Study on the Effects of Tannase on the De Astringency of Pomegranate Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
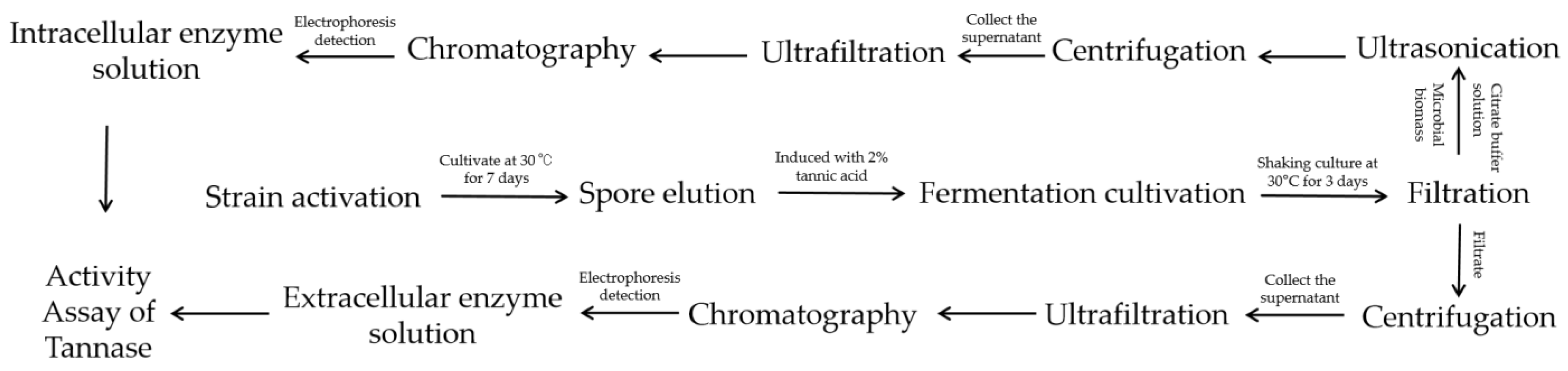
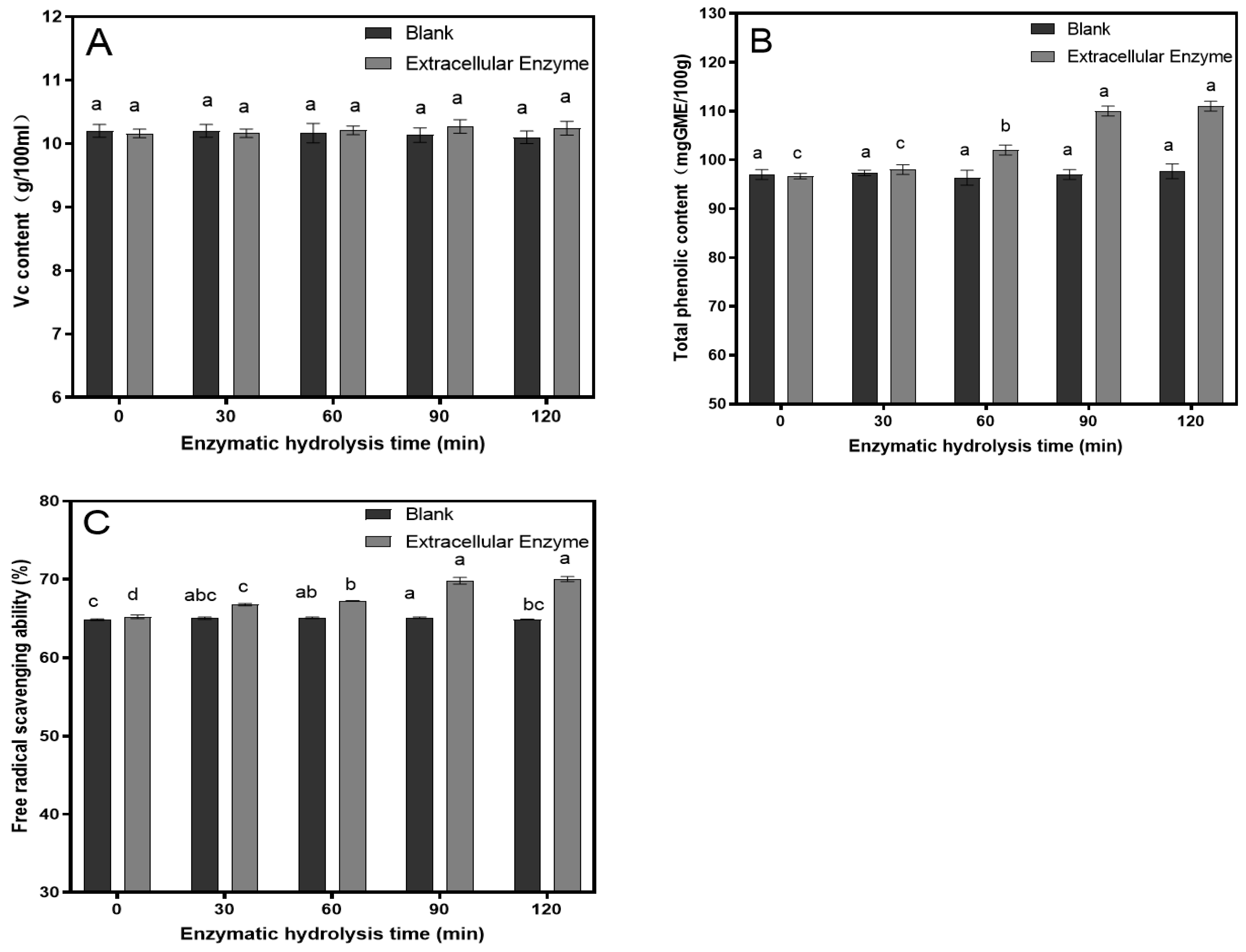
2.2. Production and Activity Assay of Tannase
2.3. Preparation Process of Pomegranate Juice
2.4. UPLC Detection of Punicalagin, Ellagic Acid, and Gallic Acid
2.5. Optimization of the Pectinase Clarification Process for Pomegranate Juice Using Orthogonal Experimentation
2.6. Rational Antioxidant Activity Index
2.7. Sensory Evaluation
3. Results and Discussion
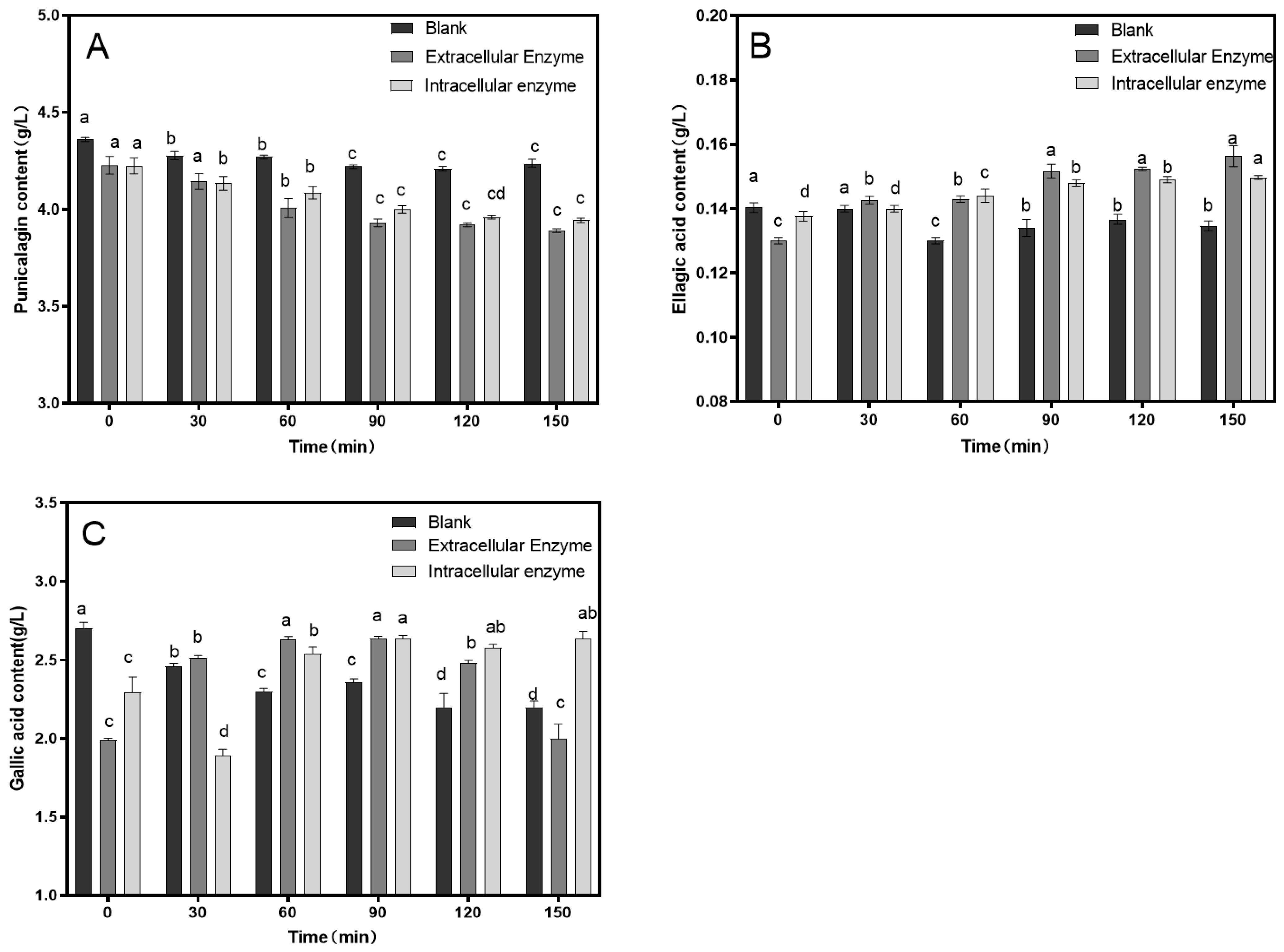
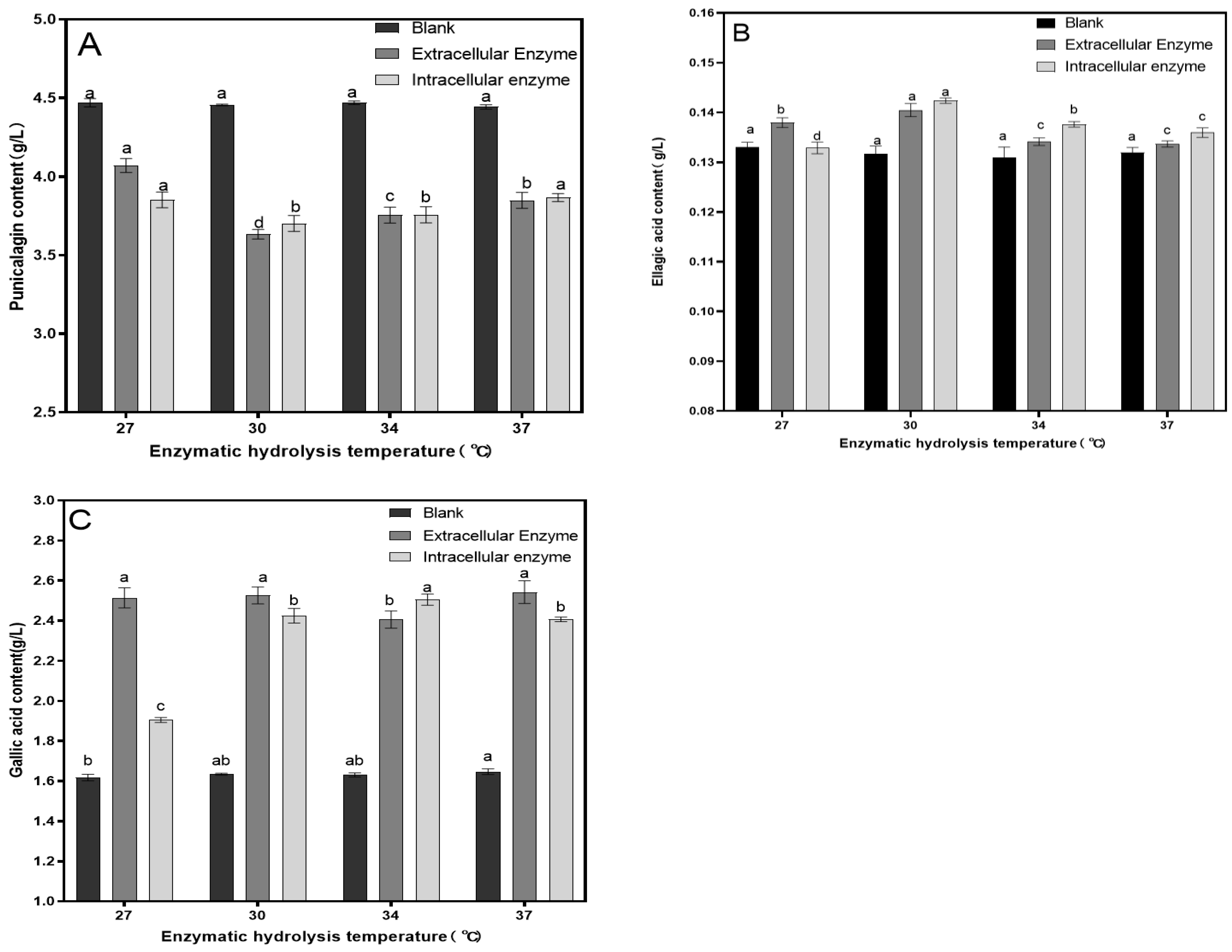
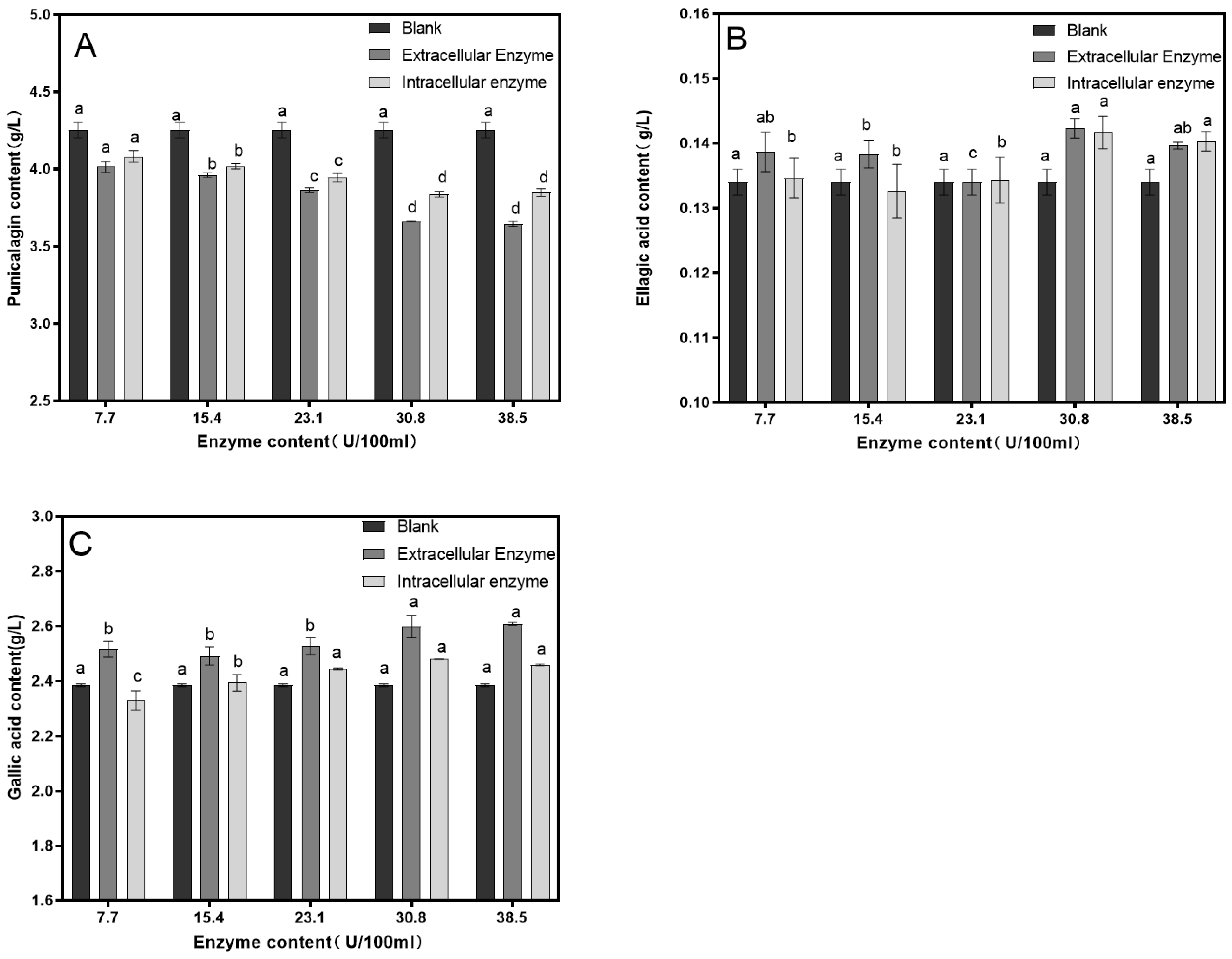
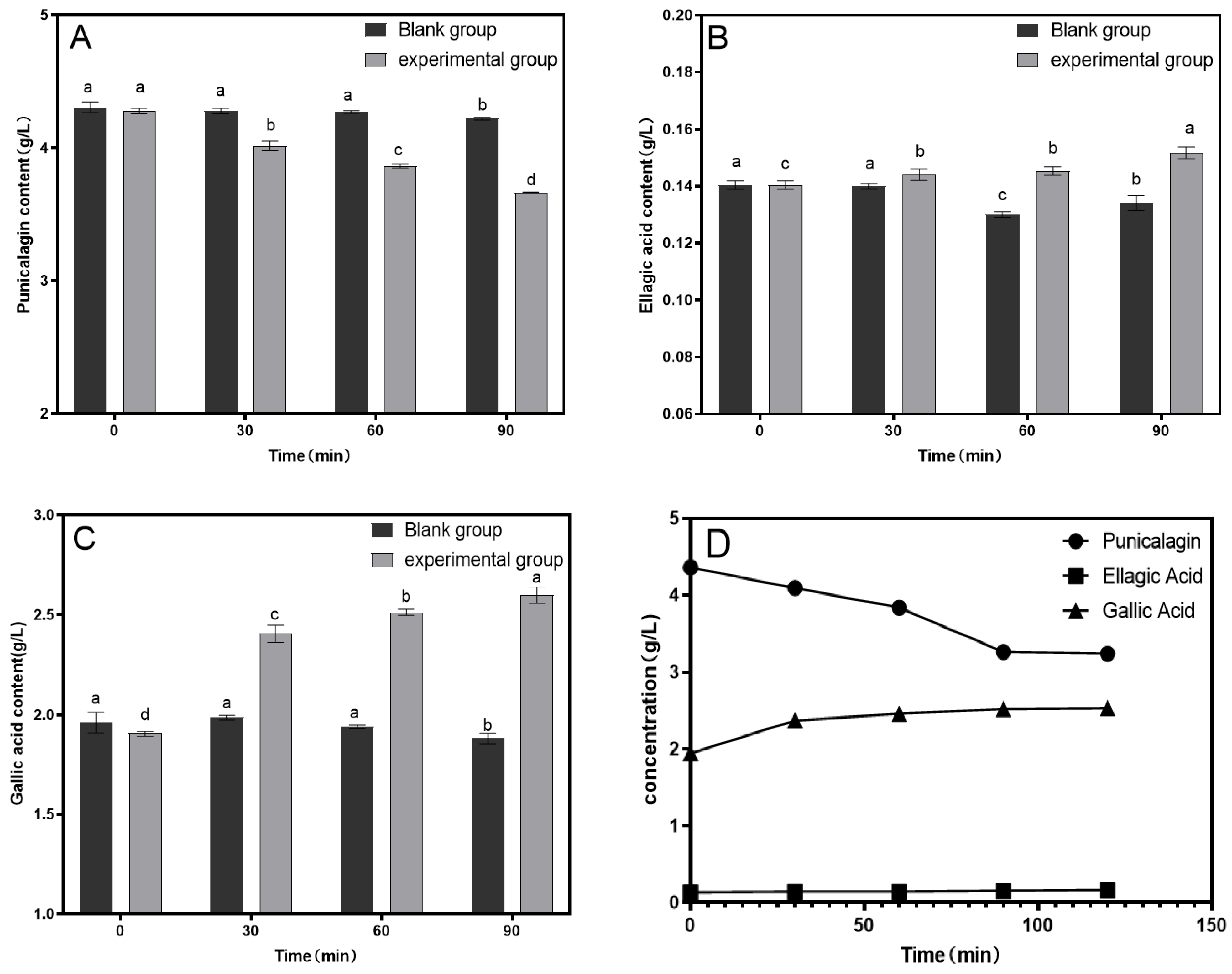
3.1. Single-Factor Experiment
3.2. Orthogonal Experimental
3.3. The Results of the Antioxidant Capacity Measurements
3.4. Results of Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escarcega, G.H.; Sánchez-Chávez, E.; Álvarez, S.P.; Caballero, M.S.; Parra, J.M.S.; Flores-Córdova, M.A.; Salazar, N.A.S.; Barrios, D.L.O. Determination of antioxidant phenolic, nutritional quality and volatiles in pomegranates (Punica granatum L.) cultivated in Mexico. Int. J. Food Prop. 2020, 23, 979–991. [Google Scholar] [CrossRef]
- Roy, B.; Singh, J.; Dewan, T. A comparative study of antioxidant activity and total phenolic content of fresh juices of some common Indian fruits with their commercial counterparts. Curr. Sci. 2020, 118, 300–304. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Miguel, M.G.; Neves, M.A.; Marreiros, A. Comparative Study of Some Physicochemical and in vitro Biological Properties of Juices of Four Pomegranate Cultivars. J. Agric. Sci. Technol. 2023, 25, 1341–1355. [Google Scholar]
