The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes
2.2. Substrate
2.3. In Vitro Static Simulation of Oro-Gastric Digestion
2.4. Free Amino Nitrogen Analysis
2.5. Amino Acid Analysis
2.6. Size Exclusion Chromatography
2.7. Gel Electrophoresis
2.8. Glycerol, Maltose, and Glucose Analyses
2.9. Fatty Acid Analysis
2.10. Statistics
3. Results and Discussion
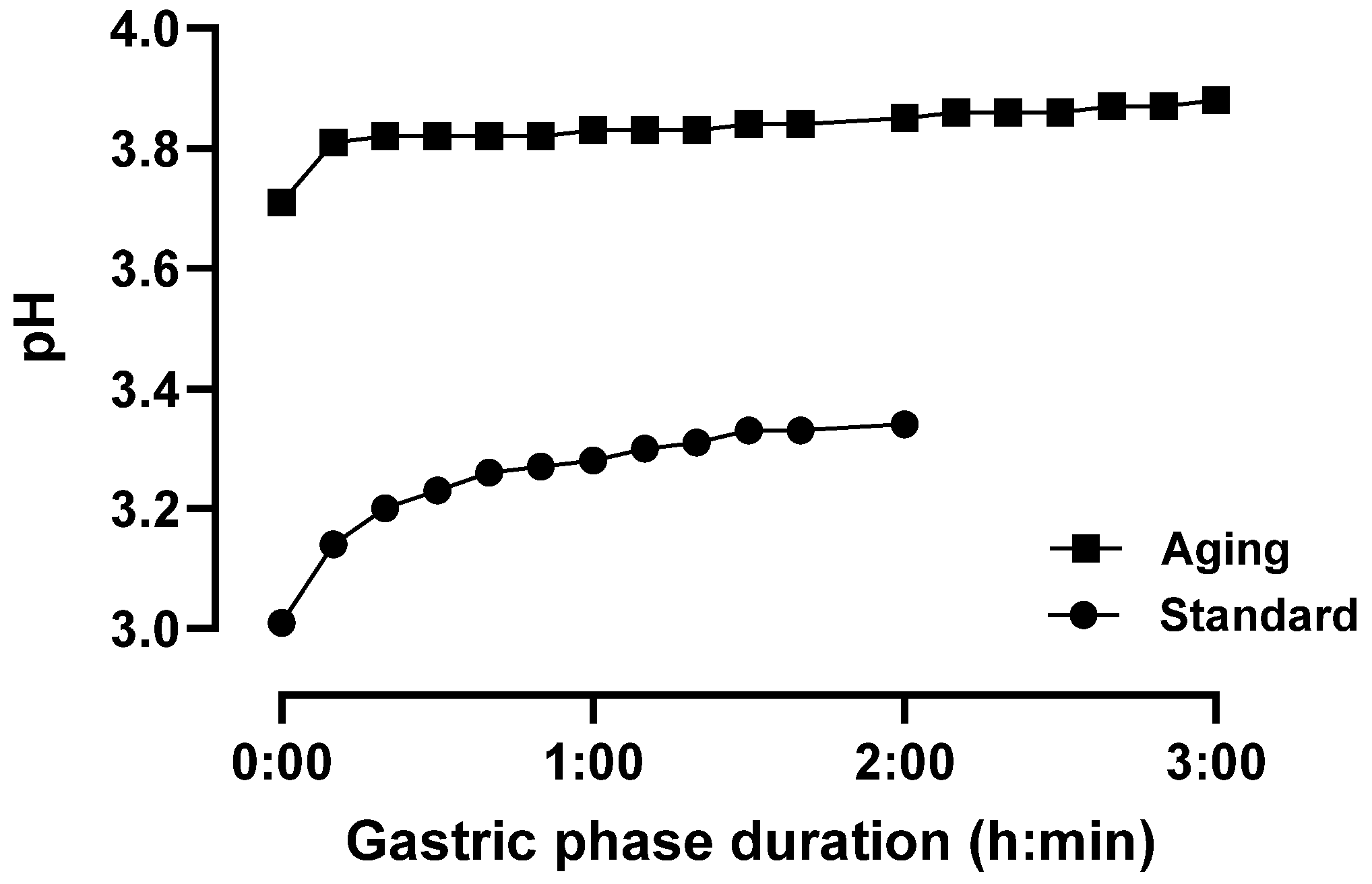
3.1. pH Response Profiling
3.2. Peptide and Amino Acid Bioaccessibility
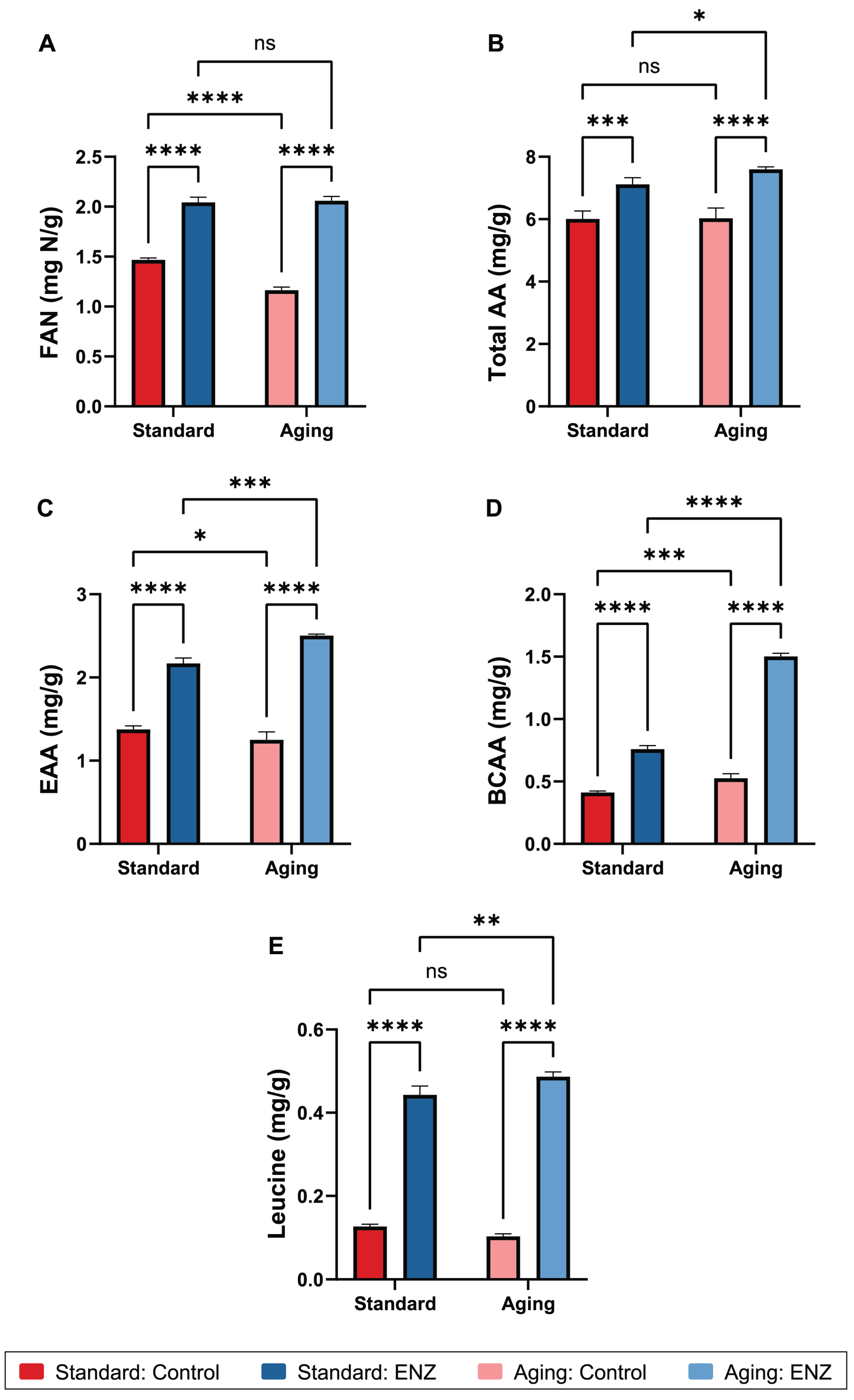
3.2.1. Free Amino Nitrogen
3.2.2. Amino Acids
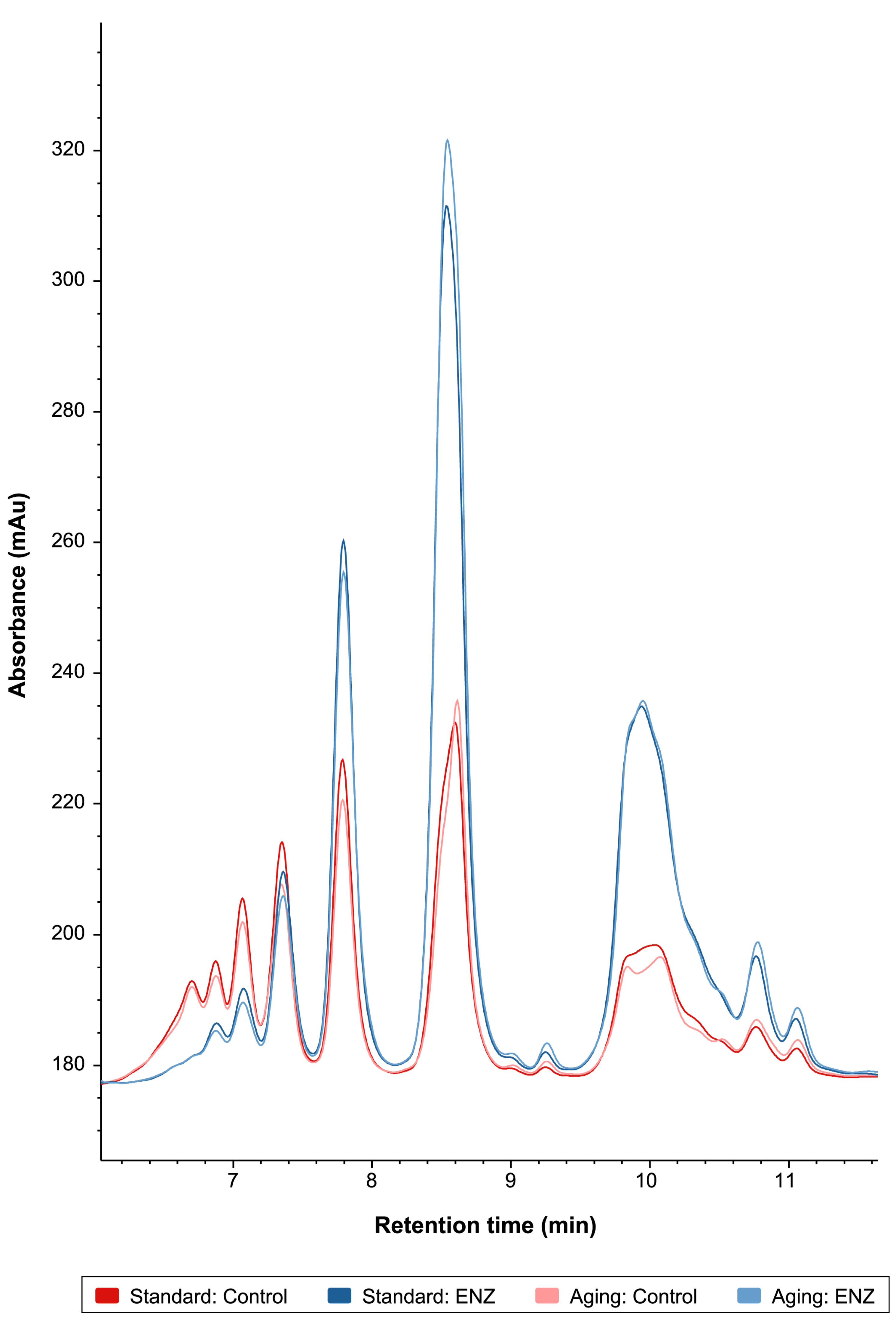
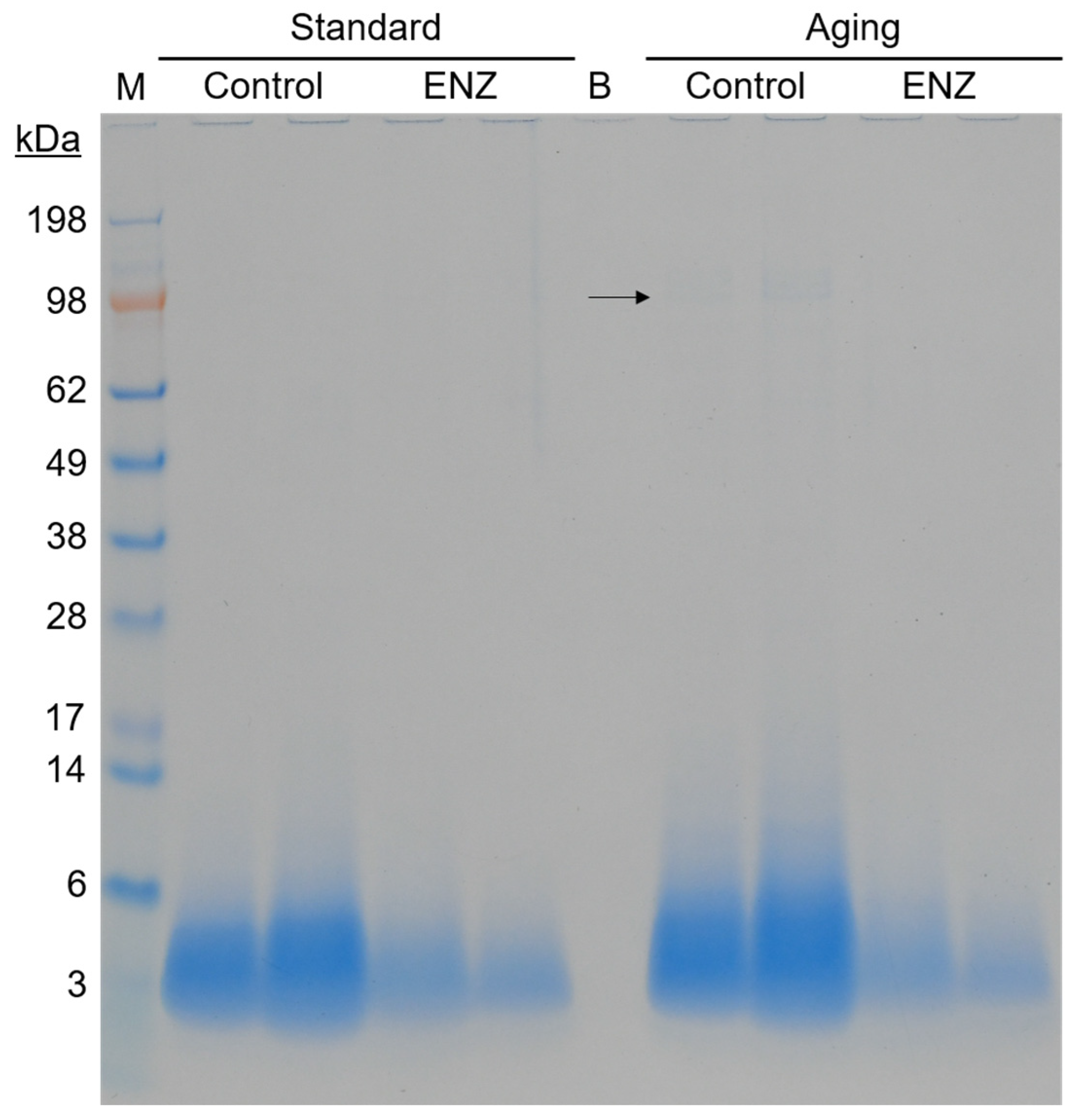
3.2.3. Peptide Profiling by Size Exclusion Chromatography and SDS-PAGE
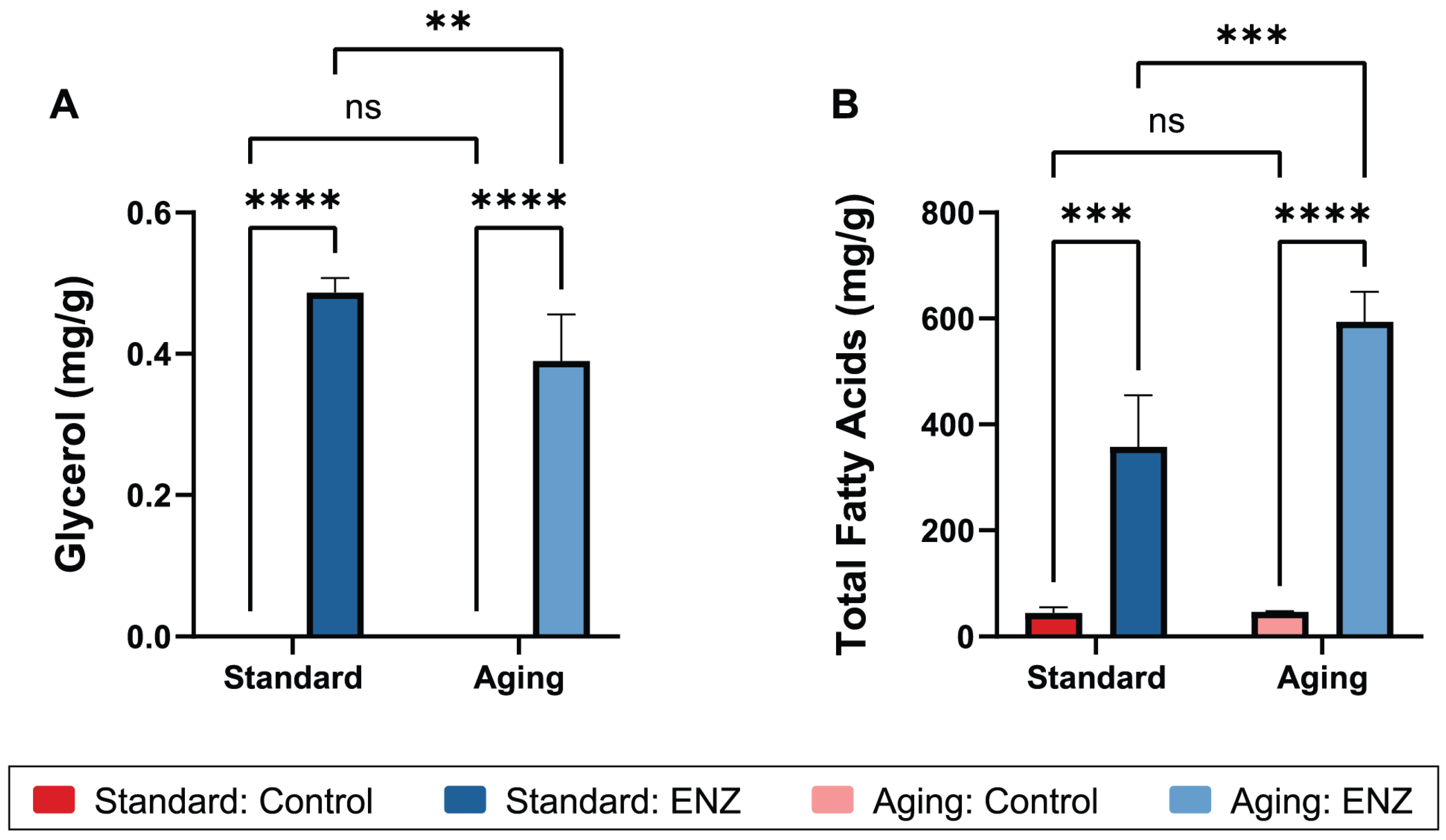
3.3. Fatty Acid and Glycerol Bioaccessibility
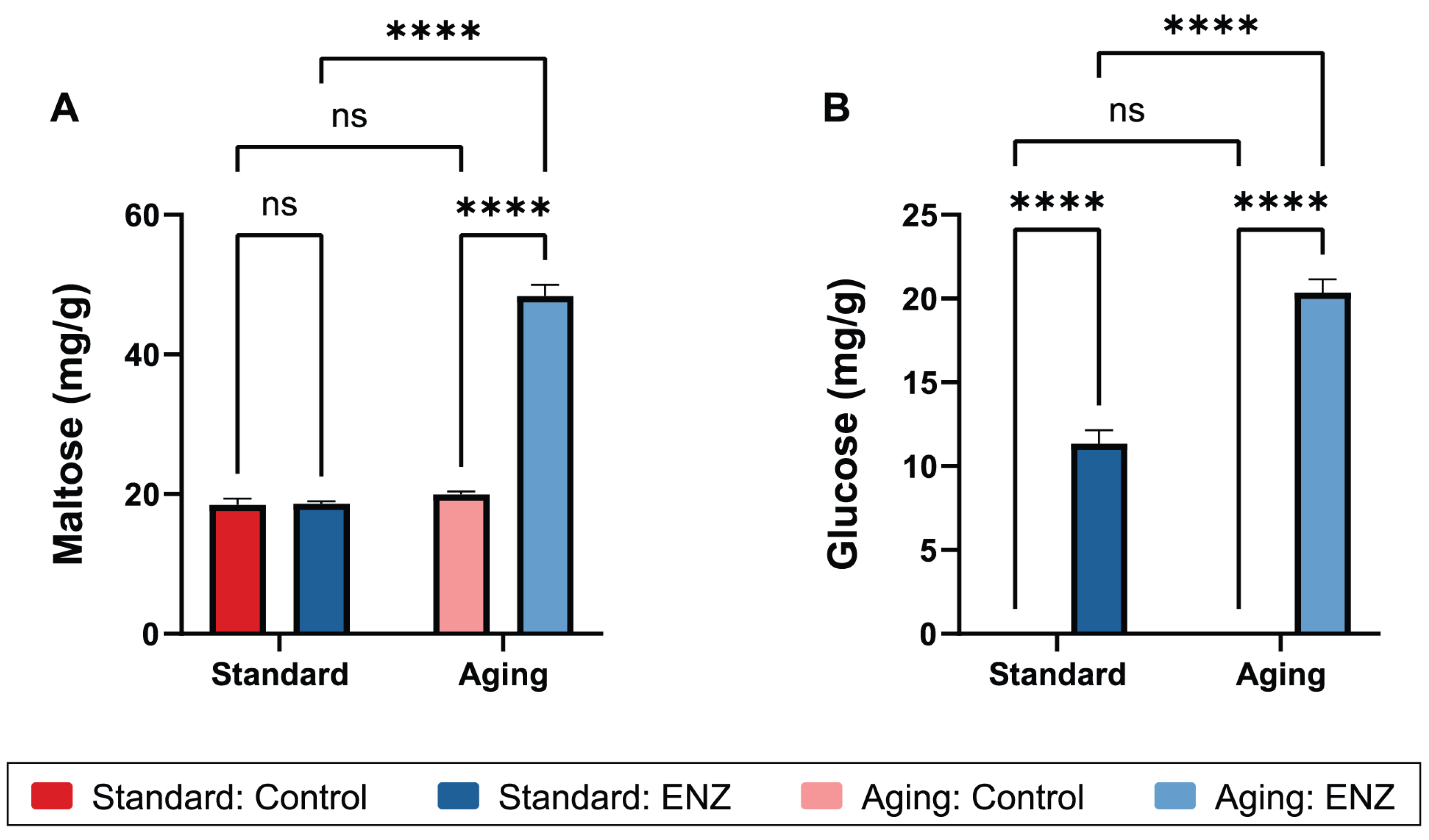
3.4. Maltose and Glucose Bioaccessibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| AmP | Aspergillus melleus protease |
| ANOVA | analysis of variance |
| BCAA | branched chain amino acid |
| CcL | Candida cylindracea lipase |
| EAA | essential amino acid |
| ENZ | mixture of six microbial enzyme preparations |
| FA | fatty acid |
| FAN | free amino nitrogen |
| GI | gastrointestinal |
| MW | molecular weight |
| NAC | N-acetyl-L-cysteine |
| OPA | o-phthaldaldehyde |
| PPA | porcine pancreatic amylase |
| PVDF | polyvinylidenedifluoride |
| RT | retention time |
| SDS-PAGE | sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| SEC | size exclusion chromatography |
References
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B. Effects of age on gastric alkaline and nonparietal fluid secretion in humans. Gerontology 1998, 44, 222–227. [Google Scholar] [CrossRef]
- Meyer, J.; Spier, E.; Neuwelt, F. Basal secretion of digestive enzymes in old age. Arch. Intern. Med. 1940, 65, 171–177. [Google Scholar] [CrossRef]
- Dreiling, D.A.; Triebling, A.T.; Koller, M. The effect of age on human exocrine pancreatic secretion. Mt. Sinai J. Med. 1985, 52, 336–339. [Google Scholar] [PubMed]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef]
