Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Separation of PLs
2.3. Lips Preparation
2.4. Determination of Particle Size and Zeta Potential of Lips
2.5. Measurement of Encapsulation Efficiency (EE)
2.6. Scanning Electron Microscopy (SEM)
2.7. Lips Membrane Property Studies
2.7.1. Micropolarity
2.7.2. Measurement of Membrane Fluidity
2.8. FTIR Spectroscopy
2.9. Differential Scanning Calorimetry (DSC)
2.10. Stability Analysis
2.10.1. Storage Stability
2.10.2. Photostability
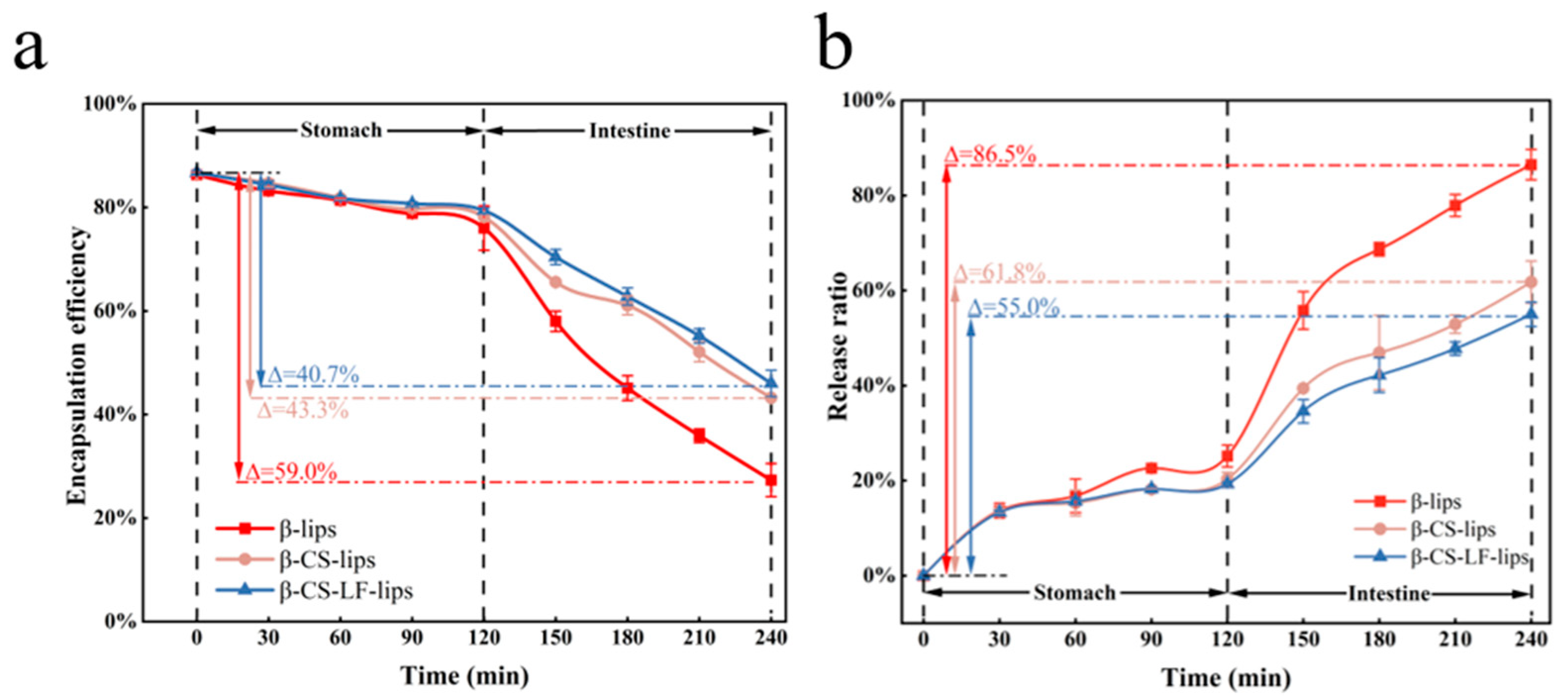
2.10.3. In Vitro Digestion Stability
Simulated Gastric Digestion
Simulation of Small Intestine Digestion
2.11. Cell Experiments
2.12. Cell Viability Assay
2.13. Measurement of Inflammatory Factor Levels
2.14. Statistical Analysis
3. Results and Discussion
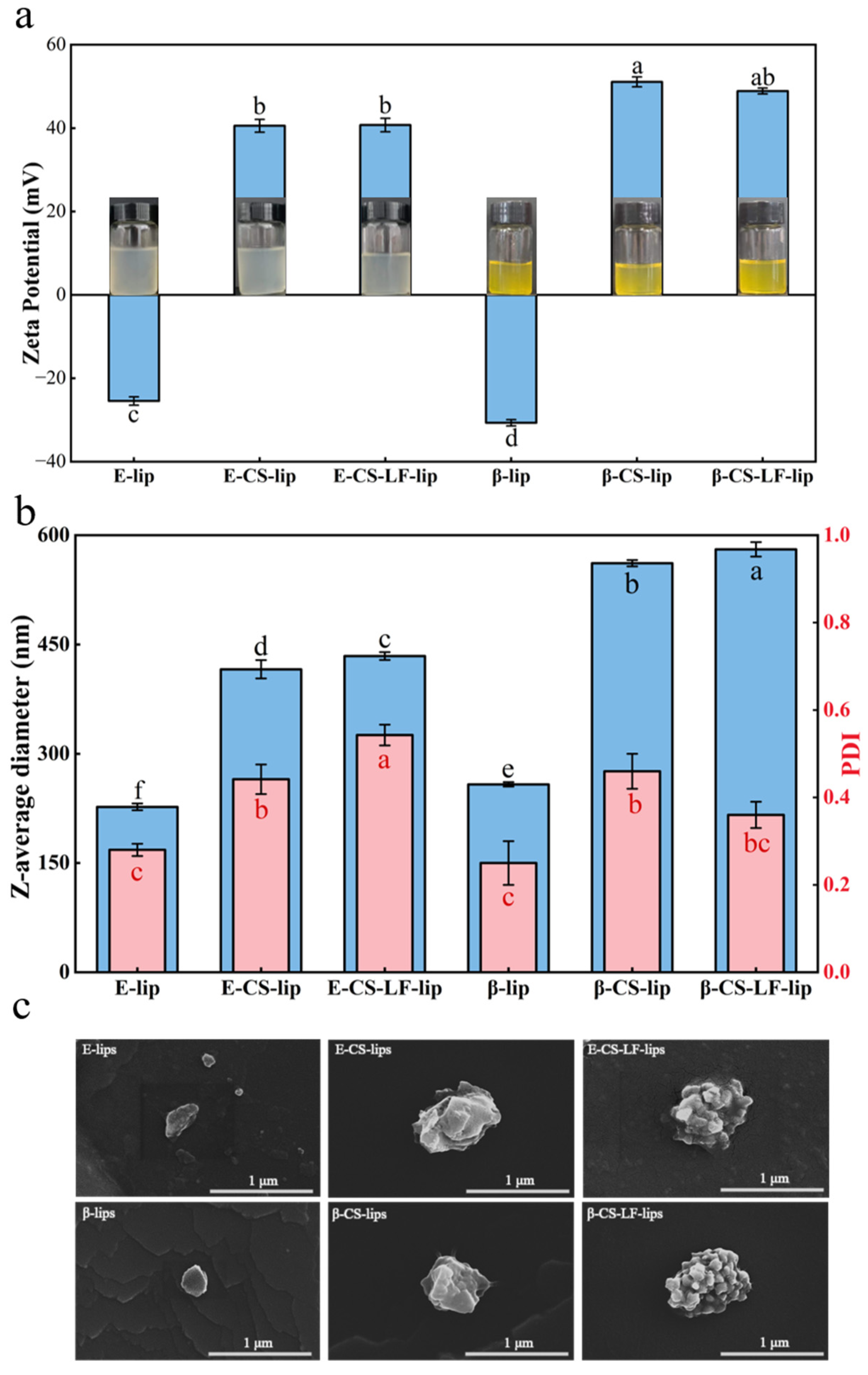
3.1. Optimization of Lips Formulation
3.2. Microstructural Observation of Lips
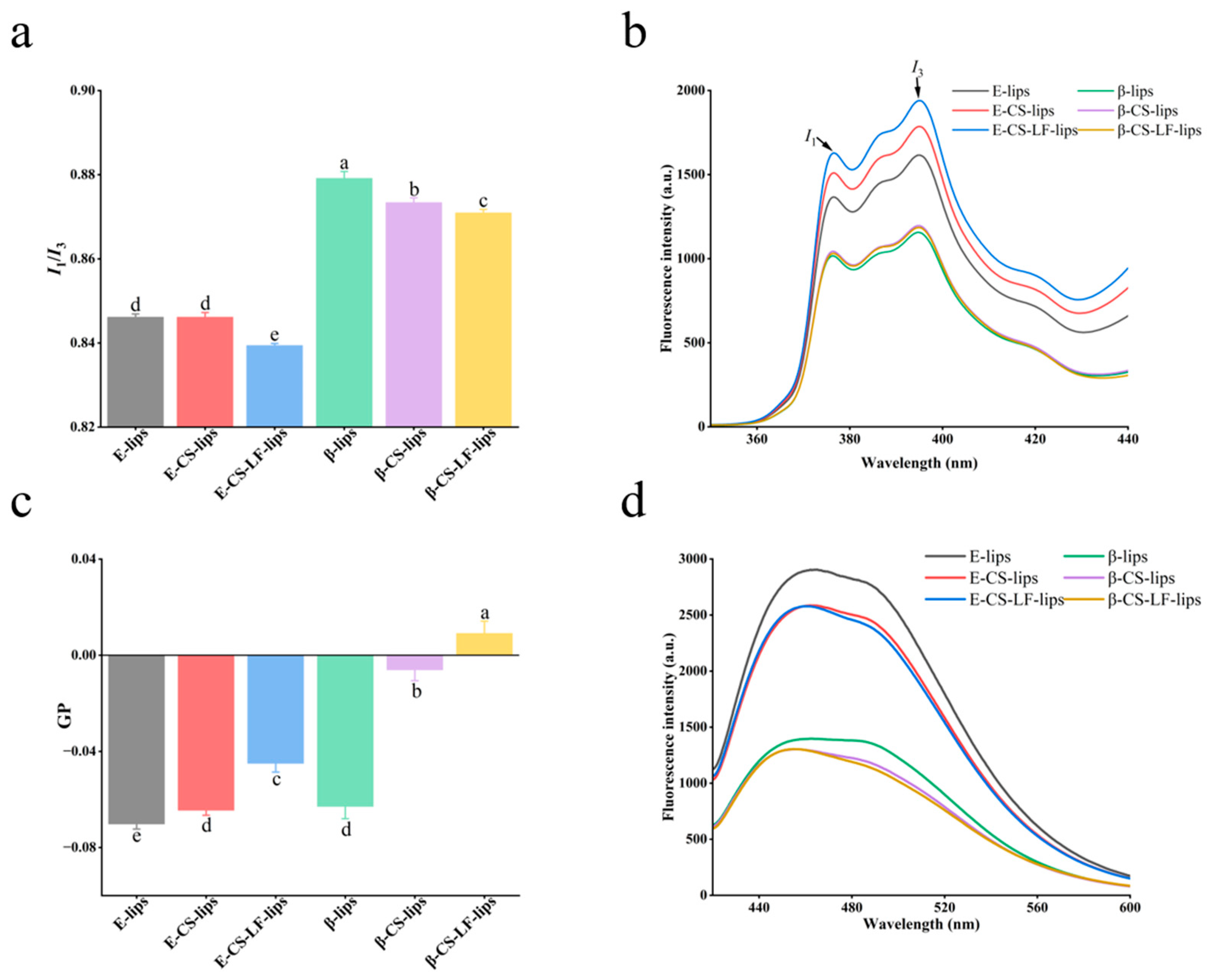
3.3. Lips Membrane Property Studies
3.3.1. Membrane Micropolarity
3.3.2. Membrane Fluidity
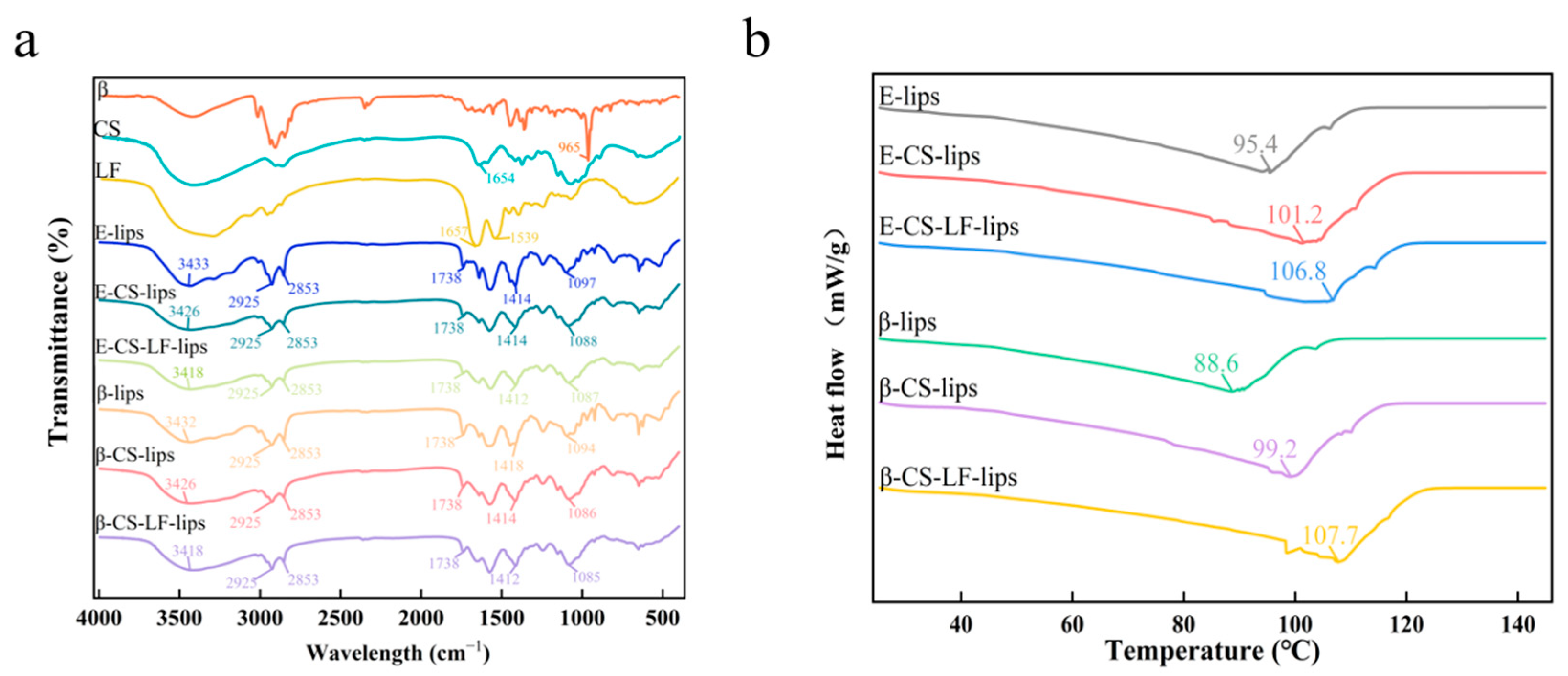
3.4. FTIR Analysis
3.5. DSC Analysis
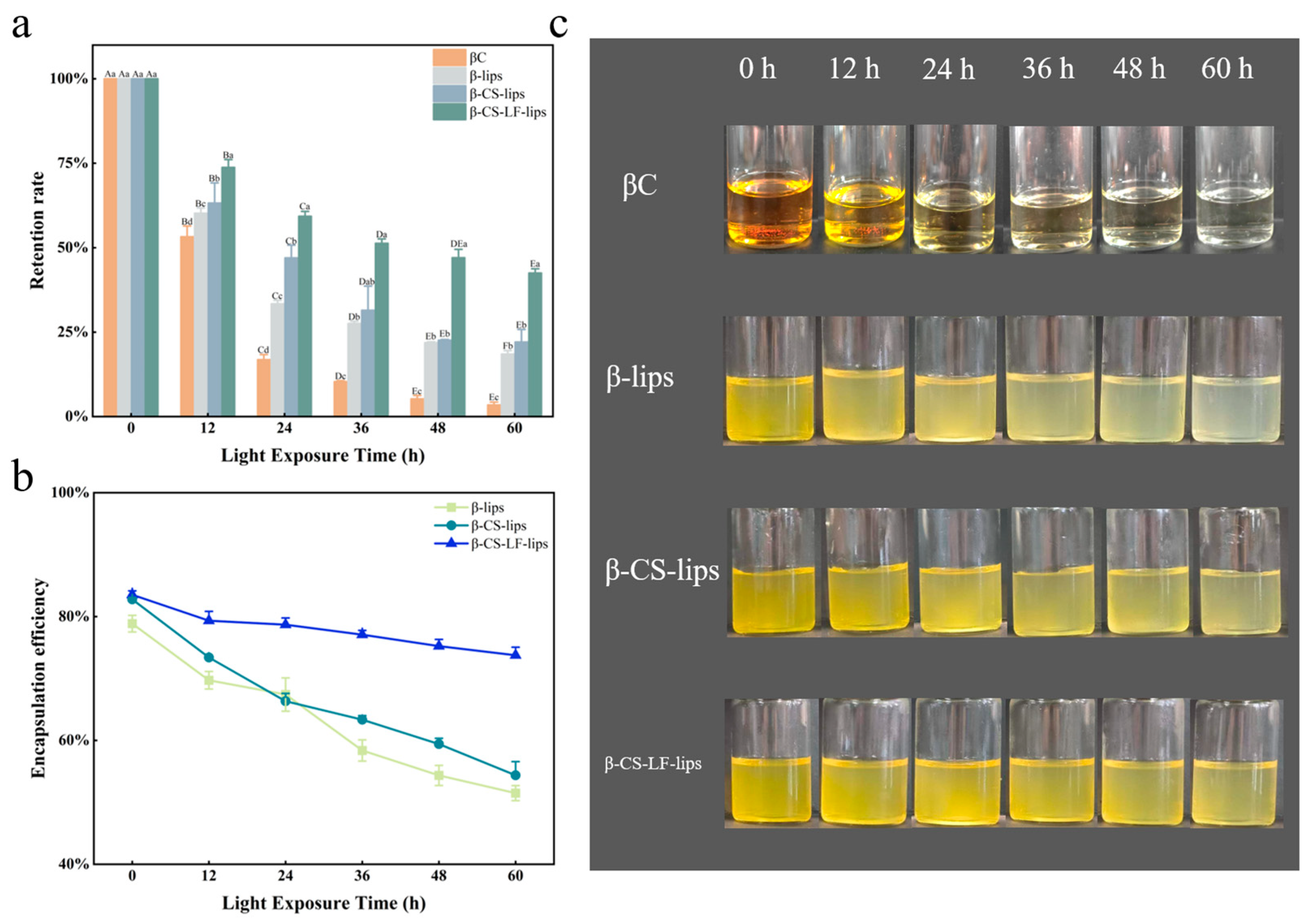
3.6. Stability Analysis
3.6.1. Storage Stability
3.6.2. Photo Stability
3.6.3. Digestive Stability
3.7. Study of Anti-Inflammatory Properties of Liposomal System
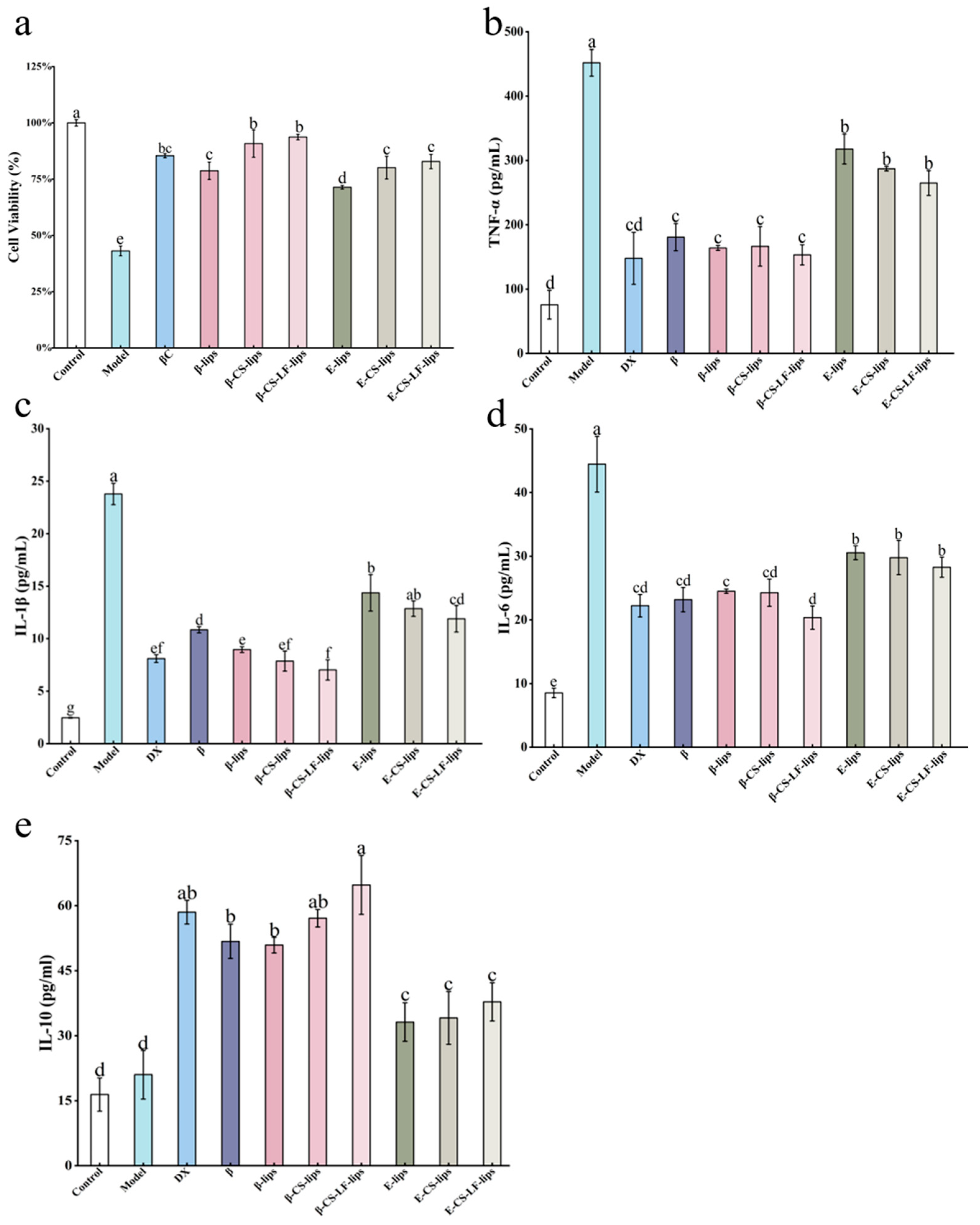
3.7.1. The Effect of Lips on LPS-Induced BV-2 Cell Viability
3.7.2. The Effect of Lips on LPS-Induced Inflammatory Factor Secretion in BV-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Lips | Liposomes |
| CS | Chitosan |
| βC | β-carotene |
| PLs | Phospholipids |
| LF | Lactoferrin |
| βC | β-carotene |
| EE | Encapsulation efficiency |
| RR | Retention rate |
| BV-2 | Mouse microglia |
| LPS | Lipopolysaccharide |
References
- Jiang, X.; Pan, D.; Tao, M.; Zhang, T.; Zeng, X.; Wu, Z.; Guo, Y. New Nanocarrier System for Liposomes Coated with Lactobacillus acidophilus S-Layer Protein to Improve Leu–Gln–Pro–Glu Absorption through the Intestinal Epithelium. J. Agric. Food Chem. 2021, 69, 7593–7602. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Wu, X.; Geng, M.; Teng, F. Layer-by-layer self-assembled liposomes prepared using sodium alginate and chitosan: Insights into vesicle characteristics and physicochemical stability. Food Hydrocoll. 2024, 149, 109606. [Google Scholar] [CrossRef]