- Masuda, M.; Horinaka, M.; Yasuda, S.; Morita, M.; Nishimoto, E.; Ishikawa, H.; Mutoh, M.; Sakai, T. Discovery of cancer-preventive juices reactivating RB functions. Environ. Health Prev. Med. 2023, 28, 54. [Google Scholar] [CrossRef]
- Faria, G.M.L.; Silva, E.K. Pulsed electric field, ultrasound and microwave heating based extraction techniques for valorization of pomegranate peel by-products: A review. J. Environ. Chem. Eng. 2024, 12, 113078. [Google Scholar] [CrossRef]
- Bett-Garber, K.L.; Watson, M.A.; Lea, J.M.; Bai, J.H.; Baldwin, E.; Raithore, S. Efficacy of monitoring the sensory taste characteristics in pomegranate juice with electronic tongue and chemical measurements. J. Food Qual. 2014, 37, 383–394. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Wang, X.; Tian, Y.; Lin, J.; Yu, D. Technical study of removing tannins from Rosa roxburghii juice using ginger protein. Guihaia 2024, 44, 1052–1059. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y. Removal of Tannins in Production of Pomegranate Juice. Food Sci. 2009, 30, 135–138. [Google Scholar]
- Corrales-Maldonado, C.G.; Vargas-Arispuro, I.; Martínez-Carrillo, J.L.; Pérez-Morales, R.; Martínez-Téllez, M.Á.; Aispuro-Hernández, E.; Arellano-Gil, M.; Castro-Espinoza, L. The gut bacteria symbionts from the monophagous insect Acrobasis nuxvorella produce tannase for the digestion of Carya illinoinensis tannins. J. Asia-Pac. Entomol. 2022, 25, 102005. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, X.Y.; He, L.P.; Qiu, Y.X.; Zhu, H.W.; Cao, Y. Novel Trends for Use of Microbial Tannases. Prep. Biochem. Biotechnol. 