- Clarkston, W.K.; Pantano, M.M.; Morley, J.E.; Horowitz, M.; Littlefield, J.M.; Burton, F.R. Evidence for the anorexia of aging: Gastrointestinal transit and hunger in healthy elderly vs. young adults. Am. J. Physiol. 1997, 272, R243–R248. [Google Scholar] [CrossRef]
- Di Francesco, V.; Zamboni, M.; Dioli, A.; Zoico, E.; Mazzali, G.; Omizzolo, F.; Bissoli, L.; Solerte, S.B.; Benini, L.; Bosello, O. Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1581–1585. [Google Scholar] [CrossRef]
- O’Donovan, D.; Hausken, T.; Lei, Y.; Russo, A.; Keogh, J.; Horowitz, M.; Jones, K.L. Effect of aging on transpyloric flow, gastric emptying, and intragastric distribution in healthy humans--impact on glycemia. Dig. Dis. Sci. 2005, 50, 671–676. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Gastric emptying in the elderly. Clin. Geriatr. Med. 2015, 31, 339–353. [Google Scholar] [CrossRef]
- Fikry, M.E. Exocrine pancreatic functions in the aged. J. Am. Geriatr. Soc. 1968, 16, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Matsumoto, S.; Harada, H.; Ochi, K.; Tanaka, J.; Seno, T.; Oka, H.; Miyake, H.; Kimura, I. Aging and Exocrine Pancreatic Function Evaluated by the Recently Standardized Secretin Test. Jpn. J. Geriatr. 1991, 28, 599–605. [Google Scholar] [CrossRef]
- Laugier, R.; Bernard, J.P.; Berthezene, P.; Dupuy, P. Changes in pancreatic exocrine secretion with age: Pancreatic exocrine secretion does decrease in the elderly. Digestion 1991, 50, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Balas, D.; Moreau, J.; Bouisson, M.; Senegas-Balas, F.; Guidet, M.; Ribet, A. Exocrine pancreatic secretion in the elderly. Int. J. Pancreatol. 1988, 3, 497–502. [Google Scholar] [CrossRef]
- Keller, J.; Layer, P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005, 54 (Suppl. S6), vi1–vi28. [Google Scholar] [CrossRef]
- Einarsson, K.; Nilsell, K.; Leijd, B.; Angelin, B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N. Engl. J. Med. 1985, 313, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Salemans, J.M.; Nagengast, F.M.; Tangerman, A.; van Schaik, A.; Hopman, W.P.; de Haan, A.F.; Jansen, J.B. Effect of ageing on postprandial conjugated and unconjugated serum bile acid levels in healthy subjects. Eur. J. Clin. Investig. 1993, 23, 192–198. [Google Scholar] [CrossRef]
