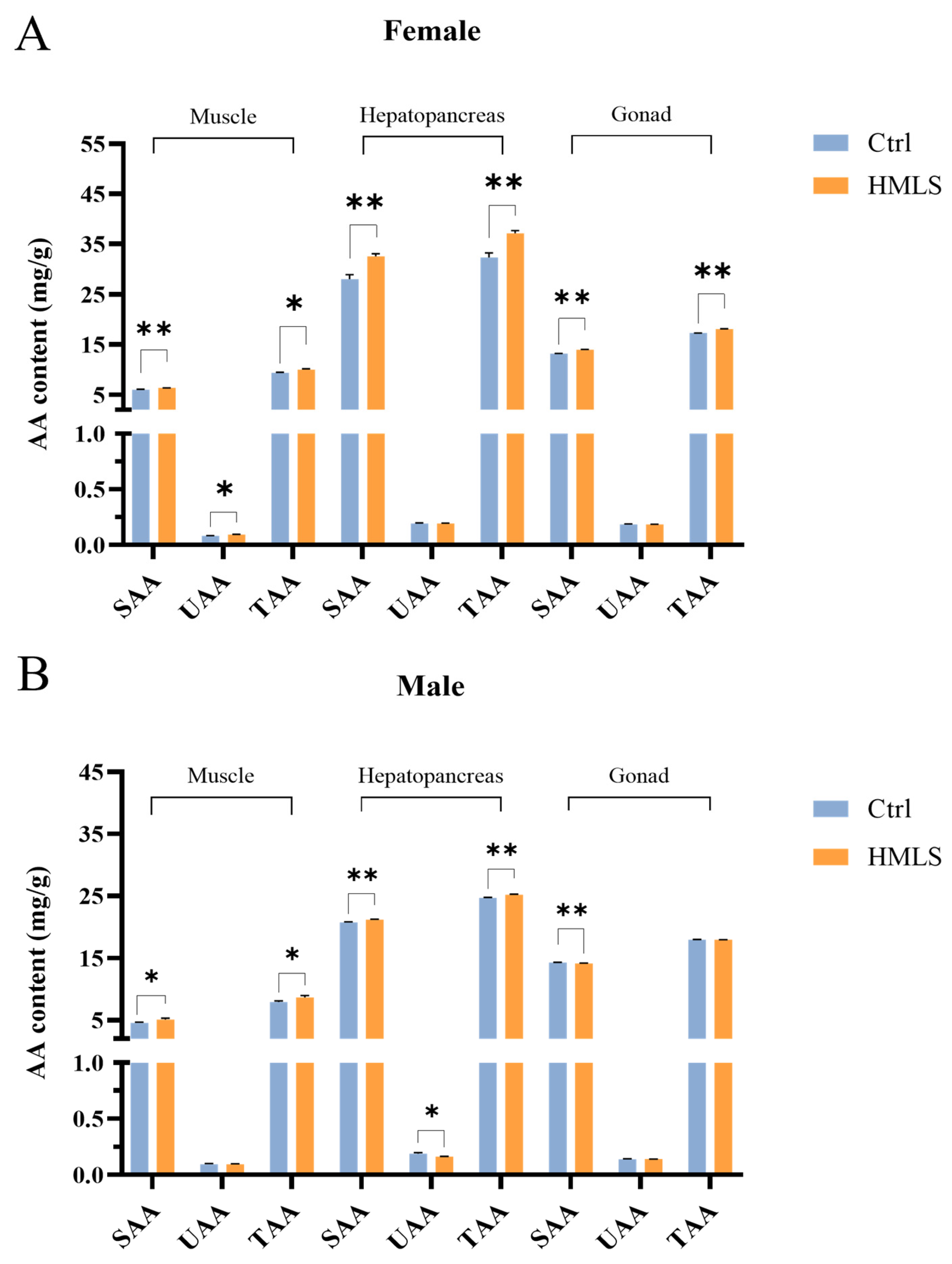
Proteins are crucial energy sources for crustaceans, and AA composition serves as an important indicator for evaluating the nutritional value of
E. sinensis [
10]. HML are rich in AAs and are considered a more environmentally sustainable alternative to traditional protein sources such as soybean meal or fishmeal [
17]. In comparison to the AA composition of soybean meal and fishmeal [
14], HML in this study are particularly abundant in Leu, His, Ala, Phe, Asp, Tyr, and Cys. To date, there are limited studies exploring the effects of dietary HML on the AA composition of
E. sinensis. However, research on other insect meals has reported that
H. illucens is an efficient source of animal protein for crustaceans [
50,
51]. The substitution of IF by
H. illucens has been shown to significantly increase the TAA content in the gonad and the DAA (SAAs and UAAs) content in the muscle and gonad of male
E. sinensis, while significantly decreasing the TAA and DAA content in the hepatopancreas of female
E. sinensis [
8]. In contrast, our study found that dietary HML significantly increased the TAA and SAA content in the edible tissues of female
E. sinensis and in the muscle and hepatopancreas of male
E. sinensis but had no significant effects on the TAA content in the gonad of male