2015, 45, 221–232. [Google Scholar] [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of phytochemical constituents, antibacterial, antioxidant, photoprotective activities and cytotoxic effect of leaves extracts and fractions of Aloe vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Boussekine, S.; Kadi, K.; Martín-García, A.I.; Kheddouma, A.; Nagaz, K.; Bensouici, C. A comparative study on chemical profile and biological activities of aerial parts (stems, flowers, leaves, pods and seeds) of Astragalus gombiformis. Biocatal. Agric. Biotechnol. 2020, 27, 101668. [Google Scholar] [CrossRef]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M.; et al. Ultrasound-Assisted Extraction, LC–MS/MS analysis, anticholinesterase, and antioxidant activities of valuable natural metabolites from Astragalus armatus Willd.: In silico molecular docking and In vitro enzymatic studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical constituents of Astragalus monspessulanus and integrative analysis for its antioxidant, photoprotective, and antityrosinase activities: Experimental and computational investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Fayez, S.; Zengin, G.; Selvi, S.; Uba, A.I.; Mollica, A.; Bouyahya, A.; Ponniya, S.K.M.; Nilofar; Lekmine, S.; et al. Chemical exploration of different extracts from Phytolacca americana leaves and their potential utilization for global health problems: In silico and network pharmacology validation. J. Biomol. Struct. Dyn. 2024, 42, 1–21. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Triki, Z.; Fergani, Z.; Lekmine, S.; Tahraoui, H.; Amrane, A.; Zamouche, M.; Kebir, M.; Assadi, A.A.; Khezami, L.; Zhang, J. Numerical Modelling and Performance Evaluation of Vacuum Membrane Distillation for Energy-Efficient Seawater Desalination: Towards Energy-Efficient Solutions. Water 2023, 15, 3612. [Google Scholar] [CrossRef]