- Sanders, L.; Goltz, S.; Maki, K.C. Resiliency of the digestive system during aging and the impact of diet. Nutr. Today 2023, 58, 165–174. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [CrossRef]
- Morley, J.E.; Silver, A.J.; Miller, D.K.; Rubenstein, L.Z. The anorexia of the elderly. Ann. N. Y. Acad. Sci. 1989, 575, 50–59. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef]
- Robinson, S.; Granic, A.; Cruz-Jentoft, A.J.; Sayer, A.A. The role of nutrition in the prevention of sarcopenia. Am. J. Clin. Nutr. 2023, 118, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Garvey, S.M.; Guice, J.L.; Hollins, M.D.; Best, C.H.; Tinker, K.M. Fungal digestive enzymes promote macronutrient hydrolysis in the INFOGEST static in vitro simulation of digestion. Food Chem. 2022, 386, 13277. [Google Scholar] [CrossRef]
- Freitas, D.; Gómez-Mascaraque, L.G.; Brodkorb, A. Digestion of protein and toxic gluten peptides in wheat bread, pasta and cereal and the effect of a supplemental enzyme mix. Front. Nutr. 2022, 9, 986272. [Google Scholar] [CrossRef] [PubMed]
- Tran Do, D.H.; Kong, F.; Penet, C.; Winetzky, D.; Gregory, K. Using a dynamic stomach model to study efficacy of supplemental enzymes during simulated digestion. LWT–Food Sci. Technol. 2016, 65, 580–588. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Ishigaki, Y.; Yamaguchi, S.; Chen, J.; Liu, X. Microbial protease supplementation improves gastric emptying and protein digestive fate of beef for the elderly under dynamic in vitro digestion. Food Res. Int. 2025, 202, 115721. [Google Scholar] [CrossRef]
- Menden, A.; Hall, D.; Broedlow, C.A.; Darcey, T.; Crawford, F.; Klatt, N.; Crynen, S.; Mullan, M.; Ait-Ghezala, G. Candida rugosa lipase alters the gastrointestinal environment in wild-type mice. Biomed. Pharmacother. 2020, 130, 110579. [Google Scholar] [CrossRef]
- Menden, A.; Hall, D.; Hahn-Townsend, C.; Broedlow, C.A.; Joshi, U.; Pearson, A.; Crawford, F.; Evans, J.E.; Klatt, N.; Crynen, S.; et al. Exogenous lipase administration alters gut microbiota composition and ameliorates Alzheimer’s disease-like pathology in APP/PS1 mice. Sci. Rep. 2022, 12, 4797. [Google Scholar] [CrossRef]
