Comparative Research of Antioxidant, Antimicrobial, Antiprotozoal and Cytotoxic Activities of Edible Suillus sp. Fruiting Body Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Material of Fruiting Bodies (Sporocarps) of the Mushrooms
2.2. Preparation of Extracts from Fruiting Bodies by Different Solvents
2.3. Determination of Total Phenolic Content (TPC) in Different Suillus sp. Extracts
2.4. Antioxidant Activity (AA) Tests by Spectrophotometric Methods, Using Antioxidant ABTS•+ Capacity and DPPH Radical Scavenging Assays
2.5. Antibacterial and Antifungal Activity Assay
2.6. Cytotoxicity Assay on MRC-5 Line Cells
2.7. Anti-Trypanosomal Assays
2.8. Antileishmanian Assay
2.9. Antiplasmodial Test
2.10. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content (TPC) in the Extracts from Fruiting Bodies of the Suillus sp. Mushrooms
3.2. Results of Antioxidant Activity (AA) of the Suillus sp. Extracts
3.3. Antimicrobial Activity (AMA) of the Suillus sp. Fruiting Bodies Extracts in Agar Disc Diffusion Test
3.4. Results of Cytotoxic, Antiprotozoal, Antitrypanosomal, Antileishmanial and Antimicrobial Activity of Mushroom Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPC | Total Phenolic Content |
| AA | Antioxidant activity |
| GAE | Gallic acid equivalent |
| AMA | Antimicrobial activity |
| ALA | Antileishmanial activity |
| APA | Antiprotozoal activity |
| CA | Cytotoxic activity |
| AMA | Antimicrobial activity |
| RPMI | Cell culture medium |
References
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.-J.; McKenzie, E.H.C.; Maharachchikumbura, S.S.N.; Buyck, B.; Zhao, C.-L.; Fan, Y.-G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Lofgren, L.A.; Stajich, J.E. Fungal biodiversity and conservation mycology in light of new technology, big data, and changing attitudes. Curr. Biol. 2021, 31, R1312–R1325. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Janowski, D.; Wilgan, R.; Leski, T.; Karliński, L.; Rudawska, M. Effective molecular identification of ectomycorrhizal fungi: Revisiting DNA isolation methods. Forests 2019, 10, 218. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Vellinga, E.C.; Bruns, T.D.; Kennedy, P.G. Phylogenetic assessment of global Suillus ITS sequences supports morphologically defined species and reveals synonymous and undescribed taxa. Mycologia 2016, 108, 1216–1228. [Google Scholar] [CrossRef]
- Tao, J.; Wang, X.; Long, Y.; Gao, Z.; Zhang, G.; Guo, Z.; Wang, G.; Xu, G.; Wang, Y.; Liu, H. Determining gene order patterns in the Suillus and Boletales through comparative analysis of their mitogenomes. Int. J. Mol. Sci. 2024, 25, 9597. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Gao, J.; Zhang, Y.; Liu, Y.; Tang, M. Effects of ectomycorrhizal fungi (Suillus variegatus) on the growth, hydraulic function, and non-structural carbohydrates of Pinus tabulaeformis under drought stress. BMC Plant Biol. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Yang, R.; Gao, J.; Liu, Y.; Zhang, H.; Wang, Y.; Tang, M. Contribution of Suillus variegatus to the ecological restoration of 10-year-old Pinus tabuliformis on the Loess Plateau. App. Soil Ecol. 2021, 167, 104044. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, X.; Feng, W.; Ding, G.; Quan, W. Effect of Ectomycorrhizal fungi on the drought resistance of Pinus massonian seedlings. J. Fungi 2023, 9, 471. [Google Scholar] [CrossRef]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- Dyshko, V.; Hilszczańska, D.; Davydenko, K.; Matić, S.; Moser, W.K.; Borowik, P.; Oszako, T. An overview of mycorrhiza in pines: Research, species, and applications. Plants 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Souna, M.; Chaouche, T.; Tefiani, C.; Azzi, R.; Habi, S. The mycorrhizal relationship between maritime pine and Aleppo pine with four species of higher fungi of the genera Suillellus and Suillus in the Ghazaouet littoral, northwestern Algeria. In Proceedings of the 4th International Electronic Conference on Forests session Forest Biodiversity, Ecosystem Services, and Earth Observations, Online, 23–25 September 2024; Available online: https://sciforum.net/manuscripts/18344/slides.pdf (accessed on 19 December 2024).