- Yi, X.; Gao, S.; Gao, X.; Zhang, X.; Xia, G.; Liu, Z.; Shi, H.; Shen, X. Glycolipids improve the stability of liposomes: The perspective of bilayer membrane structure. Food Chem. 2023, 412, 135517. [Google Scholar] [CrossRef]
- Le, N.T.T.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Hoang Thi, T.T. Soy Lecithin-Derived Liposomal Delivery Systems: Surface Modification and Current Applications. Int. J. Mol. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef]
- Tiboni, M.; Benedetti, S.; Skouras, A.; Curzi, G.; Perinelli, D.R.; Palmieri, G.F.; Casettari, L. 3D-printed microfluidic chip for the preparation of glycyrrhetinic acid-loaded ethanolic liposomes. Int. J. Pharm. 2020, 584, 119436. [Google Scholar] [CrossRef] [PubMed]
- William, B.; Noémie, P.; Brigitte, E.; Géraldine, P. Supercritical fluid methods: An alternative to conventional methods to prepare liposomes. Chem. Eng. J. 2020, 383, 123106. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, W.; Zhang, J.; Song, X.; Sun, W.; Fan, Y. The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. Int. J. Biol. Macromol. 2017, 105, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C 2016, 64, 124–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chem. 2019, 293, 315–322. [Google Scholar] [CrossRef]
- Lu, J.-M.; Jin, G.-N.; Xin, Y.; Ma, J.-W.; Shen, X.-Y.; Quan, Y.-Z.; Liu, Y.-M.; Zhou, J.-Y.; Wang, B.-Z.; Li, Y.-B.; et al. Lactoferrin-modified nanoemulsions enhance brain-targeting and therapeutic efficacy of arctigenin against Toxoplasma gondii-induced neuronal injury. Int. J. Parasitol. Drugs Drug Resist. 2025, 27, 100575. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Oshima, K.; Kuhara, T.; Shin, K.; Abe, F.; Iwatsuki, K.; Nadano, D.; Matsuda, T. A lactoferrin-receptor, intelectin 1, affects uptake, sub-cellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells. J. Biochem. 2013, 154, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, N.; Dhathathreyan, A.; Sreeram, K.J. Regulating structural and mechanical properties of pectin reinforced liposomes at fluid/solid interface. Food Hydrocoll. 2021, 111, 106225. [Google Scholar] [CrossRef]