- Liang, Q.; Yuan, M.; Xu, L.; Lio, E.; Zhang, F.; Mou, H.; Secundo, F. Application of enzymes as a feed additive in aquaculture. Mar. Life Sci.Technol. 2022, 4, 208–221. [Google Scholar] [CrossRef]
- Torres-Pitarch, A.; Manzanilla, E.G.; Gardiner, G.E.; O’Doherty, J.V.; Lawlor, P.G. Systematic review and meta-analysis of the effect of feed enzymes on growth and nutrient digestibility in grow-finisher pigs: Effect of enzyme type and cereal source. Anim. Feed Sci. Technol. 2019, 251, 153–165. [Google Scholar] [CrossRef]
- Ido, H.; Matsubara, H.; Kuroda, M.; Takahashi, A.; Kojima, Y.; Koikeda, S.; Sasaki, M. Combination of gluten-digesting enzymes improved symptoms of non-celiac gluten sensitivity: A randomized single-blind, placebo-controlled crossover study. Clin. Transl. Gastroenterol. 2018, 9, 181. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Arumugam, S.; Pande, A.; Paschapur, M.; Ali, F. Evaluation of the safety and efficacy of a multienzyme complex in patients with functional dyspepsia: A randomized, double-blind, placebo-controlled study. J. Med. Food 2018, 21, 1120–1128. [Google Scholar] [CrossRef]
- Martin-Biggers, J. A multi-digestive enzyme and herbal dietary supplement reduces bloating in a single use in healthy adults: A randomized, placebo-controlled, cross over study. Nutr. Diet. Suppl. 2024, 16, 51–57. [Google Scholar] [CrossRef]
- König, J.; Holster, S.; Bruins, M.J.; Brummer, R.J. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci. Rep. 2017, 7, 13100. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, K.J.M.; Askow, A.T.; Deutz, M.T.; McKenna, C.F.; Garvey, S.M.; Guice, J.L.; Kesler, R.M.; Barnes, T.M.; Tinker, K.M.; Paluska, S.A.; et al. Acute microbial protease supplementation increases net postprandial plasma amino acid concentrations after pea protein ingestion in healthy adults: A randomized, double-blind, placebo-controlled trial. J. Nutr. 2024, 154, 1549–1560. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food—An international consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Menard, O.; Lesmes, U.; Shani-Levi, C.S.; Araiza Calahorra, A.; Lavoisier, A.; Morzel, M.; Rieder, A.; Feron, G.; Nebbia, S.; Mashiah, L.; et al. Static in vitro digestion model adapted to the general older adult population: An INFOGEST international consensus. Food Funct. 2023, 14, 4569–4582. [Google Scholar] [CrossRef]