E. sinensis. Furthermore, varying levels of dietary defatted superworm (
Zophobas atratus) or
H. illucens larvae as alternative protein sources did not affect the TAA content in juvenile Pacific white shrimp (
Penaeus vannamei) [
52,
53]. Therefore, the effects of dietary HML on the FAA content in
E. sinensis may be gender- and tissue-specific.
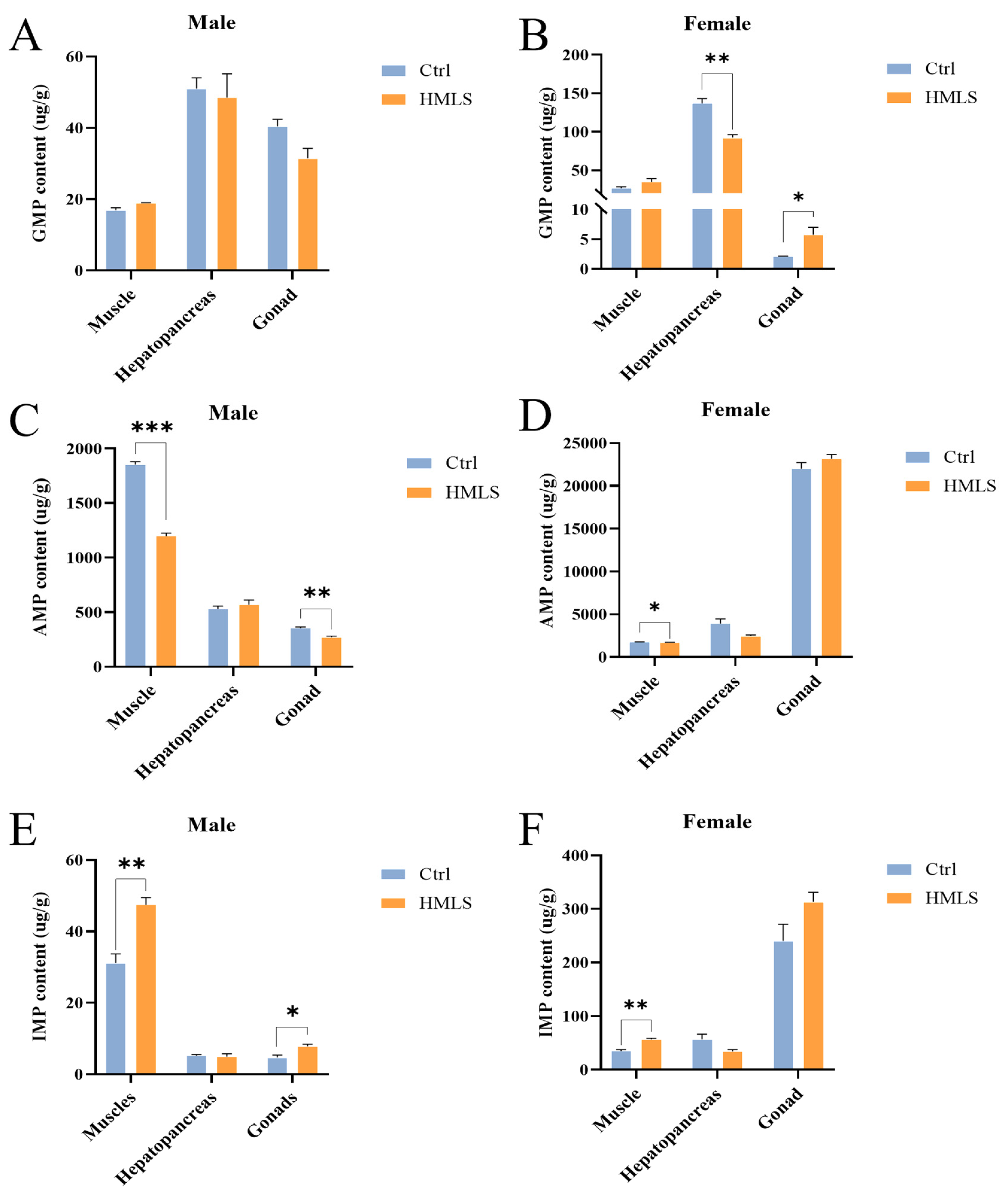
IMP serves as a taste enhancer in food, boosting sweetness while masking fishy, sour, bitter, and salty flavors [
54]. A previous study demonstrated that dietary
H. illucens significantly increases the IMP content in the muscle of both sexes and in the gonad of female
E. sinensis [
8]. Consistent with this finding, our study showed that dietary HML significantly increased the IMP content in the muscle of both sexes and in the gonads of male
E. sinensis. However, dietary HML significantly decreased the AMP content in the muscle of both sexes and in the gonad of the male, as well as the GMP content in the hepatopancreas of the female. A previous study indicates that IMP and GMP are umami-tasting compounds commonly used as flavor enhancers, while AMP can be converted into IMP by the action of AMP deaminase [
55]. Therefore, our results suggest that dietary HML in
