A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules
Abstract
:1. Introduction
2. Materials and Methods
3. Results
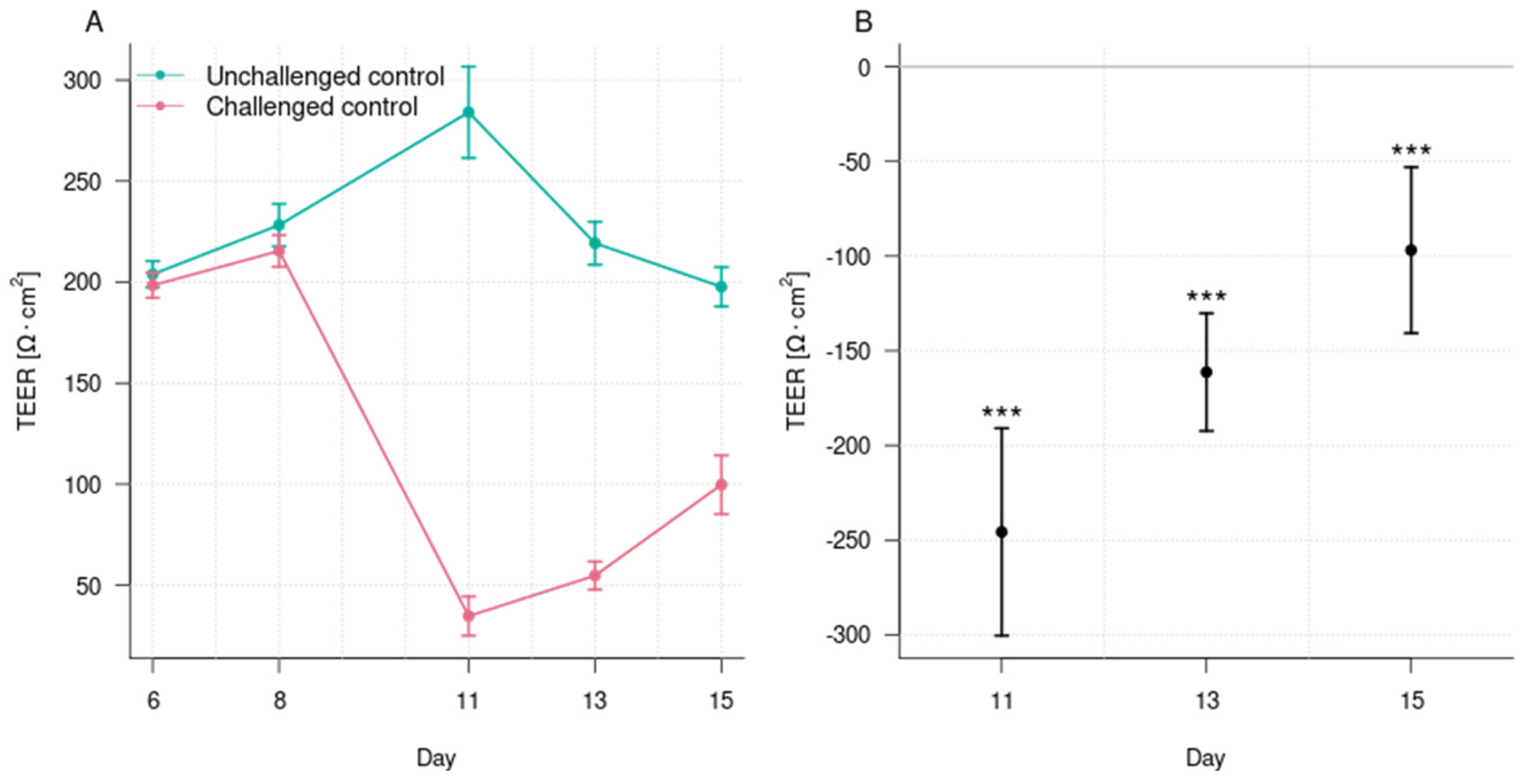
3.1. Induction of Epithelial Barrier Damage
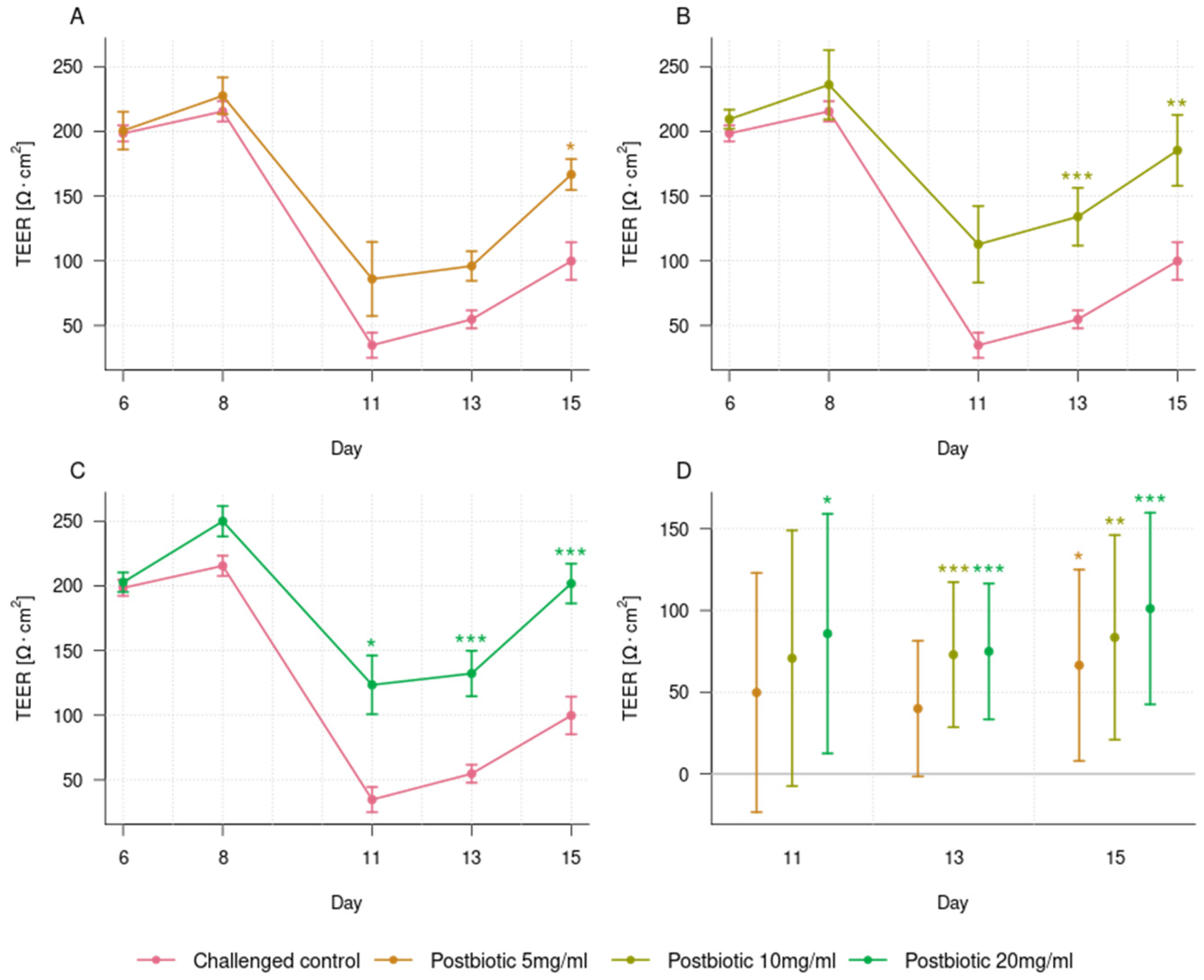
3.2. Dose-Dependent Effect of the Postbiotic in Preventing Epithelial Barrier Damage
3.3. Effect of the Postbiotic on Cytokine Release Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus Plantarum NCU116 Regulate Intestinal Barrier Function via STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Warda, A.K.; Clooney, A.G.; Ryan, F.; de Almeida Bettio, P.H.; Di Benedetto, G.; Ross, R.P.; Hill, C. A Postbiotic Consisting of Heat-Treated Lactobacilli Has a Bifidogenic Effect in Pure Culture and in Human Fermented Faecal Communities. Appl. Environ. Microbiol 2021, 87, e02459-20. [Google Scholar] [CrossRef]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus Paracasei CBA L74 Metabolic Products and Fermented Milk for Infant Formula Have Anti-Inflammatory Activity on Dendritic Cells in Vitro and Protective Effects against Colitis and an Enteric Pathogen in Vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Liévin, V.; Bernet-Camard, M.F.; Hudault, S.; Servin, A.L. Antibacterial Effect of the Adhering Human Lactobacillus acidophilus Strain LB. Antimicrob. Agents Chemother. 1997, 41, 1046–1052. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus Paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the Power of Postbiotics: A Revolutionary Approach to Nutrition for Humans and Animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Tarrerias, A.L.; Costil, V.; Vicari, F.; Létard, J.C.; Adenis-Lamarre, P.; Aisène, A.; Batistelli, D.; Bonnaud, G.; Carpentier, S.; Dalbiès, P.; et al. The Effect of Inactivated Lactobacillus LB Fermented Culture Medium on Symptom Severity: Observational Investigation in 297 Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Dig. Dis. 2011, 29, 588–591. [Google Scholar] [CrossRef]