- Boussekine, S.; Lekmine, S.; Gasmi, S.; Benkhedir, A.; Saker, H.; Lidoughi, A. The protective effect of selenium on diabetic nephropathy in wistar rats. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Brik, A.; Djeffali, O.; Ounissi, M.; Slimani, M.; Ola, M.S.; Eldahshan, O.A.; Martín-García, A.I. Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants 2024, 13, 654. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Lekmine, S.; Mameri, A.; Tahraoui, H.; Hamid, S.; Benzitoune, N.; Moula, N.; Zhang, J.; Amrane, A. Optimization of ultrasound-assisted extraction of bioactive compounds from Carthamus caeruleus L. rhizome: Integrating central composite design, Gaussian process regression, and multi-objective Grey Wolf optimization approaches. Process Biochem. 2024, 147, 476–488. [Google Scholar] [CrossRef]
- Moussa, H.; Hamid, S.; Mameri, A.; Lekmine, S.; Tahraoui, H.; Kebir, M.; Touzout, N.; Dahmoune, F.; Ola, M.S.; Zhang, J. From Green Chemistry to Healthy Environments: Silver Nanoparticles as a Dual Antioxidant and Antibacterial Agents for Advancing Biomedicine and Sustainable Wastewater Treatment. Bioengineering 2024, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Peña-Lucio, E.M.; Chávez-González, M.L.; Londoño-Hernandez, L.; Ruiz, H.A.; Martínez-Hernandez, J.L.; Govea-Salas, M.; Sukumaran, P.N.; Abdulhameed, S.; Aguilar, C.N. Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions. Fermented 2023, 9, 781. [Google Scholar] [CrossRef]
- Mostafa, H.S. Potato peels for tannase production from Penicillium commune HS2, a high tannin-tolerant strain, and its optimization using response surface methodology. Biomass Convers. Biorefiner 2022, 13, 16765–16778. [Google Scholar] [CrossRef]