- Abdulhadi, S.Y.; Gergees, R.N.; Hasan, G.Q. Molecular identification, antioxidant efficacy of phenolic compounds, and antimicrobial activity of beta-carotene isolated from fruiting bodies of Suillus sp. KIJOMS 2020, 6, 4. [Google Scholar] [CrossRef]
- Yao, L.; Lv, J.-H.; Li, J.-P.; An, X.-Y.; Cheng, G.-H.; Li, C.-T.; Li, Y. Chemical constituents from mushroom Suillus luteus (Agaricomycetes) and their bioactivities. Int. J. Med. Mushrooms 2022, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Rangel, J.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Contents of carboxylic acids and two phenolics and antioxidant activity of dried Portuguese wild edible mushrooms. J. Agric. Food Chem. 2006, 54, 8530–8537. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Bernaś, E.; Skrzypczak, A.; Kapusta, I. Vitamins, phenolics and antioxidant activity of culinary prepared Suillus luteus (L.) Roussel mushroom. LWT Food Sci. Technol. 2014, 59, 701–706. [Google Scholar] [CrossRef]
- Stojanova, M.; Pantić, M.; Karadelev, M.; Ivanovski, V.; Nikšić, M. Determination of biological activity of Suillus granulatus mushroom extracts. J. Food Meas. Charact. 2022, 16, 4564–4572. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, R.; Ho, C.-T.; Li, B.; Chen, S.; Xiao, C.; Hu, H.; Cai, M.; Chen, Z.; Xie, Y.; et al. Preparation, chemical structure, and immunostimulatory activity of a water-soluble heteropolysaccharide from Suillus granulatus fruiting bodies. Food Chem. X 2022, 13, 100211. [Google Scholar] [CrossRef]
- Zhao, H.; Yao, L.; Zhao, J.; Zhang, M.; Wang, L.A.; Lv, J.; Zhang, J. Chemical compounds, bioactivities, and potential applications of the mushroom species of genus Suillus (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2024, 26, 25–41. [Google Scholar] [CrossRef]
- Nieto, I.J.; Ávila, I.M.C. Determination of fatty acids and triterpenoid compounds from the fruiting body of Suillus luteus. Rev. Colomb. Quím. 2008, 37, 297–304. Available online: https://www.redalyc.org/articulo.oa?id=309026679002 (accessed on 19 December 2024).
- Zhang, M.Y.; Malins, L.R.; Ward, J.S.; Barrow, R.A. Total synthesis of suillusin. Org. Lett. 2018, 20, 7304–7307. [Google Scholar] [CrossRef]
- Yamaç, M.; Bilgili, F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006, 44, 660–667. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Z.; Pang, Q.; Liang, X.; Li, H.; Sui, X.; Li, C.; Song, F. Responses of bacterial community structure, diversity, and chemical properties in the rhizosphere soil on fruiting-body formation of Suillus luteus. Microorganisms 2022, 10, 2059. [Google Scholar] [CrossRef]
- Roszczenko, P.; Szewczyk-Roszczenko, O.K.; Gornowicz, A.; Iwańska, I.A.; Bielawski, K.; Wujec, M.; Bielawska, A. The anticancer potential of edible mushrooms: A Review of selected species from Roztocze, Poland. Nutrients 2024, 16, 2849. [Google Scholar] [CrossRef]
- Dong, Z.-J.; Wang, F.; Wang, R.; Yang, L.-M.; Zheng, Y.T.; Liu, J.-K. Chemical constituents of fruiting bodies from basidiomycete Suillus granulatus and their anti-HIV-1 activity. Chin. Tradit. Herb. Drugs 2007, 38, 337–339. [Google Scholar]
- Tel, G.; Deveci, E.; Küçükaydın, S.; Özler, M.A.; Duru, M.E.; Harmandar, M. Evaluation of antioxidant activity of Armillaria tabescens, Leucopaxillus gentianeus and Suillus granulatus: The mushroom species from Anatolia. Eurasian J. Anal. Chem. 2013, 8, 136–147. [Google Scholar]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Evidente, A. Structures and biological activities of alkaloids produced by mushrooms, a fungal subgroup. Biomolecules 2022, 12, 1025. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on antimicrobial activity of mushroom (basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Dai, R.; Liu, M.; Nik Nabil, W.N.; Xi, Z.; Xu, H. Mycomedicine: A unique class of natural products with potent anti-tumour bioactivities. Molecules 2021, 26, 1113. [Google Scholar] [CrossRef]
- Zhao, H.; Xiong, M.; Yang, X.; Yao, L.; Wang, Z.; Wang, L.; Li, Z.; Zhang, J.; Lv, J. Six new polyphenolic metabolites isolated from the Suillus granulatus and their cytotoxicity against HepG2 cells. Front. Nutr. 2024, 11, 1390256. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Trepa, M.; Olechowska-Jarząb, A.; Nowak, P.; Ziaja, M.; Kała, K.; Muszyńska, B. Natural compounds of fungal origin with antimicrobial activity—Potential cosmetics applications. Pharmaceuticals 2023, 16, 1200. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Vallavan, V.; Krishnasamy, G.; Zin, N.M.; Abdul Latif, M. A Review on antistaphylococcal secondary metabolites from Basidiomycetes. Molecules 2020, 25, 5848. [Google Scholar] [CrossRef]
