Integrated Metabolomic and Transcriptomic Analysis Reveals the Molecular Regulatory Mechanism of Gamma-Aminobutyric Acid Accumulation in White Quinoa (Chenopodium quinoa Willd.) in Response to Dark and Ultrasound Stress
Abstract
1. Introduction
2. Materials and Methods
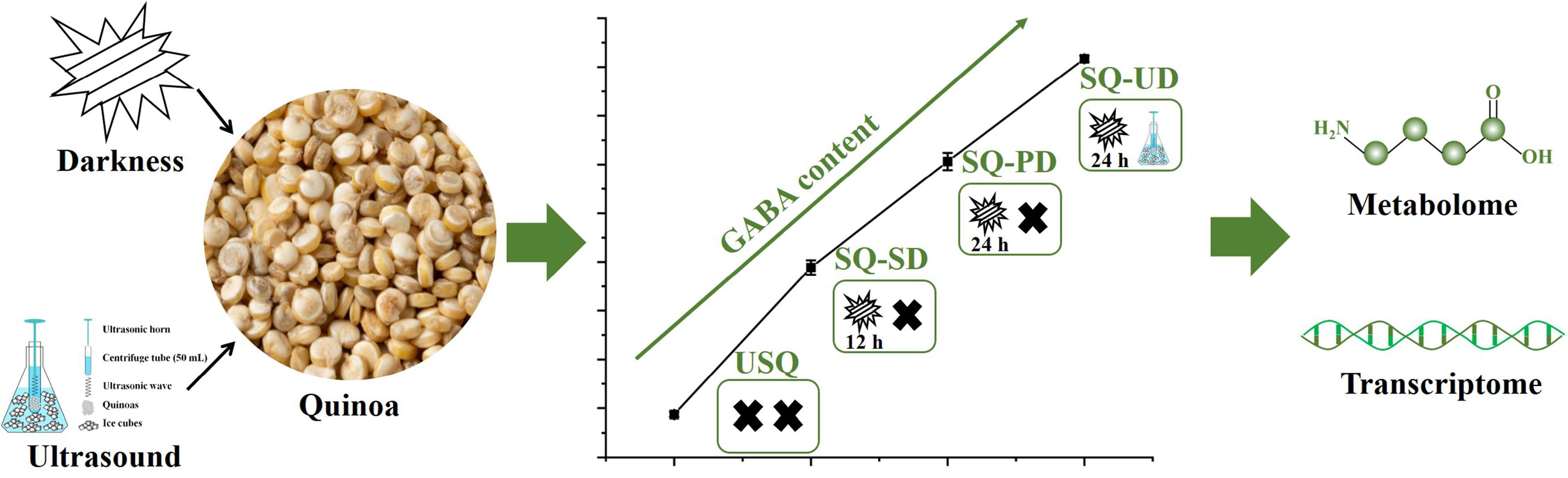
2.1. Quinoa Cultivation and Ultrasonic Treatment
2.2. Determination of GABA Content
2.3. Widely Targeted Metabolomics Analysis
2.3.1. Metabolite Extraction
2.3.2. UPLC and Electrospray Ionization (ESI)–Triple Quadrupole-Linear Ion Trap (Q TRAP)–MS/MS
2.3.3. Metabolomics Analysis
2.4. RNA Sequencing (RNA-Seq)
2.5. Differentially Expressed Genes (DEGs) and Gene Function
2.6. Quantitative Real-Time PCR (qPCR) Validation
2.7. Determination of Free Amino Acid and Nitrogen Content
2.8. Statistical Analysis
3. Results
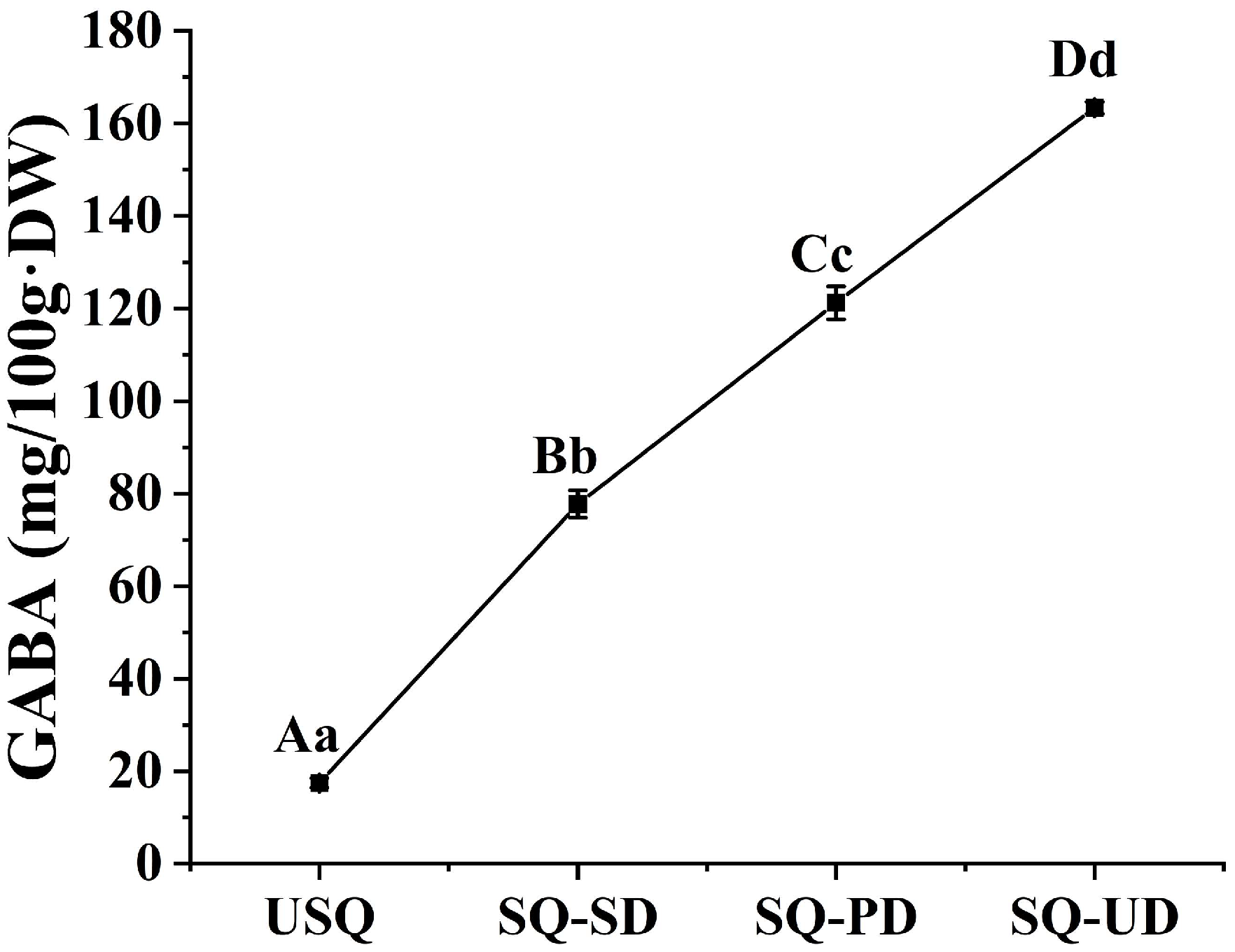
3.1. Enrichment of GABA in Quinoa Under Dark and Ultrasound Stress
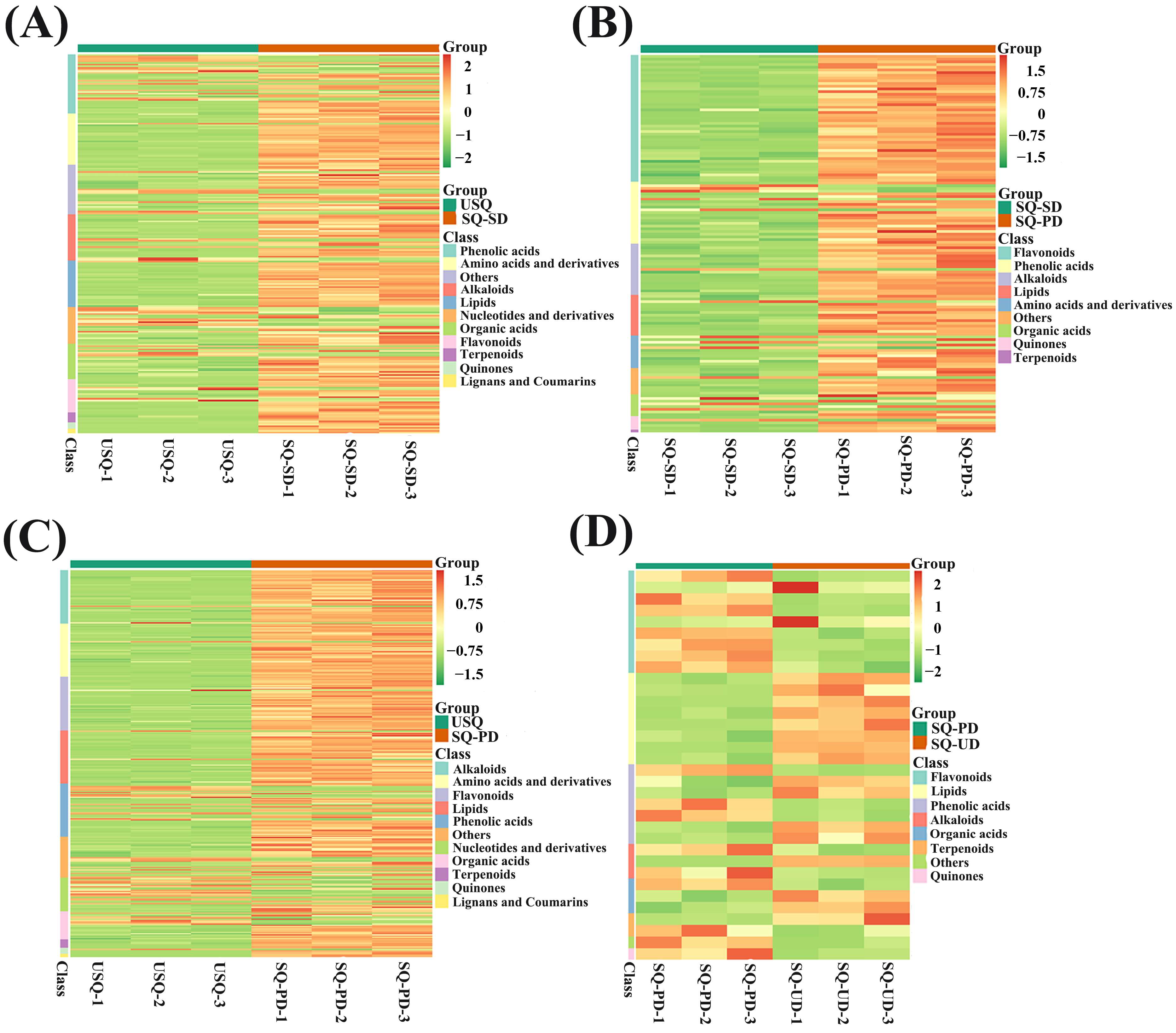
3.2. Metabolomic Analysis of Various Metabolites During GABA Enrichment in Quinoa
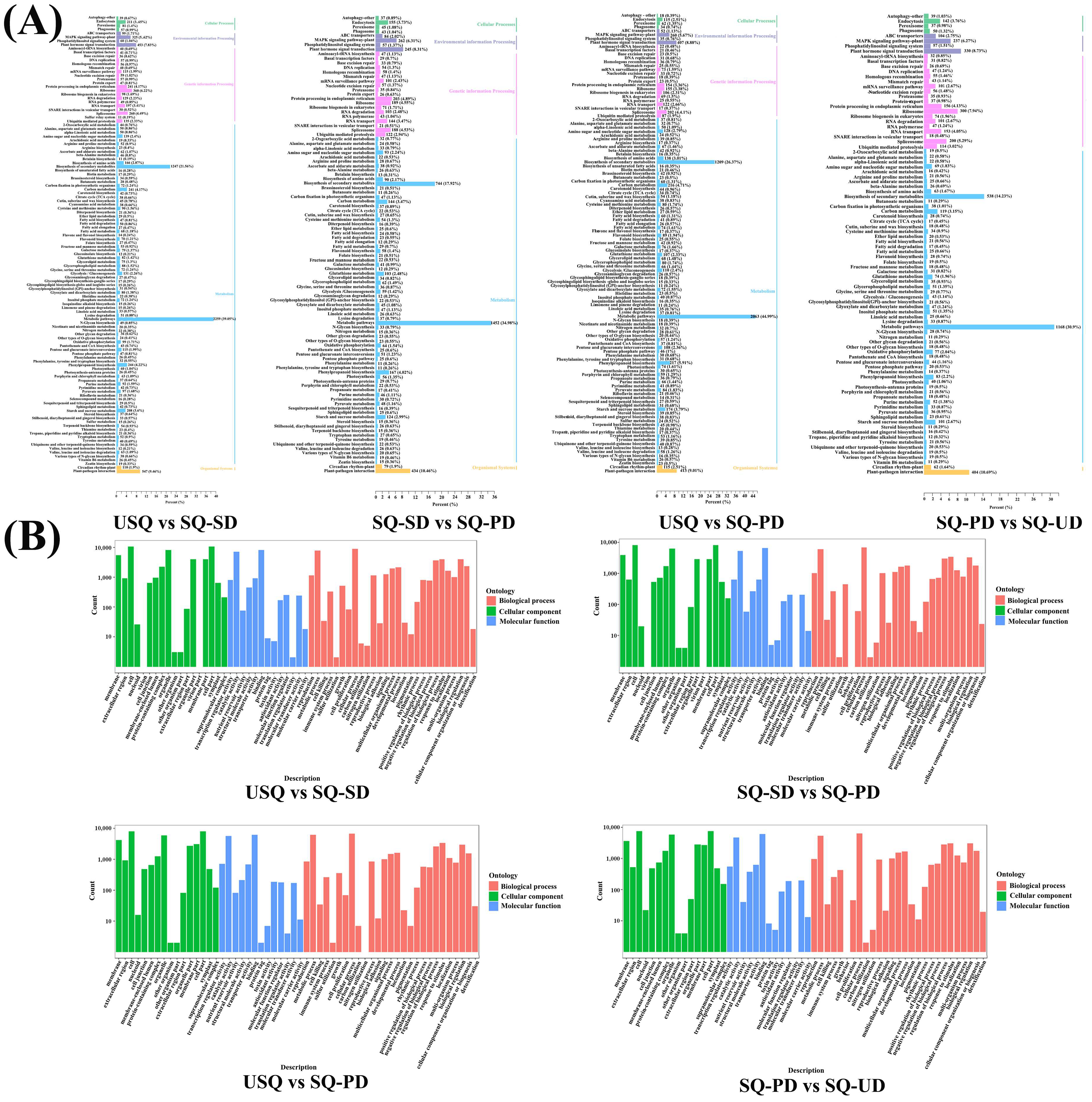
3.3. Transcriptomic Analysis of Various Genes During GABA Enrichment in Quinoa
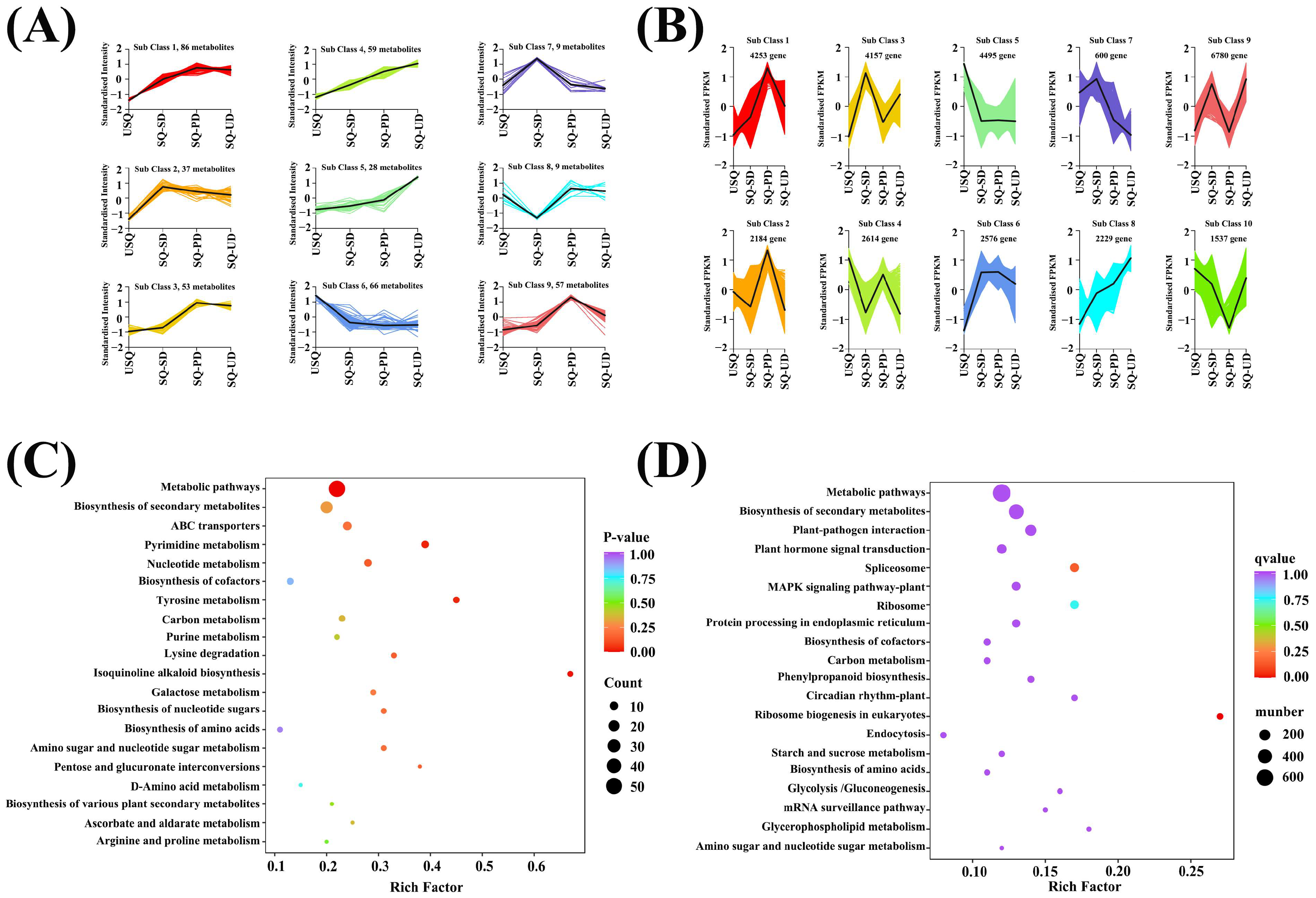
3.4. Cluster Analysis of Metabolome and Transcriptome During GABA Enrichment in Quinoa
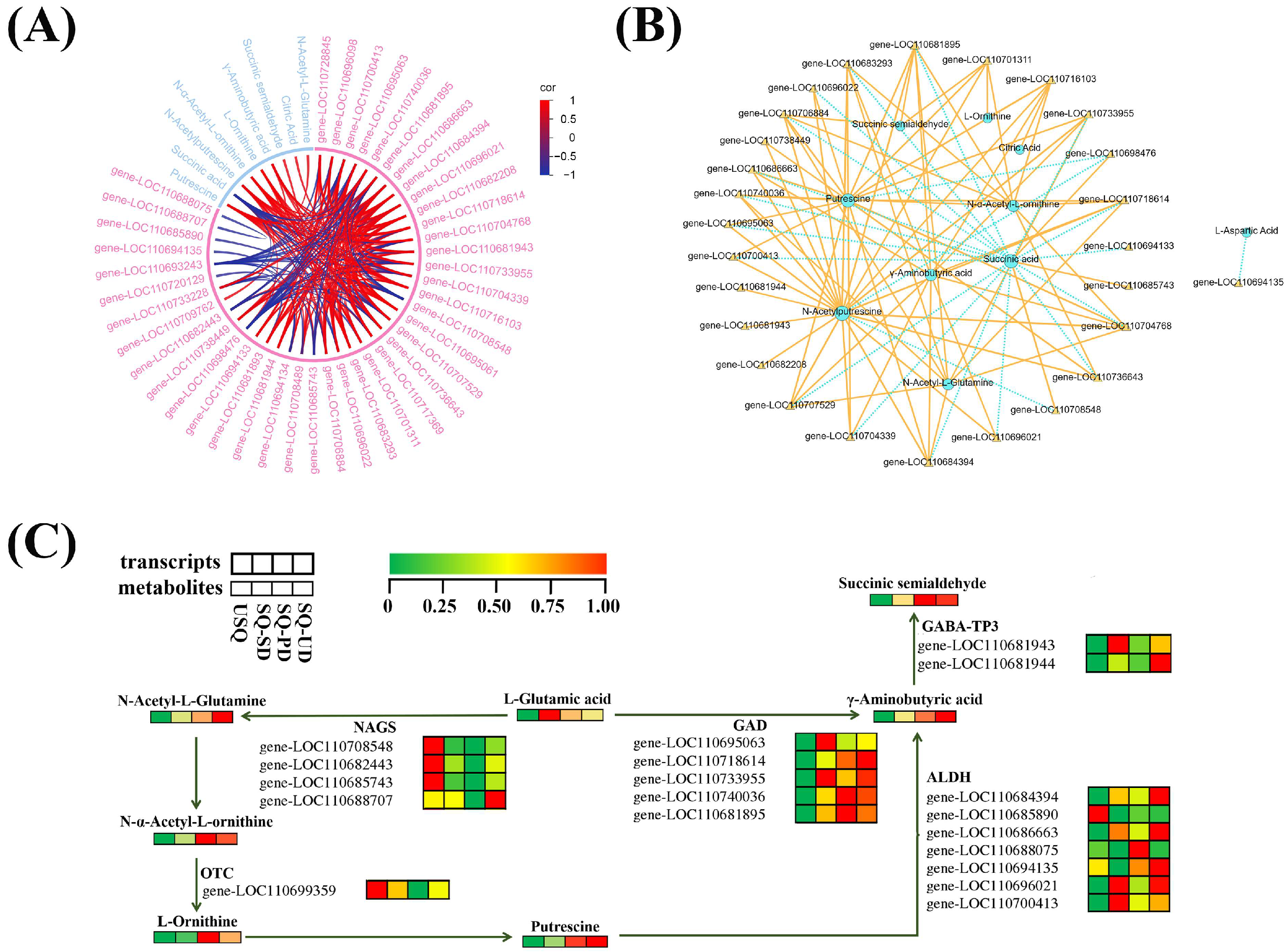
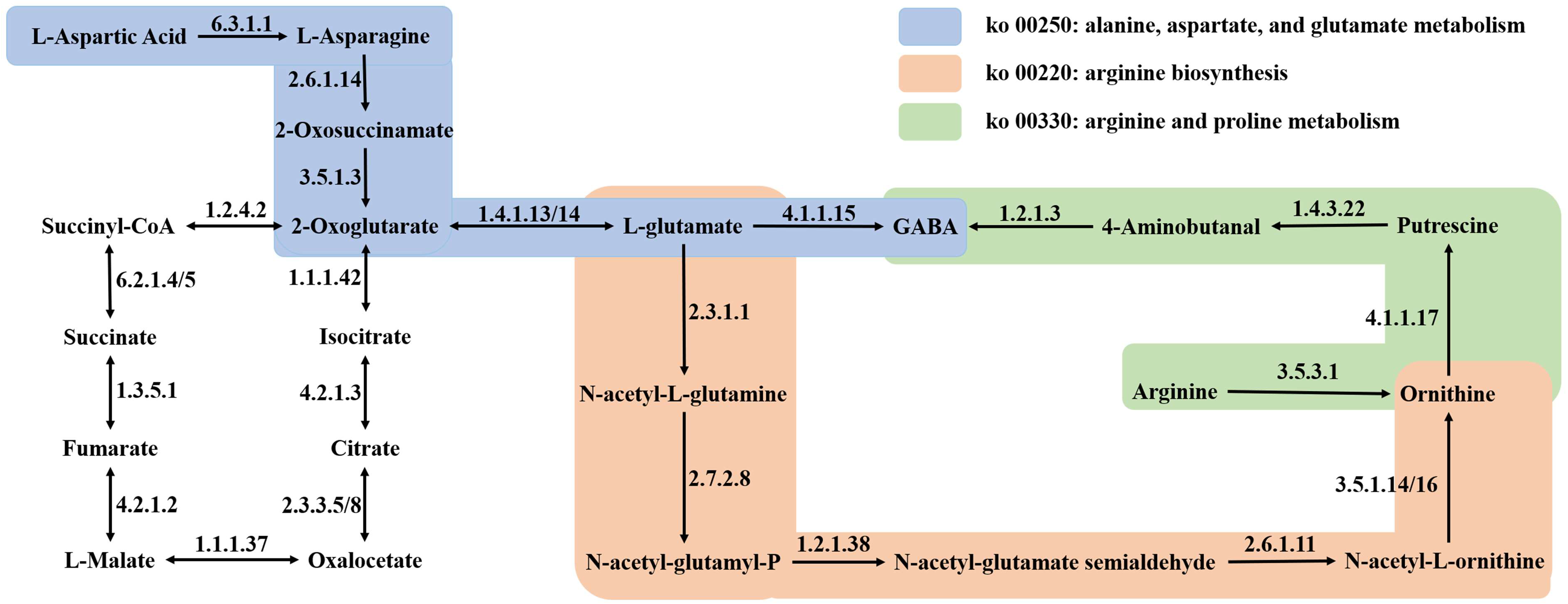
3.5. Integration of Metabolomic and Transcriptomic Profiles to Analyze GABA Biosynthesis in Quinoa
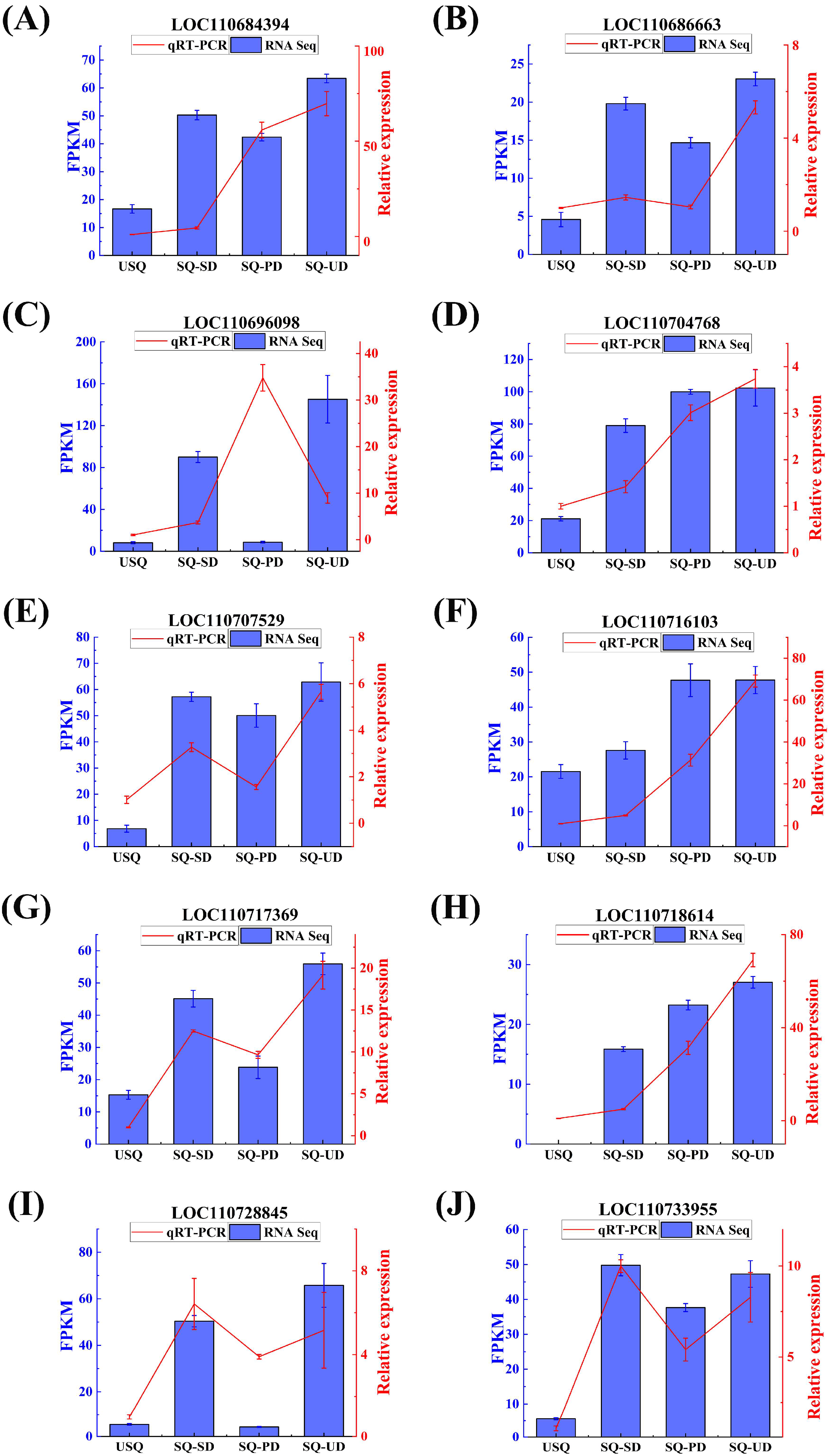
3.6. Expression Patterns and Validation of the GABA Biosynthetic Pathway Genes
3.7. Amino Acid Profile Related to GABA Enrichment in Quinoa
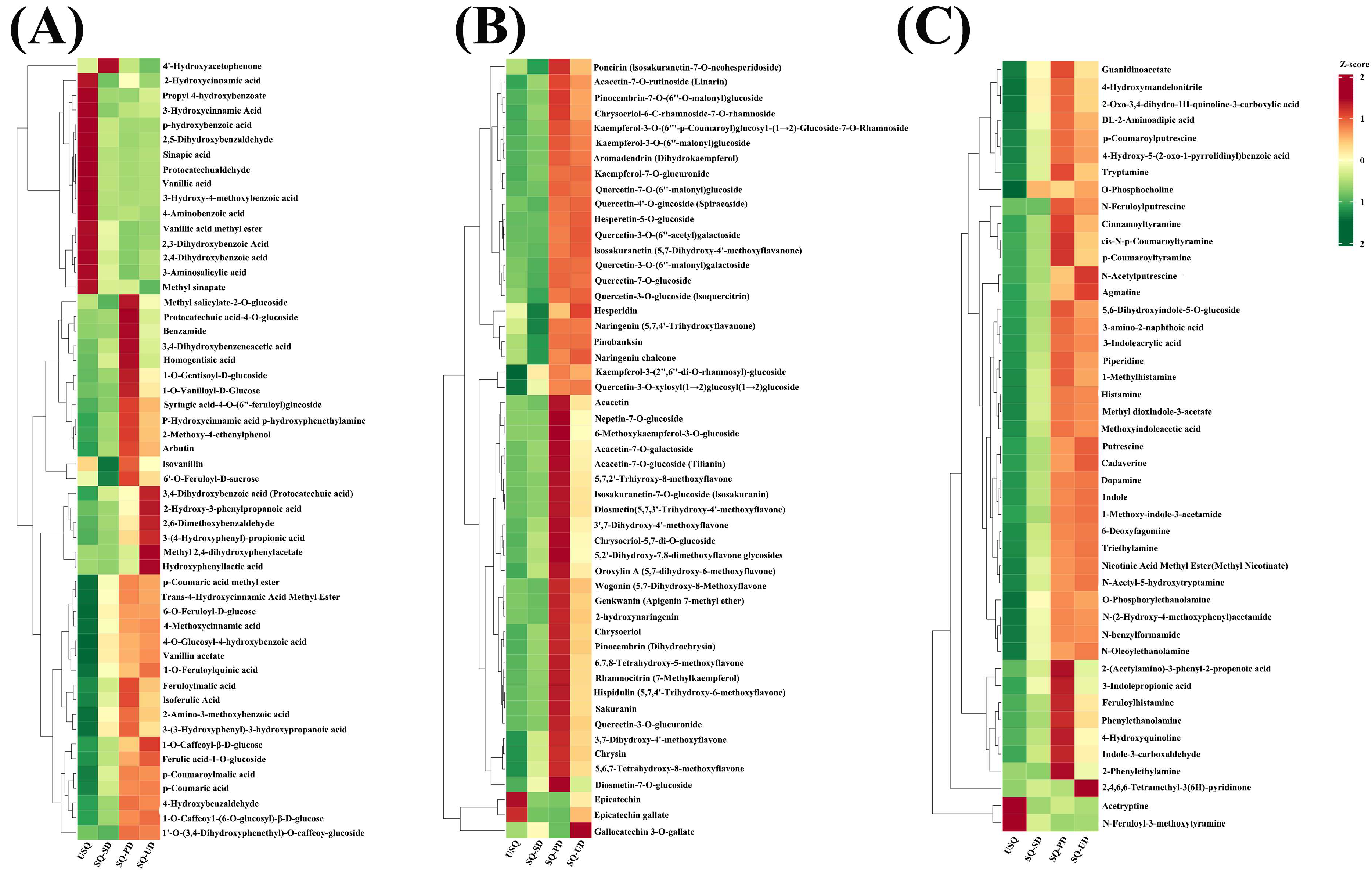
3.8. Changes in Phytochemical Content During GABA Enrichment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, S.Y.; Goh, E.L.K.; Sailor, K.A.; Kitabatake, Y.; Ming, G.L.; Song, H.J. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Douglass, A.M.; Resch, J.M.; Madara, J.C.; Kucukdereli, H.; Yizhar, O.; Grama, A.; Yamagata, M.; Yang, Z.F.; Lowell, B.B. Neural basis for fasting activation of the hypothalamic-pituitary-adrenal axis. Nature 2023, 620, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gutiérrez, L.; San Vicente, L.; Barrón, L.J.R.; Villarán, M.D.; Chávarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Yu, L.L.; Han, X.; Cen, S.; Duan, H.; Feng, S.S.; Xue, Y.Z.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Zhai, Q.X.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Zhang, M.; Meng, W.; Xie, X.; Chen, Y. Cold atmospheric plasma treatment improves the γ-aminobutyric acid content of buckwheat seeds providing a new anti-hypertensive functional ingredient. Food Chem. 2022, 388, 133064. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Lee, S.J.; Lee, S.P.; Boby, N.; Suk, K.; Birhanu, B.T.; Park, S.C. Aronia melanocarpa Extract Fermented by Lactobacillus plantarum EJ2014 Modulates Immune Response in Mice. Antioxidants 2021, 10, 1276. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villena, J.; Elean, M.; Savoy de Giori, G.; Saavedra, L.; Hebert, E.M. Immunomodulatory Properties of a γ-Aminobutyric Acid-Enriched Strawberry Juice Produced by Levilactobacillus brevis CRL 2013. Front. Microbiol. 2020, 11, 610016. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef]
- Majzoobi, M.; Wang, Z.; Teimouri, S.; Pematilleke, N.; Brennan, C.S.; Farahnaky, A. Unlocking the Potential of Sprouted Cereals, Pseudocereals, and Pulses in Combating Malnutrition. Foods 2023, 12, 3901. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Kim, S.; Shim, I.S. Production of γ-Aminobutyric Acid and Its Supplementary Role in the TCA Cycle in Rice (Oryza sativa L.) Seedlings. J. Plant Growth Regul. 2021, 40, 78–90. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lue, G.; Gao, H. GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants. Metabolites 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bashir, T.; Bae, H. Use of Ultrasonication Technology for the Increased Production of Plant Secondary Metabolites. Molecules 2017, 22, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ulanov, A.V.; Dong, M.; Yang, T.; Nemzer, B.V.; Xiong, S.; Zhao, S.; Feng, H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochemistry 2018, 40, 791–797. [Google Scholar] [CrossRef]