- Dong, X.; Wu, X.; Zhang, Y.; Wu, X.; Wan, Y.; Liu, C.; Fu, G. Breeding of tannase-producing Aspergillus carbonarius using ARTP mutagenesis and fermentation optimization. Food Ferment. Ind. 2021, 47, 15–21. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Khanongnuch, C.; Lumyong, S.; Haltrich, D.; Nguyen, T.H.; Kittibunchakul, S. Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolus ruineniae A45.2. Microb. Cell Factories 2020, 19, 95. [Google Scholar] [CrossRef]
- Wang, W. Study on the Preparation of Tannic Acid from Pomegranate Peel by Enzymatic Hydrolysis. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2022. [Google Scholar]
- Dhiman, S.; Mukherjee, G.; Singh, A.K. Recent trends and advancements in microbial tannase-catalyzed biotransformation of tannins: A review. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2018, 21, 175–195. [Google Scholar] [CrossRef]
- Jana, A.; Halder, S.K.; Banerjee, A.; Paul, T.; Pati, B.R.; Mondal, K.C.; Das Mohapatra, P.K. Biosynthesis, structural architecture and biotechnological potential of bacterial tannase: A molecular advancement. Bioresour. Technol. 2014, 157, 327–340. [Google Scholar] [CrossRef]
- Djilali, K.; Maachi, R.; Danish, M.; Lekmine, S.; Hadjadj, M.; Ait Mesbah, Z.; Benslama, O.; Tahraoui, H.; Ola, M.S.; Ali, A.A. Novel Mobile Phase for Green Chromatographic Determination of Haloperidol: Application to Commercial Pharmaceutical Products and Forced Degradation Studies. Processes 2025, 13, 260. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Bensalah, B.; Touzout, N.; Moussa, H.; Tahraoui, H.; Ola, M.S.; Hafsa, H.; Zhang, J.; Amrane, A. Bioactive Phenolics of Hyoscyamus muticus L. subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities. Int. J. Mol. Sci. 2025, 26, 370. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Ola, M.S.; Touzout, N.; Moussa, H.; Hafsa, H.; Zhang, J.; Amrane, A. Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis. Metabolites 2025, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Maity, C.; Halder, S.K.; Mondal, K.C.; Pati, B.R.; Das Mohapatra, P.K. Enhanced tannase production by Bacillus subtilis PAB2 with concomitant antioxidant production. Biocatal. Agric. Biotechnol. 2013, 2, 363–371. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Belete, A.; Yisak, H.; Chandravanshi, B.S.; Yaya, E.E. Ascorbic acid content and the antioxidant activity of common fruits commercially available in addis ababa, ethiopia. Bull. Chem. Soc. Ethiop. 2023, 37, 277–288. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Gironi, G.; Servili, M. Chemical Composition, Antioxidant Activity, and Sensory Characterization of Commercial Pomegranate Juices. Antioxidant 2021, 10, 1381. [Google Scholar] [CrossRef]
- Savikin, K.; Zdunic, G.; Jankovic, T.; Tasic, S.; Menkovic, N.; Stevic, T.; Dordevic, B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009, 64, 212–217. [Google Scholar] [CrossRef]
- Ren, G.; Yang, D. Antioxidant activity of different extracts of lotus (Nelumbo nuficera Gaertn) rhizome by gradient solvent extraction. Food Sci. Technol. 2022, 42, e03821. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Yu, G.; Pu, J.; Tian, K.; Tang, X.; Du, Y.; Wu, H.; Hu, J.; Luo, X.; et al. Comparative analysis of the phenolic contents and antioxidant activities of different parts of two pomegranate (Punica granatum L.) Cultivars: Tunisia and Qingpi. Front. Plant Sci. 2023, 14, 1265018. [Google Scholar] [CrossRef]