- Xiao, S.-D.; Zhang, D.Z.; Lu, H.; Jiang, S.H.; Liu, H.Y.; Wang, G.S.; Xu, G.M.; Zhang, Z.B.; Lin, G.J.; Wang, G.L. Multicenter, Randomized, Controlled Trial of Heat-Killed Lactobacillus acidophilus LB in Patients with Chronic Diarrhea. Adv. Ther. 2003, 20, 253–260. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Bozzetti, V.; Senger, S. Organoid Technologies for the Study of Intestinal Microbiota-Host Interactions. Trends Mol. Med. 2022, 28, 290–303. [Google Scholar] [CrossRef]
- Munoz, S.; Guzman-Rodriguez, M.; Sun, J.; Zhang, Y.-G.; Noordhof, C.; He, S.-M.; Allen-Vercoe, E.; Claud, E.C.; Petrof, E.O. Rebooting the Microbiome. Gut Microbes 2016, 7, 353–363. [Google Scholar] [CrossRef]
- Hunyady, B.; Mezey, E.; Palkovits, M. Gastrointestinal Immunology: Cell Types in the Lamina Propria—A Morphological Review. Acta Physiol. Hung. 2000, 87, 305–328. [Google Scholar] [PubMed]
- Helander, H.F.; Fändriks, L. Surface Area of the Digestive Tract-Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Basak, O.; Beumer, J.; Wiebrands, K.; Seno, H.; van Oudenaarden, A.; Clevers, H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell 2017, 20, 177–190.e4. [Google Scholar] [CrossRef]
- Salazar-Lindo, E.; Figueroa-Quintanilla, D.; Caciano, M.I.; Reto-Valiente, V.; Chauviere, G.; Colin, P.; Lacteol Study Group. Effectiveness and Safety of Lactobacillus LB in the Treatment of Mild Acute Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 571–576. [Google Scholar] [CrossRef]
- Simakachorn, N.; Pichaipat, V.; Rithipornpaisarn, P.; Kongkaew, C.; Tongpradit, P.; Varavithya, W. Clinical Evaluation of the Addition of Lyophilized, Heat-Killed Lactobacillus acidophilus LB to Oral Rehydration Therapy in the Treatment of Acute Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 68–72. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Sarrazin-Davila, L.E.; Servin, A.L. An Experimental Study and a Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Antisecretory Activity of Lactobacillus acidophilus Strain LB against Nonrotavirus Diarrhea. Pediatrics 2007, 120, e795–e803. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Bernet, M.F.; Kernéis, S.; Chauvière, G.; Fourniat, J.; Servin, A.L. Inhibition of Adhesion of Enteroinvasive Pathogens to Human Intestinal Caco-2 Cells by Lactobacillus acidophilus Strain LB Decreases Bacterial Invasion. FEMS Microbiol. Lett. 1993, 110, 299–305. [Google Scholar] [CrossRef]
- Kim, Y.; Quach, A.; Das, S.; Barrett, K.E. Potentiation of Calcium-Activated Chloride Secretion and Barrier Dysfunction May Underlie EGF Receptor Tyrosine Kinase Inhibitor-Induced Diarrhea. Physiol. Rep. 2020, 8, e14490. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Pichyangkura, R.; Muanprasat, C. Chitosan Oligosaccharide Prevents Afatinib-Induced Barrier Disruption and Chloride Secretion through Modulation of AMPK, PI3K/AKT, and ERK Signaling in T84 Cells. Polymers 2022, 14, 4255. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Montalto, M.; Maggiano, N.; Ricci, R.; Curigliano, V.; Santoro, L.; Di Nicuolo, F.; Vecchio, F.M.; Gasbarrini, A.; Gasbarrini, G. Lactobacillus acidophilus Protects Tight Junctions from Aspirin Damage in HT-29 Cells. Digestion 2004, 69, 225–228. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Amsellem, R.; Servin, A.L.; Coconnier, M.-H. Lactobacillus acidophilus (Strain LB) from the Resident Adult Human Gastrointestinal Microflora Exerts Activity against Brush Border Damage Promoted by a Diarrhoeagenic Escherichia coli in Human Enterocyte-like Cells. Gut 2002, 50, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotic Effects of Lactobacillus Fermentate on Intestinal Health, Mucosa-Associated Microbiota, and Growth Efficiency of Nursery Pigs Challenged with F18+Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef]
- Kobayashi, K.; Mochizuki, J.; Yamazaki, F.; Sashihara, T. Yogurt Starter Strains Ameliorate Intestinal Barrier Dysfunction via Activating AMPK in Caco-2 Cells. Tissue Barriers 2024, 12, 2184157. [Google Scholar] [CrossRef]