- Zamani Ghaleshahi, A.; Rajabzadeh, G. The influence of sodium alginate and genipin on physico-chemical properties and stability of WPI coated liposomes. Food Res. Int. 2020, 130, 108966. [Google Scholar] [CrossRef] [PubMed]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Zheng, J.; Xiong, H.; Zhao, L.; Xu, Y.; Bai, C. Fabricating pectin and chitosan double layer coated liposomes to improve physicochemical stability of beta-carotene and alter its gastrointestinal fate. Int. J. Biol. Macromol. 2023, 247, 125780. [Google Scholar] [CrossRef]
- Yi, X.; Gao, X.; Zhang, X.; Xia, G.; Shen, X. Preparation of liposomes by glycolipids/phospholipids as wall materials: Studies on stability and digestibility. Food Chem. 2023, 402, 134328. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2018, 84, 111–120. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, S.; Du, M. Oxidation-resistant nanoliposomes loaded with osteogenic peptides: Characteristics, stability and bioaccessibility. Food Res. Int. 2024, 177, 113843. [Google Scholar] [CrossRef]
- Bompard, J.; Rosso, A.; Brizuela, L.; Mebarek, S.; Blum, L.J.; Trunfio-Sfarghiu, A.M.; Lollo, G.; Granjon, T.; Girard-Egrot, A.; Maniti, O. Membrane Fluidity as a New Means to Selectively Target Cancer Cells with Fusogenic Lipid Carriers. Langmuir 2020, 36, 5134–5144. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-w.; Xu, H.; Sun, R.-q.; Liu, X.-l.; Ji, Z.; Zhou, D.-y.; Song, L. Engineering marine phospholipid nanoliposomes via glycerol-infused proliposomes: Mechanisms, strategies, and versatile applications in scalable food-grade nanoliposome production. Food Chem. 2024, 448, 139030. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Valoppi, F.; Comuzzo, P.; Nicoli, M.C. Microemulsions as delivery systems of lemon oil and beta-carotene into beverages: Stability test under different light conditions. J. Sci. Food Agric. 2019, 99, 7016–7020. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Erami, S.; Raftani Amiri, Z.; Jafari, S.M. Nanoliposomal encapsulation of Bitter Gourd (Momordica charantia) fruit extract as a rich source of health-promoting bioactive compounds. LWT 2019, 116, 108581. [Google Scholar] [CrossRef]