- Rababah, T.; Al-U’datt, M.; Gammoh, S.; Khatatbeh, T.; Magableh, G.; Almajwal, A.; Yücel, S.; Al-Rayyan, N. Study of the Physiochemical and Nutraceutical Properties of Sour and Sweet Pomegranate Juice in Northern Jordan. J. Food Qual. 2023, 2023, 5426321. [Google Scholar] [CrossRef]
| Level | Factor | ||
|---|---|---|---|
| A Time/(min) | B Temperature/°C | C Enzyme Dosage/(U: 100 mL) | |
| 1 | 75 | 29 | 27.7 |
| 2 | 90 | 30 | 30.8 |
| 3 | 105 | 31 | 33.9 |
| Project | Scoring Criteria | Full Marks |
|---|---|---|
| Color | Aurantiacus, Uniformly distributed | 10 |
| Taste | Moderately sweet and sour, smooth texture | 10 |
| Flavor | Mild fruit and vegetable aroma | 10 |
| Acceptability | Degree of liking | 10 |
| Experiment Number | Factor | Punicalagin Concentration (g/L) | Ellagic Acid Concentration (g/L) | Gallic Acid Concentration (g/L) | |||
|---|---|---|---|---|---|---|---|
| A Time/min | B Temperature/℃ | C Enzyme Dosage/ (mL: 100 mL) | D Blank | ||||
| 1 | 1 | 1 | 1 | 1 | 3.927 | 0.130 | 2.357 |
| 2 | 1 | 2 | 2 | 2 | 3.808 | 0.132 | 2.362 |
| 3 | 1 | 3 | 3 | 3 | 3.805 | 0.137 | 2.576 |
| 4 | 2 | 1 | 2 | 3 | 3.704 | 0.142 | 2.382 |
| 5 | 2 | 2 | 3 | 1 | 3.346 | 0.143 | 2.346 |
| 6 | 2 | 3 | 1 | 2 | 3.854 | 0.131 | 1.896 |
| 7 | 3 | 1 | 3 | 2 | 3.660 | 0.139 | 2.478 |
| 8 | 3 | 2 | 1 | 3 | 3.566 | 0.137 | 2.484 |
| 9 | 3 | 3 | 2 | 1 | 3.610 | 0.133 | 2.446 |
| K1j | 3.847 | 3.764 | 3.782 | ||||
| K2j | 3.635 | 3.573 | 3.707 | ||||
| K3j | 3.612 | 3.756 | 3.604 | ||||
| R | 0.235 | 0.191 | 0.178 | ||||
| k1j | 0.132 | 0.137 | 0.132 | ||||
| k2j | 0.139 | 0.138 | 0.135 | ||||
| k3j | 0.136 | 0.134 | 0.140 | ||||
| R | 0.007 | 0.004 | 0.008 | ||||
| k’1j | 2.432 | 2.406 | 2.246 | ||||
| k’2j | 2.208 | 2.397 | 2.396 | ||||
| k’3j | 2.469 | 2.306 | 2.467 | ||||
| R | 0.261 | 0.100 | 0.221 | ||||