- Ding, J.; Johnson, J.; Chu, Y.F.; Feng, H. Enhancement of gamma-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chem. 2019, 283, 239–247. [Google Scholar] [CrossRef]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.; Cao, J.; Liu, L.; Zhou, X.; Barba, F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 8852–8874. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.Q.; Hao, Y.; Dong, G.Q.; Du, D.X.; Huo, D.A. Annotation of genes involved in gamma-aminobutyric multiplication during coix germination by metabolome and transcriptome analysis. Food Agric. Immunol. 2023, 34, 2241668. [Google Scholar] [CrossRef]
- Xu, M.Y.; Yang, Q.W.; Bai, G.X.; Li, P.; Yan, J. Polyamine pathways interconnect with GABA metabolic processes to mediate the low-temperature response in plants. Front. Plant Sci. 2022, 13, 1035414. [Google Scholar] [CrossRef]
- Lee, X.Y.; Tan, J.S.; Cheng, L.H. Gamma aminobutyric acid (GABA) enrichment in plant-based food—A mini review. Food Rev. Int. 2023, 39, 5864–5885. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Laura Coitino, E.; Borsani, O.; Monza, J. Connecting Proline and γ-Aminobutyric Acid in Stressed Plants through Non-Enzymatic Reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhu, Y.H.; Leng, C.Q.; Wang, Q.E.; Wang, P.; Yang, R.Q. Investigation into the Relationship between Spermidine Degradation and Phenolic Compounds Biosynthesis in Barley Seedlings under Ultraviolet B Stress. Plants 2023, 12, 3533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Duan, Y.; Cao, Y.; Chen, Y.W.; Zou, Z.W.; Li, F.; Shen, Q.; Yang, X.W.; Ma, Y.C.; Fang, W.P.; et al. CsCuAOs and CsAMADH1 are required for putrescine-derived γ-aminobutyric acid accumulation in tea. Foods 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Ishaq, A.; Al-Ghamdi, A.A.; Hatamleh, A.A. Botany, Nutritional value, phytochemical composition and biological activities of quinoa. Plants 2021, 10, 2258. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Q.; Zhou, L.; Wang, J.; Ren, T.; Zheng, Y.; Lv, W.; Zhao, W. Enhancement of gamma-aminobutyric acid and the characteristics of nutrition and function in white quinoa through ultrasound stress at the pre-germination stage. Foods 2023, 13, 57. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, Z.; Zhang, F.; Zhou, X.; Sun, X.; Li, Y.; Liu, W.; Xiao, H.; Wang, N.; Lu, H.; et al. Metabolomic and transcriptomic analyses reveal the effects of self- and hetero-grafting on anthocyanin biosynthesis in grapevine. Hortic. Res. 2022, 9, uhac103. [Google Scholar] [CrossRef]
- Li, Z.T.; Zhang, F.X.; Fan, C.L.; Ye, M.N.; Chen, W.W.; Yao, Z.H.; Yao, X.S.; Dai, Y. Discovery of potential Q-marker of traditional Chinese medicine based on plant metabolomics and network pharmacology: Periplocae Cortex as an example. Phytomedicine 2021, 85, 153535. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Hu, Y.; Chen, L.; Liu, Y. Integrated comparative metabolomics and network pharmacology approach to uncover the key active ingredients of Polygonati rhizoma and their therapeutic potential for the treatment of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 934947. [Google Scholar] [CrossRef]
- Lai, J.L.; Deng, Z.X.; Ji, X.H.; Luo, X.G. Absorption and interaction mechanisms of uranium & cadmium in purple sweet potato (Ipomoea batatas L.). J. Hazard. Mater. 2020, 400, 123264. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Chu, S.; You, Y.; Chi, Y.; Wang, R.; Yang, X.; Hayat, K.; Zhang, D.; Zhou, P. Comparative cytology combined with transcriptomic and metabolomic analyses of Solanum nigrum L. in response to Cd toxicity. J. Hazard. Mater. 2022, 423, 127168. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L. Metabolomic and transcriptomic investigation of metabolic perturbations in Oryza sativa L. triggered by three pesticides. Environ. Sci. Technol. 2020, 54, 6115–6124. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Gu, M. Transcriptome analysis and differential gene expression profiling of two contrasting quinoa genotypes in response to salt stress. BMC Plant Biol. 2020, 20, 568. [Google Scholar] [CrossRef] [PubMed]