- Institute of Medicine; Otten, J.J.; Pitzi Hellwig, J.; Meyers, L.D. Chapter: Macronutrients, Healthful Diets, and Physical Activity. In Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; p. 70. [Google Scholar] [CrossRef]
- Campbell, W.W.; Deutz, N.E.P.; Volpi, E.; Apovian, C.M. Nutritional interventions: Dietary protein needs and influences on skeletal muscle of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- Chiwele, I.; Jones, B.E.; Podczeck, F. The shell dissolution of various empty hard capsules. Chem. Pharm. Bull. 2000, 48, 951–956. [Google Scholar] [CrossRef]
- Medina Hernández, M.J.; Villanueva Camañas, R.M.; Monfort Cuenca, E.; García Alvarez-Coque, M.C. Determination of the protein and free amino acid content in a sample using o-phthalaldehyde and N-acetyl-L-cysteine. Analyst 1990, 115, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Rieder, A.; Afseth, N.K.; Böcker, U.; Knutsen, S.H.; Kirkhus, B.; Mæhre, H.K.; Ballance, S.; Wubshet, S.G. Improved estimation of in vitro protein digestibility of different foods using size exclusion chromatography. Food Chem. 2021, 358, 129830. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Tinker, K.M.; Madden, E.N.; Best, C.H.; Farmar, J.G.; Garvey, S.M. Comparative evaluation of amylases in the oral phase of the INFOGEST static simulation of oro-gastric digestion. Food Res. Int. 2025, 203, 115887. [Google Scholar] [CrossRef]
- Guarrasi, V.; Mangione, M.R.; Sanfratello, V.; Martorana, V.; Bulone, D. Quantification of underivatized fatty acids from vegetable oils by HPLC with UV detection. J. Chromatogr. Sci. 2010, 48, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Brooks, F.P. Effect of diet on gastric secretion. Amer. J. Clin. Nutr. 1985, 42, 1006–1019. [Google Scholar] [CrossRef]