- Ma, L.; Gao, T.; Cheng, H.; Li, N.; Huang, W.; Liang, L. Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA. Antioxidants 2023, 12, 564. [Google Scholar] [CrossRef]
- Lan, M.; Fu, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Encapsulation of β-carotene by self-assembly of rapeseed meal-derived peptides: Factor optimization and structural characterization. LWT 2021, 138, 110456. [Google Scholar] [CrossRef]
- Guan, Y.; Ning, Y.; Xu, Z.; Zhou, C.; Zhao, Z. Chondroitin sulfate and chitosan-coated liposomes as a novel delivery system for betanin: Preparation, characterization and in vitro digestion behavior. Int. J. Biol. Macromol. 2024, 254, 128001. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Min, Q.; Ling, C.; Maiti, D.; Xu, J.; Qin, L.; Yang, K. Holo-Lactoferrin Modified Liposome for Relieving Tumor Hypoxia and Enhancing Radiochemotherapy of Cancer. Small 2019, 15, e1803703. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef]
- Liu, W.; Wei, F.; Ye, A.; Tian, M.; Han, J. Kinetic stability and membrane structure of liposomes during in vitro infant intestinal digestion: Effect of cholesterol and lactoferrin. Food Chem. 2017, 230, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Song, F.-f.; Tian, S.-j.; Yang, G.-l.; Sun, X.-y. Effect of phospholipid/flaxseed oil ratio on characteristics, structure change, and storage stability of liposomes. LWT 2022, 157, 113040. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int. J. Biol. Macromol. 2021, 168, 59–66. [Google Scholar] [CrossRef]
- Gunther, G.; Malacrida, L.; Jameson, D.M.; Gratton, E.; Sánchez, S.A. LAURDAN since Weber: The Quest for Visualizing Membrane Heterogeneity. Acc. Chem. Res. 2021, 54, 976–987. [Google Scholar] [CrossRef]
- Niaz, T.; Mackie, A. Effect of beta glucan coating on controlled release, bioaccessibility, and absorption of beta-carotene from loaded liposomes. Food Funct. 2024, 15, 1627–1642. [Google Scholar] [CrossRef]