- Gutsch, A.; Sergeant, K.; Keunen, E.; Prinsen, E.; Guerriero, G.; Renaut, J.; Hausman, J.F.; Cuypers, A. Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? Bmc Plant Biol. 2019, 19, 271. [Google Scholar] [CrossRef]
- Pham Van, H.; Nguyen Thi Hoang, Y.; Nguyen Thi Lan, P.; Nguyen Phung Ha, T.; Nguyen Thi Thu, T. Nutritional composition, enzyme activities and bioactive compounds of mung bean (Vigna radiata L.) germinated under dark and light conditions. LWT-Food Sci. Technol. 2020, 133, 110100. [Google Scholar] [CrossRef]
- Miao, J.; Lin, H.; Zhang, S.; Huo, J.; Deng, S. Effect on amino acid and mineral content of the loach (Misgurnus anguillicaudatus) by adding Fe(II) chelating hairtail protein hydrolysates (Fe (II)-HPH) to the feed. Food Sci. Nutr. 2020, 8, 1575–1582. [Google Scholar] [CrossRef]
- Li, B.Z.; Fan, R.N.; Fan, Y.T.; Liu, R.A.; Zhang, H.; Chen, T.T.; Liu, J.; Li, H.; Zhao, X.; Song, C.P. The flavonoid biosynthesis regulator PFG3 confers drought stress tolerance in plants by promoting flavonoid accumulation. Environ. Exp. Bot. 2022, 196, 104792. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Zhao, G.C.; Xie, M.X.; Wang, Y.C.; Li, J.Y. Molecular mechanisms underlying γ-aminobutyric acid (GABA) accumulation in giant embryo rice seeds. J. Agric. Food Chem. 2017, 65, 4883–4889. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Li, C.; Niu, M.; Dong, H.; Zhao, S.; Jia, C.; Xu, Y. Accelerated accumulation of γ-aminobutyric acid and modifications on its metabolic pathways in black rice grains by germination under bold stress. Foods 2023, 12, 1290. [Google Scholar] [CrossRef]
- Liao, J.; Wu, X.; Xing, Z.; Li, Q.; Duan, Y.; Fang, W.; Zhu, X. γ-Aminobutyric acid (GABA) accumulation in tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 2017, 65, 3013–3018. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Wan, X.Z.; Zhao, Z.X.; Liu, H.Q.; Wen, Y.X.; Wu, W.H.; Ge, X.D.; Zhao, C. Metabolomic analysis and pathway profiling of paramylon production in Euglena gracilis grown on different carbon sources. Int. J. Biol. Macromol. 2023, 246, 125661. [Google Scholar] [CrossRef]