- Chun, S.; Gopal, J.; Muthu, M. Antioxidant activity of mushroom extracts/polysaccharides—Their antiviral properties and plausible antiCOVID-19 properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Nandi, S.; Sikder, R.; Rapior, S.; Arnould, S.; Simal-Gandara, J.; Acharya, K. A review for cancer treatment with mushroom metabolites through targeting mitochondrial signaling pathway: In vitro and in vivo evaluations, clinical studies and future prospects for mycomedicine. Fitoterapia 2024, 172, 105681. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Budiene, J.; Nedveckyte, I.; Garjonyte, R. Antioxidant and toxic activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don essential oils and extracts. Molecules 2022, 27, 1311. [Google Scholar] [CrossRef]
- Balouirin, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Al-Sokari, S.S.; Mothana, R.; Hamed, M.; Wagih, M.; Paul Cos, P.; Maes, L. In vitro antiprotozoal activity of five plant extracts from Albaha region. World J. Pharm. Res. 2016, 5, 338–346. [Google Scholar]
- Aytar, E.C.; Akata, İ.; Açik, L. Antioxidant, antimicrobial and anti-proliferative activity of Suillus luteus (L.) Roussel extracts. Ank. Univ. Eczaci. Fak. Derg. 2020, 44, 373–387. [Google Scholar] [CrossRef]
- Volcão, L.M.; Fernandes, C.L.F.; Ribeiro, A.C.; Brum, R.L.; Eslabão, C.F.; Badiale-Furlong, E.; Ramos, D.F.; Bernardi, E.; da Silva Júnior, F.M.R. Bioactive extracts of Russula xerampelina and Suillus granulatus in the in vitro control of Pseudomonas aeruginosa phytopathogenic. S. Afr. J. Bot. 2021, 140, 218–225. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutic 2023, 15, 1615. [Google Scholar] [CrossRef]
- Tringali, C.; Geraci, C.; Nicolosi, G.; Verbist, J.-F.; Roussakis, C. An antitumor principle from Suillus granulatus. J. Nat. Prod. 1989, 52, 844–845. [Google Scholar] [CrossRef]
- Tomasi, S.; Lohézic-Le Dévéhat, F.; Sauleau, P.; Bézivin, C.; Boustie, J. Cytotoxic activity of methanol extracts from Basidiomycete mushrooms on murine cancer cell lines. Pharmazie 2004, 59, 290–294. [Google Scholar]
- Santos, T.D.; Tavares, C.; Sousa, D.; Vaz, J.A.; Calhelha, R.C.; Martins, A.; Almeida, G.M.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Suillus luteus methanolic extract inhibits cell growth and proliferation of a colon cancer cell line. Food Res. Int. 2013, 53, 476–481. [Google Scholar] [CrossRef]
- Santos, T.D.; Oliveira, M.; Sousa, D.; Lima, R.T.; Martins, A.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Suillus luteus Methanolic extract inhibits proliferation and increases expression of P-H2A.X in a non-small cell lung cancer cell line. J. Funct. Foods 2014, 6, 100–106. [Google Scholar] [CrossRef]
- León, F.; Brouard, I.; Torres, F.; Quintana, J.; Rivera, A.; Estévez, F.; Bermejo, J. A new ceramide from Suillus luteus and its cytotoxic activity against human melanoma cells. Chem. Biodivers. 2008, 5, 120–125. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential beneficial effects and pharmacological properties of ergosterol, a common bioactive compound in edible mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Department of Agriculture, Fisheries and Forestry. BICON. 2024. New Permitted Species Under the ‘Mushrooms for Human Consumption’ Case. Available online: https://bicon.agriculture.gov.au/ViewElement/Element/WhatsChangedNotice?elementPk=2205072 (accessed on 26 February 2025).
- Awang, N.A.; Ali, A.M.; Abdulrahman, M.D.; Mat, N. Edible bitter mushroom from Besut, Malaysia. J. Agrobiotech. 2018, 9, 70–79. Available online: https://journal.unisza.edu.my/agrobiotechnology/index.php/agrobiotechnology/article/download/135/153 (accessed on 18 December 2024).
- Pudil, F.; Uvíra, R.; Janda, V. Volatile Compounds in stinkhorn (Phallus impudicus L. ex Pers.) at different stages of growth. Eur. Sci. J. 2014, 10, 163–171. Available online: https://core.ac.uk/download/pdf/236408512.pdf (accessed on 20 December 2024).