- Wang, W.-D.; Chen, C.; Fu, X. Glycation mechanism of lactoferrin–chitosan oligosaccharide conjugates with improved antioxidant activity revealed by high-resolution mass spectroscopy. Food Funct. 2020, 11, 10886–10895. [Google Scholar] [CrossRef]
- Korkmaz, F.; Severcan, F. Effect of progesterone on DPPC membrane: Evidence for lateral phase separation and inverse action in lipid dynamics. Arch. Biochem. Biophys. 2005, 440, 141–147. [Google Scholar] [CrossRef]
- Chen, J.; He, C.-q.; Lin, A.-h.; Gu, W.; Chen, Z.-p.; Li, W.; Cai, B.-c. Thermosensitive liposomes with higher phase transition temperature for targeted drug delivery to tumor. Int. J. Pharm. 2014, 475, 408–415. [Google Scholar] [CrossRef]
- Pignatello, R.; Toth, I.; Puglisi, G. Structural effects of lipophilic methotrexate conjugates on model phospholipid biomembranes. Thermochim. Acta 2001, 380, 255–264. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Protein release kinetics for core–shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 2008, 29, 1207–1215. [Google Scholar] [CrossRef]
- Chen, L.; Liang, R.; Wang, Y.; Yokoyama, W.; Chen, M.; Zhong, F. Characterizations on the Stability and Release Properties of β-ionone Loaded Thermosensitive Liposomes (TSLs). J. Agric. Food Chem. 2018, 66, 8336–8345. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhao, D.; Huang, Q.; Zhao, X.; Chen, Q.; Rao, H.; Guo, L.; Hao, J. Preparation of pectin-coated and chitosan-coated phenylethanoside liposomes: Studies on characterization, stability, digestion and release behavior. Int. J. Biol. Macromol. 2024, 261, 129442. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Cho, Y.-H.; McClements, D.J. Theoretical Stability Maps for Guiding Preparation of Emulsions Stabilized by Protein–Polysaccharide Interfacial Complexes. Langmuir 2009, 25, 6649–6657. [Google Scholar] [CrossRef]
- Qiang, M.; Pang, X.; Ma, D.; Ma, C.; Liu, F. Effect of Membrane Surface Modification Using Chitosan Hydrochloride and Lactoferrin on the Properties of Astaxanthin-Loaded Liposomes. Molecules 2020, 25, 610. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-carotene-loaded oil droplets in caseinate/alginate microparticles: Enhancement of carotenoid stability and bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Bai, C.; Peng, H.; Xiong, H.; Liu, Y.; Zhao, L.; Xiao, X. Carboxymethylchitosan-coated proliposomes containing coix seed oil: Characterisation, stability and in vitro release evaluation. Food Chem. 2011, 129, 1695–1702. [Google Scholar] [CrossRef]