- Wei, T.L.; Wang, Z.X.; He, Y.F.; Xue, S.; Zhang, S.Q.; Pei, M.S.; Liu, H.N.; Yu, Y.H.; Guo, D.L. Proline synthesis and catabolism-related genes synergistically regulate proline accumulation in response to abiotic stresses in grapevines. Sci. Hortic. 2022, 305, 111373. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Suravajhala, P.; Rathnagiri, P.; Sreenivasulu, N. Intriguing role of proline in redox potential conferring high temperature stress tolerance. Front. Plant Sci. 2022, 13, 867531. [Google Scholar] [CrossRef]
- Zhuang, W.B.; Li, Y.H.; Shu, X.C.; Pu, Y.T.; Wang, X.J.; Wang, T.; Wang, Z. The classification, molecular structure and biological biosynthesis of flavonoids, and their roles in biotic and abiotic stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hou, Y.Y.; Zhu, X.; Zheng, Y.H.; Jin, P. Physiological and metabolomic analyses of hot water treatment on amino acids and phenolic metabolisms in peach cold tolerance. Postharvest Biol. Technol. 2021, 179, 111593. [Google Scholar] [CrossRef]
- Zhou, X.R.; Gong, F.S.; Dong, J.W.; Lin, X.R.; Cao, K.; Xu, H.W.; Zhou, X.F.; Igamberdiev, A.U. Abscisic acid affects phenolic acid content to increase tolerance to UV-B stress in rhododendron chrysanthum Pall. Int. J. Mol. Sci. 2024, 25, 1234. [Google Scholar] [CrossRef]
- Wei, B.; Hou, K.; Zhang, H.; Wang, X.; Wu, W. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L. Under drought stress. Ind. Crops Prod. 2020, 151, 112465. [Google Scholar] [CrossRef]
- Lorenzo, C.D. Taking stress with much more than a pinch of salt: OsGRF7 regulates salinity resistance in rice through arbutin biosynthesis. Plant Cell 2024, 36, 2753–2754. [Google Scholar] [CrossRef]
- Aimvijarn, P.; Payuhakrit, W.; Charoenchon, N.; Okada, S.; Suwannalert, P. Riceberry rice germination and UVB radiation enhance protocatechuic acid and vanillic acid to reduce cellular oxidative stress and suppress B16F10 melanogenesis relating to F-actin rearrangement. Plants 2023, 12, 484. [Google Scholar] [CrossRef]