E. sinensis promote the conversion of AMP to IMP in the muscle of both sexes and the gonad of the male. Furthermore, proteins in the feed affect the composition of AAs and nucleotides in
E. sinensis, which contribute to both the nutritive value and unique flavor [
3,
56,
57]. Previous studies have highlighted that TAV serves as an indicator for evaluating the taste impacts of AAs and nucleotides on the flavor of
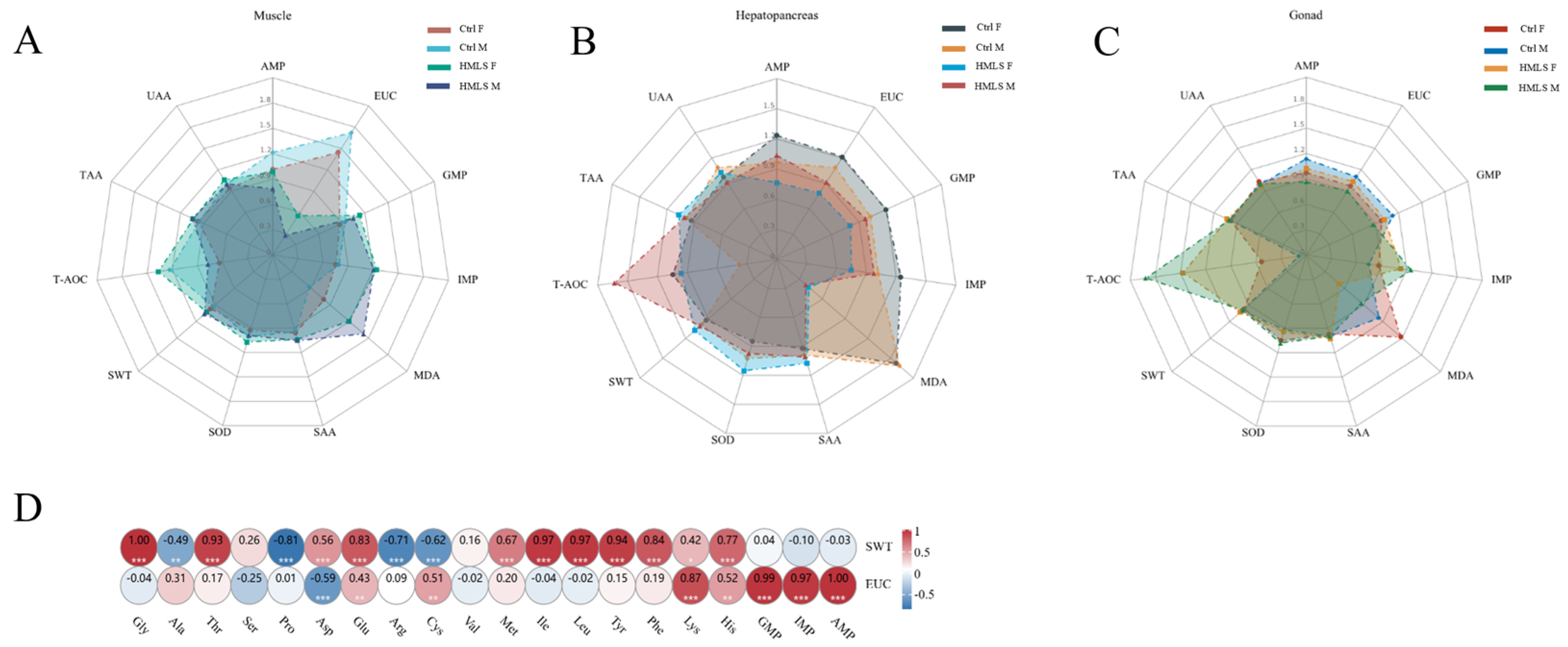
E. sinensis [
31,
58]. The umami taste of IMP and GMP is considered stronger than that of glutamic acid (Glu) [
54]. In the present study, the TAVs of UAAs, such as Glu and Asp, in the edible tissues of both the Ctrl and HMLS groups were below 1.0, with AMP primarily contributing to the umami taste in the female gonads (TAV greater than 1.0). Contrary to the results observed in
E. sinensis fed
H. illucens [
8], IMP may be the primary contributor to the umami flavor in
E. sinensis. Moreover, dietary HML for
E. sinensis significantly decreased the EUC in the muscle and hepatopancreas of both sexes, as well as in the gonad of the male.
Previous studies have shown that certain SAAs, consisting of arginine (Arg), Ala, glycine (Gly), proline (Pro), and threonine (Thr), can have effects on the sweetness characteristics of edible tissues in
E. sinensis [
8]. However, limited research has focused on the impact of dietary HML on the sweetness or bitterness of edible tissues in aquatic species. Gly, for example, is known to provide a fragrant sweetness, reduce bitterness, and eliminate off-flavors in shrimp and crab [
54]. In our study, the TAV of Gly was found to exceed 1.0 in all edible tissues. After HML feeding, the SWT of the muscle, hepatopancreas, and female gonad increased significantly, while the SWT in the male gonad decreased significantly. Similar changes in the SWT were observed in the muscle and male gonad of
E. sinensis after feeding with
H. illucens [
8]. Additionally, valine (Val) is typically considered a bitter amino acid, characterized by weak bitterness and a subtle sweet taste [
59]. A recent study suggested that a reduction in L-valine content in the muscle of
Megalobrama amblycephala was significantly associated with increased sweetness [
60]. However, in this study, the TAV of Val was greater than 1.0 across all edible tissues and showed a significant increase after HML feeding. Correlation analysis revealed no significant association between the Val content and either the EUC or SWT, suggesting that the increased Val content following HML feeding did not contribute to the sweetness in the edible tissues of
E. sinensis.