- Royer, J.; Shanklin, J.; Balch-Kenney, N.; Mayorga, M.; Houston, P.; de Jong, R.M.; McMahon, J.; Laprade, L.; Blomquist, P.; Berry, T.; et al. Rhodoxanthin synthase from honeysuckle; a membrane diiron enzyme catalyzes the multistep conversion of β-carotene to rhodoxanthin. Sci. Adv. 2020, 6, eaay9226. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Zhang, J.; Chen, C.; Xu, J.; Xu, B. Vitamin C and β-carotene co-loaded in marine and egg nanoliposomes. J. Food Eng. 2023, 340, 111315. [Google Scholar] [CrossRef]
- Roy, B.; Kojima, R.; Shah, O.; Shieh, M.; Das, E.; Ezzatpour, S.; Sato, E.; Hirata, Y.; Lindahl, S.; Matsuzawa, A.; et al. Generation of thiyl radicals in a spatiotemporal controlled manner by light: Applied for the cis to trans isomerization of unsaturated fatty acids/phospholipids. Redox Biol. 2025, 79, 103475. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, P.; Honisch, C.; Hussain, R.; Siligardi, G. Free Radicals and ROS Induce Protein Denaturation by UV Photostability Assay. Int. J. Mol. Sci. 2021, 22, 6512. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The Effect of Cannabidiol on UV-Induced Changes in Intracellular Signaling of 3D-Cultured Skin Keratinocytes. Int. J. Mol. Sci. 2021, 22, 1501. [Google Scholar] [CrossRef]
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of thermal and UV-light stability of β-carotene-loaded nanoemulsions by water-soluble chitosan coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163. [Google Scholar] [CrossRef]
- Kowalonek, J. Studies of chitosan/pectin complexes exposed to UV radiation. Int. J. Biol. Macromol. 2017, 103, 515–524. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, S.; Liu, M.; Guo, J.; Chen, Y.; Shen, X.; Deng, X.; Lu, J. Preventive effects of lactoferrin on acute alcohol-induced liver injury via iron chelation and regulation of iron metabolism. J. Dairy Sci. 2024, 107, 5316–5329. [Google Scholar] [CrossRef]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Yang, G.; Lee, H.E.; Moon, S.J.; Ko, K.M.; Koh, J.H.; Seok, J.K.; Min, J.K.; Heo, T.H.; Kang, H.C.; Cho, Y.Y.; et al. Direct Binding to NLRP3 Pyrin Domain as a Novel Strategy to Prevent NLRP3-Driven Inflammation and Gouty Arthritis. Arthritis Rheumatol. 2020, 72, 1192–1202. [Google Scholar] [CrossRef]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Inagawa, H. Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo. Int. J. Mol. Sci. 2023, 24, 14387. [Google Scholar] [CrossRef]
- Ren, D.; Fu, Y.; Wang, L.; Liu, J.; Zhong, X.; Yuan, J.; Jiang, C.; Wang, H.; Li, Z. Tetrandrine ameliorated Alzheimer’s disease through suppressing microglial inflammatory activation and neurotoxicity in the 5XFAD mouse. Phytomedicine 2021, 90, 153627. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q. Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling. Front. Immunol. 2023, 14, 1128358. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Stephen-Victor, E.; Fickenscher, H.; Bayry, J. IL-26: An Emerging Proinflammatory Member of the IL-10 Cytokine Family with Multifaceted Actions in Antiviral, Antimicrobial, and Autoimmune Responses. PLoS Pathog. 2016, 12, e1005624. [Google Scholar] [CrossRef]
- Lang, G.P.; Li, C.; Han, Y.Y. Rutin pretreatment promotes microglial M1 to M2 phenotype polarization. Neural Regen. Res. 2021, 16, 2499–2504. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of BV-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- da Silva, R.F.; Lappalainen, J.; Lee-Rueckert, M.; Kovanen, P.T. Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 2016, 248, 170–178. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Han, L.; Liu, Y.; Shao, K.; Ye, L.; Lou, J.; Jiang, C.; Pei, Y. Brain-Targeting Mechanisms of Lactoferrin-Modified DNA-Loaded Nanoparticles. J. Cereb. Blood Flow Metab. 2009, 29, 1914–1923. [Google Scholar] [CrossRef]

| βC Load | Z-Average Diameter (nm) | Zeta Potential (mV) | PDI | EE |
|---|---|---|---|---|
| E-lips | 167 ± 8 c | −25 ± 1 a | 0.20 ± 0.03 c | - |
| 0.5% | 231 ± 5 b | −30.6 ± 0.3 b | 0.24 ± 0.04 b | 77.21 ± 2.22% b |