- Liu, H.; Cheng, Z.; Xie, J. Formation of special odors driven by volatile compounds during the growth and maturation in edible fungi (Phallus impudicus). Food Chem. X 2024, 22, 101288. [Google Scholar] [CrossRef]
- Prakofjewa, J.; Sartori, M.; Kalle, R.; Łuczaj, Ł.; Karbarz, M.; Mattalia, G.; Šarka, P.; Prūse, B.; Stryamets, N.; Anegg, M.; et al. “But how true that is, I do not know”: The influence of written sources on the medicinal use of fungi across the western borderlands of the former Soviet Union. IMA Fungus 2024, 15, 22. Available online: https://imafungus.biomedcentral.com/articles/10.1186/s43008-024-00156-7 (accessed on 12 February 2025). [CrossRef]
- Motiejūnaitė, J.; Džekčioriūtė, V.; Kutorga, E.; Kasparavičius, J.; Iršėnaitė, R. Diversity of ethnomycological knowledge and mushroom foraging culture in a small nation: Case of Lithuania. J. Ethnobiol. Ethnomed. 2024, 20, 88. [Google Scholar] [CrossRef]
- Mendoza García, M.; Hernández Nava, R.M.; Villegas Villarreal, E.C.; Díaz Montserrat, Q.; Acosta-Urdapilleta, M.d.L.; Díaz-Godínez, G.; Téllez-Téllez, M. Effect of pH on the radial growth rate and pigment production of two strains of Pycnoporus. In Proceedings of the Conference: Biotechnology Summit 2014, Santa María Huatulco, Oaxaca, Mexico, 8–10 October 2014; Fernández-Luqueño, F., López-Valdez, F., Lozano-Muñiz, S., Eds.; Centro de Investigación y de Estudios Avanzados del I.P.N. Cinvestav: Ciudad de México, Mexico, 2014; pp. 194–198. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Díaz-Godínez, G. Mushroom Pigments and Their Applications. In Biomolecules from Natural Sources: Advances and Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 82–100. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Villegas, E.; Rodríguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Díaz-Godínez, G. Mycosphere Essay 11: Fungi of Pycnoporus: Morphological and molecular identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 7, 1500–1525. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. HPLC and NMR studies of phenoxazone alkaloids from Pycnoporus cinnabarinus. Nat. Prod. Commun. 2009, 4, 489–498. [Google Scholar] [CrossRef]
- Nemadziva, B.; Kudanga, T. Chapter 10—Laccases in organic synthesis. In Bacterial Laccases: Engineering, Immobilization, Heterologous Production, and Industrial Applications; Yadav, D., Kudanga, T., Eds.; Academic Press: London, UK; Cambridge, MA, USA; Elsevier Inc.: San Diego, CA, USA; Oxford, UK, 2024. [Google Scholar] [CrossRef]
- Fazio, F.; Lionetto, L.; Molinaro, G.; Bertrand, H.O.; Acher, F.; Ngomba, R.T.; Goudet, C. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol. Pharmacol. 2012, 81, 643–656. [Google Scholar] [CrossRef]
- Göçenoğlu, A.; Pazarlioglu, N. Cinnabarinic acid: Enhanced production from Pycnoporus cinnabarinus, characterization, structural and functional properties. Hacet. J. Biol. Chem. 2014, 42, 281–290. Available online: https://dergipark.org.tr/en/download/article-file/1727972 (accessed on 19 December 2024). [CrossRef]
- Shittu, O.B.; Alofe, F.V.; Onawunmi, G.O.; Ogundaini, A.O.; Tiwalade, T.A. Mycelial growth and antibacterial metabolite production by wild mushrooms. Afr. J. Biomed. Res. 2006, 8, 157–162. [Google Scholar] [CrossRef]
- Järvinen, P.; Nybond, S.; Marcourt, L.; Queiroz, E.F.; Wolfender, J.-L.; Mettälä, A.; Karp, M.; Vuorela, H.; Vuorela, P.; Hatakka, A.; et al. Cell-based bioreporter assay coupled to HPLC micro-fractionation in the evaluation of antimicrobial properties of the basidiomycete fungus Pycnoporus cinnabarinus. Pharm. Biol. 2016, 54, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, V.; Lougarre, A.; Ali-Ahmed, D.; Rahbé, Y.; Guillot, J.; Chavant, L.; Fournier, D.; Paquereau, L. Xerocomus chrysenteron lectin: Identification of a new pesticidal protein. Biochim. Biophys. Acta Gen. Subj. 2003, 1621, 292–298. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.-J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef]
- Martinčič, R.; Mravljak, J.; Švajger, U.; Perdih, A.; Anderluh, M.; Novič, M. In silico discovery of novel potent antioxidants on the basis of pulvinic acid and coumarine derivatives and their experimental evaluation. PLoS ONE 2015, 10, e0140602. [Google Scholar] [CrossRef]
- Orywal, K.; Socha, K.; Nowakowski, P.; Zoń, W.; Mroczko, B.; Perkowski, M. Dried wild-grown mushrooms can be considered a source of selected minerals. Nutrients 2022, 14, 2750. [Google Scholar] [CrossRef]
- Birck, C.; Damian, L.; Marty-Detraves, C.; Lougarre, A.; Schulze-Briese, C.; Koehl, P.; Fournier, D.; Paquereau, L.; Samama, J.-P. A new lectin family with structure similarity to actinoporins revealed by the crystal structure of Xerocomus chrysenteron lectin XCL. J. Mol. Biol. 2004, 344, 1409–1420. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the Eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. Int. J. Environ. Res. Public Health 2019, 16, 3614. [Google Scholar] [CrossRef]