- Furone, F.; Bellomo, C.; Carpinelli, M.; Nicoletti, M.; Hewa-Munasinghege, F.N.; Mordaa, M.; Mandile, R.; Barone, M.V.; Nanayakkara, M. The Protective Role of Lactobacillus Rhamnosus GG Postbiotic on the Alteration of Autophagy and Inflammation Pathways Induced by Gliadin in Intestinal Models. Front. Med. 2023, 10, 1085578. [Google Scholar] [CrossRef]
- Conte, M.; Nigro, F.; Porpora, M.; Bellomo, C.; Furone, F.; Budelli, A.L.; Nigro, R.; Barone, M.V.; Nanayakkara, M. Gliadin Peptide P31-43 Induces mTOR/NFkβ Activation and Reduces Autophagy: The Role of Lactobacillus paracasei CBA L74 Postbiotc. Int. J. Mol. Sci. 2022, 23, 3655. [Google Scholar] [CrossRef]
- Cho, Y.; Sung, M.-H.; Kang, H.-T.; Lee, J.H. Establishment of an Apical-Out Organoid Model for Directly Assessing the Function of Postbiotics. J. Microbiol. Biotechnol. 2024, 34, 2184–2191. [Google Scholar] [CrossRef]
- Lee, H.; Jung, K.B.; Kwon, O.; Son, Y.S.; Choi, E.; Yu, W.D.; Son, N.; Jeon, J.H.; Jo, H.; Yang, H.; et al. Limosilactobacillus reuteri DS0384 Promotes Intestinal Epithelial Maturation via the Postbiotic Effect in Human Intestinal Organoids and Infant Mice. Gut Microbes 2022, 14, 2121580. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532.e2. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Oglio, F.; Bruno, C.; Coppola, S.; De Michele, R.; Masino, A.; Carucci, L. Evidence on the Preventive Effects of the Postbiotic Derived from Cow’s Milk Fermentation with Lacticaseibacillus paracasei CBA L74 against Pediatric Gastrointestinal Infections. Microorganisms 2022, 11, 10. [Google Scholar] [CrossRef]
- Warda, A.K.; de Almeida Bettio, P.H.; Hueston, C.M.; Di Benedetto, G.; Clooney, A.G.; Hill, C. Oral Administration of Heat-Treated Lactobacilli Modifies the Murine Microbiome and Reduces Citrobacter Induced Colitis. Front. Microbiol. 2020, 11, 69. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and Postbiotic Activity in Health and Disease: Comparison on a Novel Polarised Ex-Vivo Organ Culture Model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]