- Xie, C.; Wang, P.; Gu, Z.X.; Yang, R.Q. Spermidine alleviates oxidative damage and enhances phenolic compounds accumulation in barley seedlings under UV-B stress. J. Sci. Food Agric. 2023, 103, 648–656. [Google Scholar] [CrossRef]
| Gene ID | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| LOC110718614 | CAGCAAGTGTCTGTTTTTCTAATCG | CGCGTTCTCTTGGCAGTTTT |
| LOC110733955 | ATACGGCTTGGCTTTGAGGT | GAGCCATGGACCACTATTTTGC |
| LOC110684394 | AGGCATCTTATGACAAGGTAAAAGC | CCTGTGCAGAAGCGTCAAAG |
| LOC110686663 | TGTGAGTGAAAGTGTTGTCCCA | ACGACCAAGGAGGTTCCAC |
| LOC110681943 | ACATCAGTGCAGCAAACAATAGTC | TAGGGGTGCTAACACAAGCAG |
| LOC110704768 | CCAAAAGTCGGGATGCTTGC | GCAGTTGCTCATCTTTCTGTCC |
| LOC110707529 | GTGTTGTCATCCTTCCCGACA | AGTGGAGGATTTCATACCTGAACC |
| LOC110728845 | AATGGGAGAGGGGGACTAGAAT | CAAGGACTGCCCTCGTCTAC |
| LOC110696098 | ACTCCAACCTCACTTGCCATT | TTTCCCTGCTGAGTGCCTG |
| LOC110717369 | TTTCCCGTGTTGTCCTCATCC | CAACACTTGGACCACCTGGA |
| LOC110716103 | GAGATGTGGGGTGCCACTTT | TTGCTTTGTGTAAGTCAACGAGAC |
| EF1α | GTACGCATGGGTGCTTGACAAACTC | ATCAGCCTGGGAGGTACCAGTAAT |
| Amino Acids (mg/g) | USQ | SQ-SD | SQ-PD | SQ-UD |
|---|---|---|---|---|
| Aspartate | 0.242 ± 0.020 Aa | 0.329 ± 0.048 Bb | 0.295 ± 0.017 ABb | 0.418 ± 0.014 Cc |
| Threonine | 0.173 ± 0.016 Aa | 0.189 ± 0.010 AaB | 0.222 ± 0.011 Bb | 0.322 ± 0.012 Cc |
| Serine | 0.321 ± 0.018 Aa | 0.474 ± 0.018 Bb | 0.415 ± 0.008 Cc | 0.371 ± 0.014 Dd |
| Glutamate | 0.555 ± 0.024 Aa | 0.839 ± 0.044 Bb | 0.721 ± 0.017 Cc | 0.627 ± 0.011 Ad |
| Glycine | 0.177 ± 0.012 Aa | 0.097 ± 0.008 Bb | 0.103 ± 0.014 Bb | 0.176 ± 0.012 Aa |
| Alanine | 0.245 ± 0.023 Aa | 0.263 ± 0.013 Aa | 0.367 ± 0.018 Bb | 0.467 ± 0.010 Cc |
| Cysteine | 0.149 ± 0.008 Aa | 0.130 ± 0.010 Ab | 0.184 ± 0.010 Bc | 0.263 ± 0.008 Cd |
| Valine | 0.313 ± 0.011 Aa | 0.327 ± 0.024 Aa | 0.394 ± 0.004 Bb | 0.548 ± 0.013 Cc |
| Methionine | 0.195 ± 0.011 Aa | 0.190 ± 0.012 Aa | 0.200 ± 0.009 Aa | 0.405 ± 0.006 Bb |
| Isoleucine | 0.242 ± 0.015 Aa | 0.256 ± 0.017 Aa | 0.324 ± 0.018 Bb | 0.439 ± 0.011 Cc |
| Leucine | 0.305 ± 0.007 Aa | 0.323 ± 0.014 Aa | 0.427 ± 0.009 Bb | 0.613 ± 0.009 Cc |
| Tyrosine | 0.294 ± 0.007 Aa | 0.306 ± 0.006 Aa | 0.377 ± 0.008 Bb | 0.601 ± 0.015 Cc |