- Šušaníková, I.; Kvasnicová, A.; Brzková, Ž.; Ďuriška, O.; Mučaji, P. New biological findings of ethanol and chloroform extracts of fungi Suillellus rubrosanguineus and Tylopilus felleus. Interdiscip. Toxicol. 2018, 11, 204–208. [Google Scholar] [CrossRef]
- Sterniša, M.; Sabotič, J.; Klančnik, A. A novel approach using growth curve analysis to distinguish between antimicrobial and anti-biofilm activities against Salmonella. Int. J. Food Microbiol. 2022, 364, 109520. [Google Scholar] [CrossRef]
- Grzybek, J.; Zgorniak-Nowosielska, I.; Kasprowicz, A.; Zawilinska, B.; Kohlmunzer, S. Antitumor activity of a fungal glucan tylopilan and Propionibacterium acnes preparation. Acta Soc. Bot. Pol. 2014, 63, 293–298. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.-S. Clinical and physiological perspectives of β-glucans: The past, present, and future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef]
- Kohlmünzer, S.; Grzybek, J.; Wegiel, J. Biological activity of polysaccharides from the mycelial culture of Tylopilus felleus (Bull.: Fr.) P. Karst. Acta Pol. Pharm. 1992, 49, 31–34. [Google Scholar] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Mleczek, P.; Goliński, P.; Gąsecka, M.; Sobieralski, K.; Dawidowicz, L.; Szymańczyk, M. Bioaccumulation of elements in three selected mushroom species from southwest Poland. J. Environ. Sci. Health B 2015, 50, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kojta, A.K.; Falandysz, J. Metallic elements (Ca, Hg, Fe, K, Mg, Mn, Na, Zn) in the fruiting bodies of Boletus badius. Food Chem. 2016, 200, 206–214. [Google Scholar] [CrossRef]
- Falandysz, J.; Zhang, J.; Wang, Y.Z.; Saba, M.; Krasińska, G.; Wiejak, A.; Li, T. Evaluation of mercury contamination in fungi Boletus species from Latosols, Lateritic Red Earths, and Red and Yellow Earths in the Circum-Pacific Mercuriferous Belt of Southwestern China. PLoS ONE 2015, 10, e0143608. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, T.; Krasińska, G.; Apanel, A.; Wang, Y.; Pankavec, S. Evaluation of the radioactive contamination in fungi genus Boletus in the region of Europe and Yunnan Province in China. Appl. Microbiol. Biotechnol. 2015, 99, 8217–8224. [Google Scholar] [CrossRef][Green Version]
- Harkut, O.; Alexa, P.; Uhlář, R. Radiocaesium contamination of mushrooms at high- and low-level Chernobyl exposure sites and its consequences for public health. Life 2021, 11, 1370. [Google Scholar] [CrossRef]
- Strumińska-Parulska, D.; Falandysz, J. A review of the occurrence of alpha-emitting radionuclides in wild mushrooms. Int. J. Environ. Res. Public Health 2020, 17, 8220. [Google Scholar] [CrossRef] [PubMed]
- Andronikov, A.V.; Andronikova, I.E.; Sebek, O.; Martinkova, E.; Stepanova, M. Accumulation and within-mushroom distribution of elements in red cracking bolete (Xerocomellus chrysenteron) collected over the extended period from compositionally contrasting substrates. Envrion. Monit. Assess. 2023, 195, 1157. [Google Scholar] [CrossRef]
- Šíma, J.; Vondruška, J.; Svoboda, L.; Šeda, M.; Rokos, L. The accumulation of risk and essential elements in edible mushrooms Chlorophyllum rhacodes, Suillus grevillei, Imleria badia, and Xerocomellus chrysenteron growing in the Czech Republic. Chem. Biodivers. 2019, 16, e1800478. [Google Scholar] [CrossRef] [PubMed]
- Dryżałowska, A.; Falandysz, J. Bioconcentration of mercury by mushroom Xerocomus chrysenteron from the spatially distinct locations: Levels, possible intake and safety. Ecotoxicol. Environ. Saf. 2014, 107, 97–102. [Google Scholar] [CrossRef]
- Demková, L.; Árvay, J.; Hauptvogl, M.; Michalková, J.; Šnirc, M.; Harangozo, Ľ.; Bobuľská, L.; Bajčan, D.; Kunca, V. Mercury content in three edible wild-growing mushroom species from different environmentally loaded areas in Slovakia: An ecological and human health risk assessment. J. Fungi 2021, 7, 434. [Google Scholar] [CrossRef]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chem. 2022, 367, 130670. [Google Scholar] [CrossRef] [PubMed]
- Solek, P.; Shemedyuk, N.; Gzhytskyj, S.; Shemedyuk, A.; Dudzinska, E.; Koziorowski, M. Risk of wild fungi treatment failure: Phallus impudicus-induced telomere damage triggers p21/p53 and p16-dependent cell cycle arrest and may contribute to male fertility reduction in vitro. Ecotoxicol. Environ. Saf. 2021, 209, 111782. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 334. [Google Scholar] [CrossRef]
- Buko, V.; Bakunovich, A.; Astrowski, A.; Moroz, V.; Puchkova, T.; Kastsianevich, A.; Tomulewicz, M. Polysaccharides of mushroom Phallus impudicus Mycelium: Immunomodulating and wound healing properties. Mod. Food Sci. Technol. 2019, 35, 30–37. [Google Scholar] [CrossRef]
- Khan, A.R.; Fiaz, M.; Khan, R.A.; Wahab, Z.; Khan, J.B.; Khan, I.; Jan-Khan, M.W. Anti-oxidant and hypoglyceamic potential of Phallus impudicus (l. ex pers) (stink horn) mushroom in alloxan induced diabetic rats. J. Crit. Rev. 2020, 7, 4180–4185. Available online: https://www.researchgate.net/publication/347881166 (accessed on 21 March 2025).