- Mennah-Govela, Y.A.; Cai, H.; Chu, J.; Kim, K.; Maborang, M.K.; Sun, W.; Bornhorst, G.M. Buffering capacity of commercially available foods is influenced by composition and initial properties in the context of gastric digestion. Food Funct. 2020, 11, 2255–2267. [Google Scholar] [CrossRef]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Salelles, L.; Floury, J.; Le Feunteun, S. Pepsin activity as a function of pH and digestion time on caseins and egg white proteins under static in vitro conditions. Food Funct. 2021, 12, 12468–12478. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.H. Studies on gastric proteolysis. I. The proteolytic activity of human gastric juice and pig and calf gastric mucosal extracts below pH5. Biochem. J. 1959, 71, 73–83. [Google Scholar] [CrossRef]
- Deng, R.; Nau, F.; Lucas, T.; Maillard, M.; Leduc, A.; Ossemond, J.; Musse, M.; Le Feunteun, S. First assessments of nutrient bioaccessibility with an INFOGEST semi-dynamic gastric digestion in vitro protocol adapted to model proton pump inhibitor use. Food Res. Int. 2025, 203, 115833. [Google Scholar] [CrossRef]
- Sánchez-García, J.; Muñoz-Pina, S.; García-Hernández, J.; Tárrega, A.; Heredia, A.; Andrés, A. Protein digestibility and ACE inhibitory activity of fermented flours in older adults and standard gastrointestinal simulation. Food Res. Int. 2024, 180, 114080. [Google Scholar] [CrossRef] [PubMed]
- Lavoisier, A.; Morzel, M.; Chevalier, S.; Henry, G.; Jardin, J.; Harel-Oger, M.; Garric, G.; Dupont, D. In vitro digestion of two protein-rich dairy products in the ageing gastrointestinal tract. Food Funct. 2023, 14, 9377–9390. [Google Scholar] [CrossRef]
- Lavoisier, A.; Chevalier, S.; Henry, G.; Ossemond, J.; Harel-Oger, M.; Garric, G.; Dupont, D.; Morzel, M. Impact of age on the digestion of cream cheese formulated with opposite caseins to whey proteins ratios: An in vitro study. Food Res. Int. 2024, 190, 114621. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Hong, X.; Tao, X.; Zhang, J.; Zhang, J.; Hou, Y.; Wu, T.; Liu, X.; Zhou, P. Calcium binding affects in vitro gastrointestinal digestion of bovine α-lactalbumin under infant, adult and elderly conditions. Int. Dairy J. 2024, 154, 105943. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, F.; Lin, Z.; Sun, X.; Zeng, X.; Liu, H.; Li, Y.; Yuan, Z.; Su, Y.; Wang, C.; et al. In vitro digestion mimicking conditions in adults and elderly reveals digestive characteristics of pork from different cooking ways. Food Res. Int. 2024, 183, 114204. [Google Scholar] [CrossRef]