| Variable [pg/mL] | Challenged Control | Challenged Postbiotics 5 mg/mL | p-Value | Challenged Postbiotics 10 mg/mL | p-Value | Challenged Postbiotics 20 mg/mL | p-Value |
|---|---|---|---|---|---|---|---|
| CCL2 | 3450 ± 299 | 2010 ± 144 | 0.013 | 1018 ± 97 | 0.013 | 101 ± 20 | 0.009 |
| CX3CL1 | 1186 ± 63 | 591 ± 49 | 0.009 | 489 ± 38 | 0.013 | 164 ± 17 | 0.009 |
| CXCL1 | 5828 ± 237 | 4443 ± 153 | 0.009 | 2596 ± 141 | 0.013 | 564 ± 66 | 0.009 |
| CXCL10 | 62 ± 19 | 35 ± 10 | 0.290 | 7 ± 0.3 | 0.013 | 24 ± 8 | 0.074 |
| CXCL5 | 122 ± 6 | 125 ± 4 | 1.000 | 125 ± 6 | 0.837 | 52 ± 10 | 0.009 |
| IL-8 | 2238 ± 282 | 3224 ± 751 | 0.459 | 1630 ± 193 | 0.197 | 395 ± 65 | 0.009 |
| IL-10 | 19 ± 4 | 11 ± 2 | 0.216 | 3 ± 0.3 | 0.013 | 8 ± 2 | 0.032 |
| IL-11 | 5840 ± 330 | 4553 ± 315 | 0.051 | 2598 ± 227 | 0.013 | 290 ± 52 | 0.009 |
| IL-23 | 784 ± 151 | 519 ± 71 | 0.216 | 172 ± 10 | 0.013 | 277 ± 87 | 0.017 |
| IL-4 | 25 ± 2 | 20 ± 2 | 0.185 | 14 ± 1 | 0.013 | 3 ± 1 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercamondi, C.I.; Bendik, I.; Eckhardt, E.; Mak, T.; Seifert, N.; Kuratli, K.; Richard, N.; Tamasi, B.; Mussler, B.; Wintergerst, E. A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules. Foods 2025, 14, 1173. https://doi.org/10.3390/foods14071173
Cercamondi CI, Bendik I, Eckhardt E, Mak T, Seifert N, Kuratli K, Richard N, Tamasi B, Mussler B, Wintergerst E. A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules. Foods. 2025; 14(7):1173. https://doi.org/10.3390/foods14071173
Chicago/Turabian StyleCercamondi, Colin I., Igor Bendik, Erik Eckhardt, Tim Mak, Nicole Seifert, Karin Kuratli, Nathalie Richard, Balint Tamasi, Bernd Mussler, and Eva Wintergerst. 2025. "A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules" Foods 14, no. 7: 1173. https://doi.org/10.3390/foods14071173
APA StyleCercamondi, C. I., Bendik, I., Eckhardt, E., Mak, T., Seifert, N., Kuratli, K., Richard, N., Tamasi, B., Mussler, B., & Wintergerst, E. (2025). A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules. Foods, 14(7), 1173. https://doi.org/10.3390/foods14071173