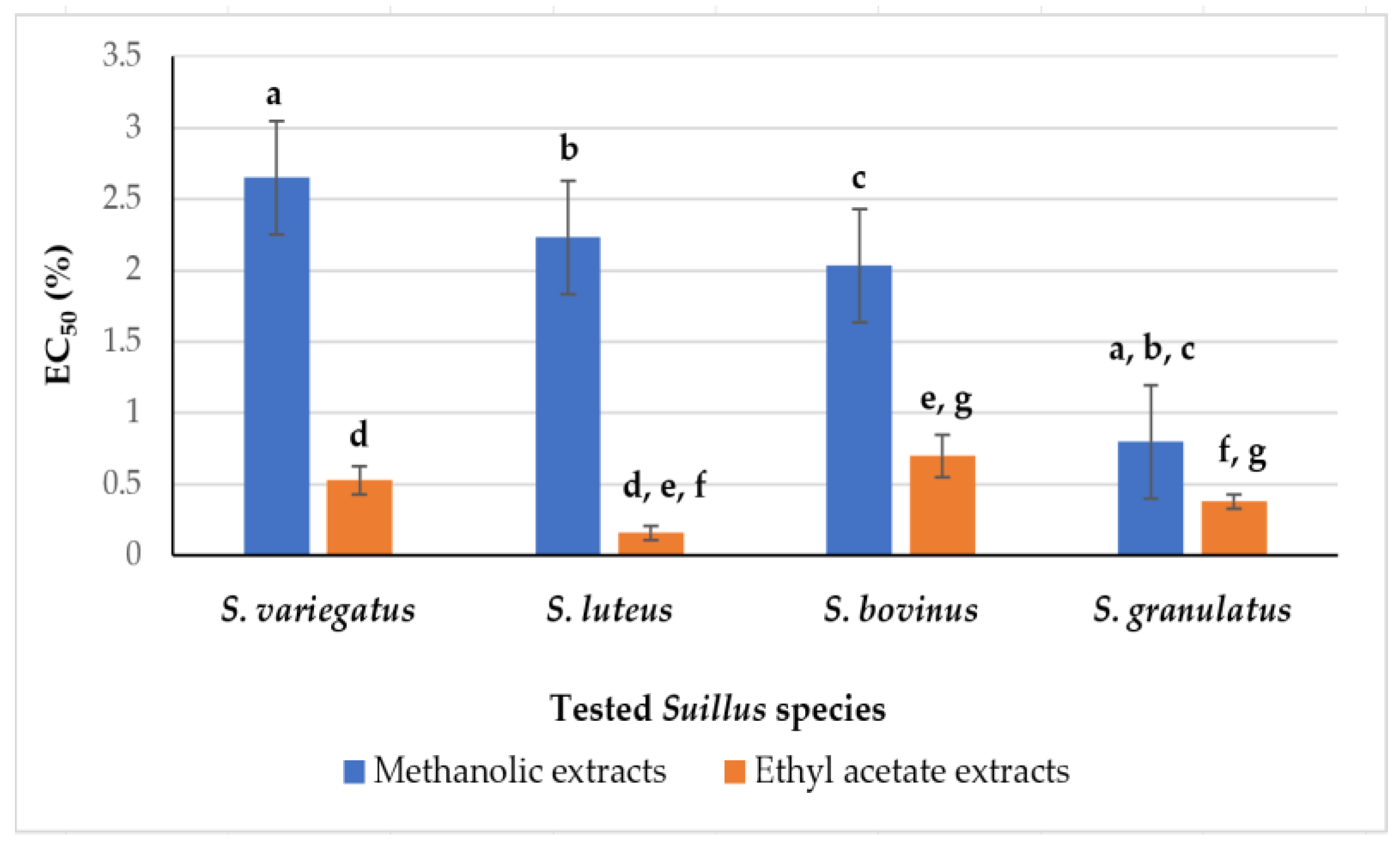

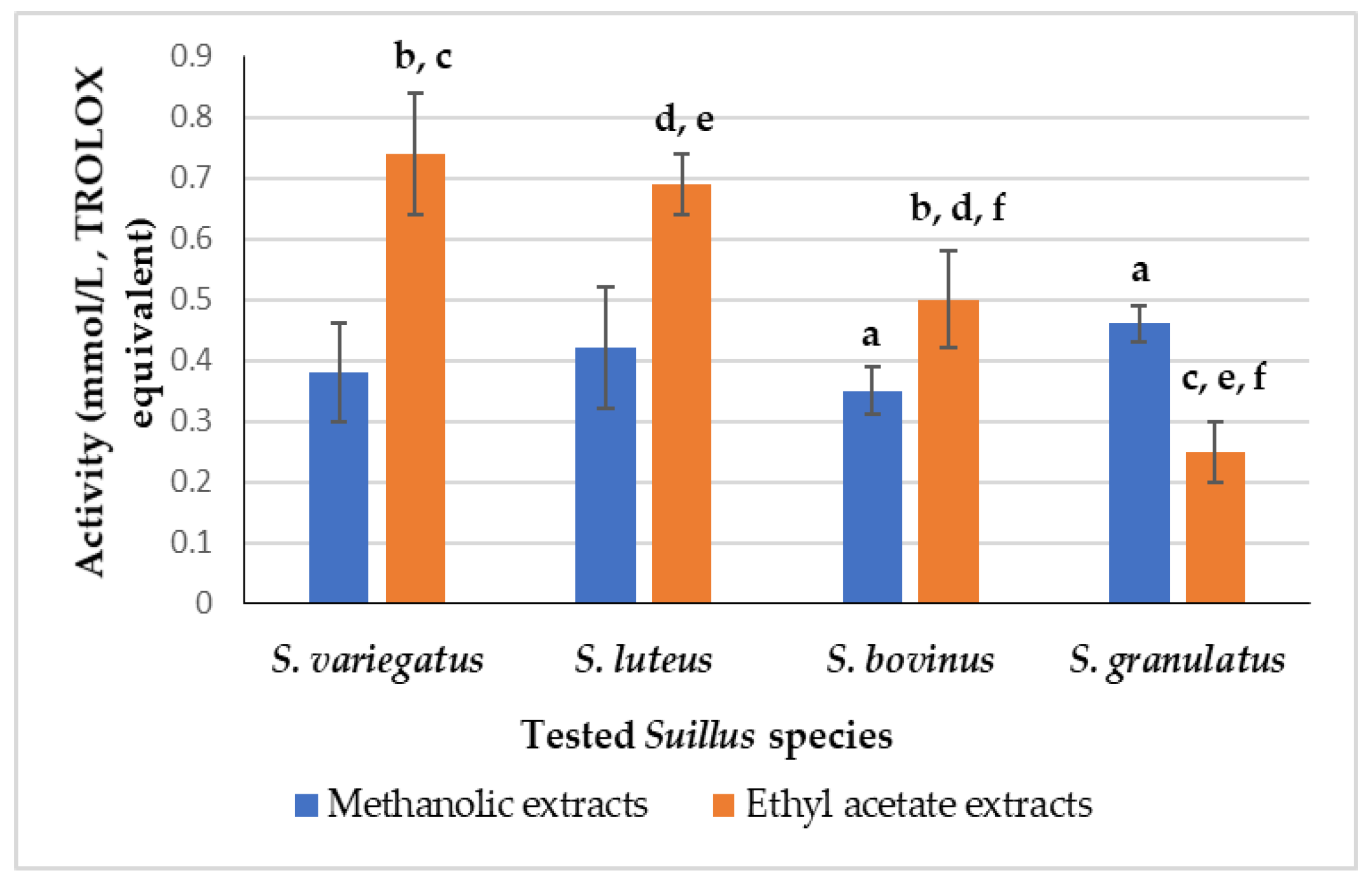
| Suillus Species | Methanol/Water | Ethyl Acetate |
|---|---|---|
| S. variegatus | 340.11 ± 8.98 | 310.03 ± 9.68 |
| S. luteus | 393.47 ± 9.83 | 104.90 ± 3.55 |
| S. bovinus | 248.32 ± 5.45 | 178.22 ± 4.12 |
| S. granulatus | 580.77 ± 13.10 | 250.15 ± 6.34 |
| Extracts and Standards | Tested Bacteria and Fungi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | B. mycoides | B. cereus | P. aeruginosa | E. coli | P. vulgaris | C. albicans | Rh. rubra | M. guill. | A. niger | ||
| S. variegatus | a | 0 (C) | 40.6 ± 4.4 | 0 | 10.3 ± 0.4 | 18.2 ± 3.1 | C | 12.1 ± 0.3 | 12.5 ± 1.3 | 11.7 ± 2.3 | 10.5 ± 0.2 | 0 |
| b | 0 | 18.1 ± 2.7 | 0 | 10.4 ± 0.2 | 13.6 ± 2.4 | 0 | 12.1± 0.5 | 11.6 ± 1.3 | 10.7 ± 1.3 | C | 0 | |
| S. luteus | a | 15.2 ± 3.7 | 0 | 30.2 ± 1.8 | 18.0 ± 1.4 | 15.6 ± 1.4 | 13.1 ± 1.0 | 14.6 ± 1.1 | 16.1 ± 0.9 | 12.5 ± 0.6 | C | 0 |
| b | C | 0 | 25.4 ± 2.2 | 12.3 ± 0.7 | 14.6 ± 1.4 | 12.3 ± 0.7 | 12.0 ± 0.6 | 13.3 ± 2.2 | 12.5 ± 0.5 | C | 0 | |
| S. bovinus | a | 0 | 0 (C) | 0 | 11.4 ± 0.3 | 15.7± 2.3 | 22.3 ± 3.6 | 17.7± 2.5 | 16.3 ± 2.8 | 12.5 ± 0.5 | 14.3 ± 2.5 | 0 |
| b | 0 | 0 | 0 | 10.5 ± 0.6 | 13.1 ± 0.9 | 16.1 ± 1.4 | 13.4 ± 1.7 | 12.0 ± 1.1 | 11.9 ± 1.0 | 11.0 ± 0.4 | 0 | |