- Qiu, Z.; Shi, Y.; Zheng, Y.; Shi, W.; Zhang, L.; Yin, M.; Wang, X. Comparison of in vitro digestive characteristics of proteins from different sources in simulated elderly gastrointestinal conditions. Food Chem. 2025, 463, 141299. [Google Scholar] [CrossRef]
- Ribes, S.; Arnal, M.; Talens, P. Influence of food oral processing, bolus characteristics, and digestive conditions on the protein digestibility of turkey cold meat and fresh cheese. Food Res. Int. 2023, 173, 113297. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.; Salcedo, L.; Talens, P.; Ribes, S. Role of food texture, oral processing responses, bolus properties, and digestive conditions on the nutrient bioaccessibility of al dente and soft-cooked red lentil pasta. Foods 2024, 13, 2341. [Google Scholar] [CrossRef]
- García Arteaga, V.; Kraus, S.; Schott, M.; Muranyi, I.; Schweiggert-Weisz, U.; Eisner, P. Screening of twelve pea (Pisum sativum L.) cultivars and their isolates focusing on the protein characterization, functionality, and sensory profiles. Foods 2021, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Ye, Q.; Wang, Y.; Selomulya, C. Modulating digestibility and mitigating beany flavor of pea protein. Food Rev. Int. 2024, 40, 3129–3158. [Google Scholar] [CrossRef]
- Duijsens, D.; Verkempinck, S.H.E.; Somers, E.; Hendrickx, M.E.G.; Grauwet, T. From static to semi-dynamic in vitro digestion conditions relevant for the older population: Starch and protein digestion of cooked lentils. Food Funct. 2024, 15, 591–607. [Google Scholar] [CrossRef]
- Hernández-Olivas, E.; Muñoz-Pina, S.; Andrés, A.; Heredia, A. Impact of elderly gastrointestinal alterations on in vitro digestion of salmon, sardine, sea bass and hake: Proteolysis, lipolysis and bioaccessibility of calcium and vitamins. Food Chem. 2020, 326, 127024. [Google Scholar] [CrossRef]
- Garvey, S.; Lange, K.; Van Den Ende, T.; Tinker, K. Microbial enzymes enhance macronutrient digestibility in a dynamic digestion simulation. In Proceedings of the 8th International Conference on Food Digestion, Porto, Portugal, 8–11 April 2024. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05211440 (accessed on 4 March 2025).