| Time/min | 0 | 30 | 60 | 90 | 120 | |
|---|---|---|---|---|---|---|
| Project | ||||||
| Color | Bright (7.24 ± 0.6) | Bright (7.20 ± 0.4) | Bright and uniform (7.12 ± 0.3) | Bright and uniform (7.02 ± 0.3) | Bright but slightly deep (6.80 ± 0.4) | |
| Taste | Distinct bitterness (5.40 ± 0.3) | Distinct bitterness (6.13 ± 0.4) | Medium bitterness (7.48 ± 0.5) | Mild astringency (8.53 ± 0.2) | Mild astringency (8.60 ± 0.5) | |
| Flavor | Marked characteristic flavor (7.79 ± 0.4) | Marked characteristic flavor (7.20 ± 0.3) | Marked characteristic flavor (6.87 ± 0.3) | Slightly reduced characteristic flavor 6.80 ± 0.4) | Diminished distinctive flavor 6.58 ± 0.2) | |
| Acceptability | Unacceptable (4.48 ± 0.2) | Slightly acceptable (5.24 ± 0.4) | Moderately acceptable (6.93 ± 0.6) | Acceptable (7.92 ± 0.3) | Acceptable (7.94 ± 0.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, L.; Wang, H.; Chen, Z.; Li, X.; Ji, Y.; Yu, J.; Song, P. Study on the Effects of Tannase on the De Astringency of Pomegranate Juice. Foods 2025, 14, 985. https://doi.org/10.3390/foods14060985
Zhu G, Wang L, Wang H, Chen Z, Li X, Ji Y, Yu J, Song P. Study on the Effects of Tannase on the De Astringency of Pomegranate Juice. Foods. 2025; 14(6):985. https://doi.org/10.3390/foods14060985
Chicago/Turabian StyleZhu, Guida, Longwen Wang, Han Wang, Zihan Chen, Xue Li, Yi Ji, Jing Yu, and Ping Song. 2025. "Study on the Effects of Tannase on the De Astringency of Pomegranate Juice" Foods 14, no. 6: 985. https://doi.org/10.3390/foods14060985
APA StyleZhu, G., Wang, L., Wang, H., Chen, Z., Li, X., Ji, Y., Yu, J., & Song, P. (2025). Study on the Effects of Tannase on the De Astringency of Pomegranate Juice. Foods, 14(6), 985. https://doi.org/10.3390/foods14060985