| S. granulatus | a | 24.7 ± 3.3 | 24.4 ± 2.4 | 28.5 ± 3.3 | 18.3 ± 1.8 | 11.7 ± 1.3 | 18.4 ± 0.6 | 16.1 ± 0.4 | 13.3 ± 1.4 | C | 13.6 ± 0.4 | 0 |
| b | 14.3 ± 1.7 | 9.3 ± 1.4 | 23.0 ± 0.4 | 14.9 ± 0.7 | 9.9 ± 0.3 | 10.0 ± 1.0 | 12.6 ± 0.5 | 12.1 ± 0.6 | C | 12.1 ± 0.6 | 0 | |
| Chloramphenicol | 24.5 ± 0.5 | 14.8 ± 1.2 | 28.2 ± 3.2 | 26.4 ± 1.2 | 20.2 ± 1.8 | 13.6 ± 1.4 | 32.5 ± 1.2 | |||||
| Tetracycline | 16.4 ± 15.2 | 11.8 ± 1.8 | 20.0 ± 2.2 | 19.6 ± 0.7 | 17.7 ± 2.2 | 17.7± 2.4 | 27.6 ± 1.7 | |||||
| Nystatin | 25.0 ± 2.8 | 16.7 ± 2.0 | 24.4 ± 3.2 | 31.1 ± 2.0 | ||||||||
| Extract | MRC-5 | T. cruzi | T. brucei | L. infantum | P. falciparum | C. albicans | S. aureus | E. coli |
|---|---|---|---|---|---|---|---|---|
| S. variegatus | >128 | >128 | >128 | >128 | 12.70 | >128 | >128 | >128 |
| S. luteus | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| S. bovinus | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| S. granulatus | 64.45 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| X. badius | >128 | 71.01 | >128 | >128 | >128 | >128 | >128 | >128 |
| X. chrysenteron | 64.45 | 17.59 | 16.92 | >128 | >128 | >128 | 84.94 | >128 |
| X. subtomentosus | >128 | 72.33 | >128 | >128 | >128 | >128 | >128 | >128 |
| T. felleus | 64.45 | 5.54 | >128 | >128 | >128 | >128 | >128 | >128 |