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Garvey, S.M.; Tinker, K.M.; Hollins, M.D.; Guice, J.L.; Schuler, C. Fungal Enzyme Mixtures and Uses Thereof. U.S. Patent Application 18/688,983, 4 March 2024. Available online: https://patentcenter.uspto.gov/applications/18688983 (accessed on 4 March 2025).
- Domínguez de María, P.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Understanding Candida rugosa lipases: An overview. Biotechnol. Adv. 2006, 24, 180–196. [Google Scholar] [CrossRef]
- Yeşiloğlu, Y.; Şit, L. Biochemical properties of free and immobilized Candida rugosa lipase onto Al2O3: A comparative study. Artif. Cells Blood Substitut. Immobil. Biotechnol. 2011, 39, 247–251. [Google Scholar] [CrossRef]
- Freitas, D.; Le Feunteun, S.; Panouillé, M.; Souchon, I. The important role of salivary α-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef]
| Primary Enzyme Activity | CAS No. | EC No. | Source Organism | Activity per Dose 2 | Optimum pH (Range) 3 |
|---|---|---|---|---|---|
| Protease | 9025-49-4 | 3.4.23.18 | Aspergillus oryzae | 60,000 HUT | 3.0 (3.0–6.0) |
| Protease | 9025-49-4 | 3.4.23.18 | Aspergillus niger | 300 SAP | 3.0 (1.5–4.5) |
| Protease | 9074-07-1 | 3.4.21.63 | Aspergillus melleus | 50 LAPU | 7.5 (5.5–10.0) |
| Lipase | 9001-62-1 | 3.1.1.3 | Candida cylindracea | 3000 FIP | 7.0 (3.0–9.0) |
| Amylase | 9000-90-2 | 3.2.1.1 | Aspergillus oryzae | 10,000 SKB 4 | 5.0 (3.5–6.5) |
| Glucoamylase | 9032-08-0 | 3.2.1.3 | Aspergillus niger | 25 AGU | 4.5 (3.0–6.0) |
| Analyte (Units) | Gastric Digesta Concentration 1 | p Values 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Simulation Condition 2 | Control | ENZ | ptreatment | pcondition | pinteraction | Control vs. ENZ 4 | Standard vs. Aging 4 | |||
| Standard | Aging | Control | ENZ | |||||||
| FAN (mg N/g) | Standard | 1.467 ± 0.021 | 2.043 ± 0.051 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.6095 |
| Aging | 1.163 ± 0.031 | 2.060 ± 0.044 | ||||||||
| Total AA (mg/g) | Standard | 6.020 ± 0.256 | 7.123 ± 0.210 | <0.0001 | 0.1077 | 0.1345 | 0.0005 | <0.0001 | 0.9204 | 0.0395 |
| Aging | 6.040 ± 0.327 | 7.603 ± 0.081 | ||||||||
| EAA (mg/g) | Standard | 1.376 ± 0.041 | 2.169 ± 0.064 | <0.0001 | 0.0188 | 0.0002 | <0.0001 | <0.0001 | 0.0374 | 0.0002 |
| Aging | 1.250 ± 0.096 | 2.505 ± 0.016 | ||||||||
| BCAA (mg/g) | Standard | 0.412 ± 0.013 | 0.760 ± 0.028 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0009 | <0.0001 |
| Aging | 0.525 ± 0.037 | 1.503 ± 0.024 | ||||||||
| Leucine (mg/g) | Standard | 0.127 ± 0.006 | 0.443 ± 0.021 | <0.0001 | 0.206 | 0.0018 | <0.0001 | <0.0001 | 0.0528 | 0.0029 |
| Aging | 0.103 ± 0.006 | 0.487 ± 0.012 | ||||||||
| Glycerol (mg/g) | Standard | 0 | 0.443 ± 0.255 | <0.0001 | 0.0410 | 0.0410 | <0.0001 | <0.0001 | >0.9999 | 0.0088 |
| Aging | 0 | 0.357 ± 0.212 | ||||||||
| Total FA (mg/g) | Standard | 44.5 ± 10.4 | 357.7 ± 97.6 | <0.0001 | 0.0067 | 0.0073 | 0.0001 | <0.0001 | 0.9668 | 0.0009 |
| Aging | 46.5 ± 0.9 | 593.5 ± 57.0 | ||||||||
| Maltose (mg/g) | Standard | 18.49 ± 0.893 | 18.62 ± 0.339 | <0.0001 | <0.0001 | <0.0001 | 0.8677 | <0.0001 | 0.0890 | <0.0001 |
| Aging | 19.99 ± 0.390 | 48.39 ± 1.593 | ||||||||
| Glucose (mg/g) | Standard | 0 | 11.20 ± 0.81 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | >0.9999 | <0.0001 |
| Aging | 0 | 20.09 ± 0.79 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garvey, S.M.; Madden, E.N.; Qu, Y.; Best, C.H.; Tinker, K.M. The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence. Foods 2025, 14, 937. https://doi.org/10.3390/foods14060937
Garvey SM, Madden EN, Qu Y, Best CH, Tinker KM. The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence. Foods. 2025; 14(6):937. https://doi.org/10.3390/foods14060937
Chicago/Turabian StyleGarvey, Sean M., Erin N. Madden, Yunyao Qu, Caroline H. Best, and Kelly M. Tinker. 2025. "The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence" Foods 14, no. 6: 937. https://doi.org/10.3390/foods14060937
APA StyleGarvey, S. M., Madden, E. N., Qu, Y., Best, C. H., & Tinker, K. M. (2025). The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence. Foods, 14(6), 937. https://doi.org/10.3390/foods14060937