| Phenylalanine | 0.239 ± 0.011 Aa | 0.232 ± 0.016 Aa | 0.298 ± 0.012 Bb | 0.522 ± 0.011 Cc |
| Histidine | 0.189 ± 0.010 Aa | 0.287 ± 0.012 Bb | 0.329 ± 0.008 Cc | 0.367 ± 0.010 Dd |
| Lysine | 0.281 ± 0.015 Aa | 0.283 ± 0.012 Aa | 0.420 ± 0.012 Bb | 0.697 ± 0.011 Cc |
| Arginine | 0.604 ± 0.014 Aa | 0.729 ± 0.015 Bb | 0.807 ± 0.013 Cc | 0.601 ± 0.018 Aa |
| Proline | 0.309 ± 0.012 Aa | 0.298 ± 0.011 Aa | 0.395 ± 0.004 Bb | 0.524 ± 0.010 Cc |
| Total amino acids | 4.832 ± 0.010 Aa | 5.553 ± 0.021 Bb | 6.278 ± 0.046 Cc | 7.957 ± 0.013 Dd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Zhou, Q.; Sun, Y.; Zhou, L.; Li, D.; Ren, T.; Zheng, Y.; Zhao, W.; Wang, J. Integrated Metabolomic and Transcriptomic Analysis Reveals the Molecular Regulatory Mechanism of Gamma-Aminobutyric Acid Accumulation in White Quinoa (Chenopodium quinoa Willd.) in Response to Dark and Ultrasound Stress. Foods 2025, 14, 1186. https://doi.org/10.3390/foods14071186
Wu M, Zhou Q, Sun Y, Zhou L, Li D, Ren T, Zheng Y, Zhao W, Wang J. Integrated Metabolomic and Transcriptomic Analysis Reveals the Molecular Regulatory Mechanism of Gamma-Aminobutyric Acid Accumulation in White Quinoa (Chenopodium quinoa Willd.) in Response to Dark and Ultrasound Stress. Foods. 2025; 14(7):1186. https://doi.org/10.3390/foods14071186
Chicago/Turabian StyleWu, Mengying, Qian Zhou, Yasai Sun, Liangfu Zhou, Dongyao Li, Ting Ren, Yu Zheng, Wen Zhao, and Jie Wang. 2025. "Integrated Metabolomic and Transcriptomic Analysis Reveals the Molecular Regulatory Mechanism of Gamma-Aminobutyric Acid Accumulation in White Quinoa (Chenopodium quinoa Willd.) in Response to Dark and Ultrasound Stress" Foods 14, no. 7: 1186. https://doi.org/10.3390/foods14071186
APA StyleWu, M., Zhou, Q., Sun, Y., Zhou, L., Li, D., Ren, T., Zheng, Y., Zhao, W., & Wang, J. (2025). Integrated Metabolomic and Transcriptomic Analysis Reveals the Molecular Regulatory Mechanism of Gamma-Aminobutyric Acid Accumulation in White Quinoa (Chenopodium quinoa Willd.) in Response to Dark and Ultrasound Stress. Foods, 14(7), 1186. https://doi.org/10.3390/foods14071186