| P. impudicus | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| P. cinnabarinus | 13.05 | 3.93 | 1.32 | 21.53 | 1.46 | >128 | 69.71 | >128 |
| Tamoxifen | 10.5 | |||||||
| Benznidazole | 2.65 | |||||||
| Suramin | 0.03 | |||||||
| Miltefosine | 3.3 | |||||||
| Chloroquine | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judžentienė, A.; Šarlauskas, J. Comparative Research of Antioxidant, Antimicrobial, Antiprotozoal and Cytotoxic Activities of Edible Suillus sp. Fruiting Body Extracts. Foods 2025, 14, 1130. https://doi.org/10.3390/foods14071130
Judžentienė A, Šarlauskas J. Comparative Research of Antioxidant, Antimicrobial, Antiprotozoal and Cytotoxic Activities of Edible Suillus sp. Fruiting Body Extracts. Foods. 2025; 14(7):1130. https://doi.org/10.3390/foods14071130
Chicago/Turabian StyleJudžentienė, Asta, and Jonas Šarlauskas. 2025. "Comparative Research of Antioxidant, Antimicrobial, Antiprotozoal and Cytotoxic Activities of Edible Suillus sp. Fruiting Body Extracts" Foods 14, no. 7: 1130. https://doi.org/10.3390/foods14071130
APA StyleJudžentienė, A., & Šarlauskas, J. (2025). Comparative Research of Antioxidant, Antimicrobial, Antiprotozoal and Cytotoxic Activities of Edible Suillus sp. Fruiting Body Extracts. Foods, 14(7), 1130. https://doi.org/10.3390/foods14071130