| 1.0% | 258 ± 3 ab | −30.7 ± 0.8 b | 0.25 ± 0.05 b | 84.91 ± 0.50% a |
| 1.5% | 268 ± 6 a | −31.9 ± 0.3 b | 0.28 ± 0.04 a | 70.39 ± 1.99% c |
| 2.0% | 267 ± 7 a | −30.5 ± 0.7 b | 0.25 ± 0.02 b | 68.95 ± 5.14% c |
| CS Concentration | Z-Average Diameter (nm) | Zeta Potential (mV) | PDI | EE |
|---|---|---|---|---|
| 0% | 259 ± 3 f | −33.0 ± 0.9 e | 0.27 ± 0.01 e | 77.21 ± 2.22% b |
| 0.5% | 483 ± 5 e | 44.7 ± 0.4 d | 0.52 ± 0.01 bc | 76.98 ± 2.03% b |
| 0.6% | 508 ± 8 d | 47.4 ± 0.4 c | 0.55 ± 0.01 ab | 80.71 ± 0.96% a |
| 0.7% | 533 ± 13 c | 49.1 ± 1.2 bc | 0.44 ± 0.01 c | 81.53 ± 0.07% a |
| 0.8% | 562 ± 4 b | 51.1 ± 1.2 a | 0.46 ± 0.04 cd | 81.85 ± 0.30% a |
| 0.9% | 565 ± 12 b | 51.0 ± 1.0 ab | 0.53 ± 0.00 ab | 82.74 ± 0.92% a |
| 1.0% | 818 ± 6 a | 50.2 ± 1.3 ab | 0.59 ± 0.08 a | 82.21 ± 1.14% a |
| LF Concentration | Z-Average Diameter (nm) | Zeta Potential (mV) | PDI | EE |
|---|---|---|---|---|
| 0% | 555 ± 13 b | 51.0 ± 0.3 a | 0.47 ± 0.03 b | 79.92 ± 0.78% c |
| 0.125% | 552 ± 16 b | 49.9 ± 0.1 ab | 0.46 ± 0.03 b | 81.05 ± 0.48% bc |
| 0.25% | 566 ± 6 a | 49.2 ± 1.3 b | 0.45 ± 0.02 b | 80.92 ± 0.53% bc |
| 0.375% | 581 ± 10 a | 48.9 ± 0.7 b | 0.36 ± 0.03 c | 82.10 ± 0.72% ab |
| 0.5% | 574 ± 10 a | 48.4 ± 1.2 b | 0.49 ± 0.01 ab | 83.10 ± 1.17% a |
| 0.1% | 571 ± 4 a | 49.2 ± 1.2 b | 0.52 ± 0.04 a | 82.72 ± 0.36% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Yi, X.; Gao, X.; Long, Z.; Guo, J.; Xia, G.; Shen, X. Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies. Foods 2025, 14, 968. https://doi.org/10.3390/foods14060968
Gao S, Yi X, Gao X, Long Z, Guo J, Xia G, Shen X. Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies. Foods. 2025; 14(6):968. https://doi.org/10.3390/foods14060968
Chicago/Turabian StyleGao, Shuxin, Xiangzhou Yi, Xia Gao, Zhengsen Long, Jingfeng Guo, Guanghua Xia, and Xuanri Shen. 2025. "Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies" Foods 14, no. 6: 968. https://doi.org/10.3390/foods14060968
APA StyleGao, S., Yi, X., Gao, X., Long, Z., Guo, J., Xia, G., & Shen, X. (2025). Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies. Foods, 14(6), 968. https://doi.org/10.3390/foods14060968





