Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention
Abstract
1. Introduction
2. Materials and Methods
3. Chemical Properties, Metabolism, Bioavailability, and Absorption
3.1. GSLs and Derivatives
3.2. Metabolic Fate of Glucosinolates in Humans: Bioavailability and Absorption
3.3. Impact of Food Processing and Cooking Methods on Glucosinolate Stability and Bioavailability in Cruciferous Vegetables
4. Dietary Sources and Role of Glucosinolates in Chronic Disease Prevention
4.1. Dietary Sources and Recommendations for Optimal Intake
| Author | Dietary Sources | Derivatives | Recommendations for Optimal Intake | Anti-Cancer Properties | Mechanisms of Anti-Cancer Activity | Anti-Inflammatory Effects | Cardio Protective Effects |
|---|---|---|---|---|---|---|---|
| Lund E. [74] | Cruciferous vegetables (broccoli, cabbage, kale, Brussels sprouts) | Isothiocyanates, Nitriles | 100–200 g/day cruciferous vegetables | ↓ cancer risk (lung, gastrointestinal) | Modulates xenobiotic-metabolizing enzymes, induces apoptosis and cell cycle arrest | ↓ IL-6, TNF-α | Improves lipid profiles, reduces oxidative stress |
| Keum Y. [81] | Cruciferous vegetables (broccoli, watercress, Brussels sprouts, cabbage) | Phenethyl-isothiocyanate, Sulforaphane | 3–5 servings/week | ↓ cancer risk (lung, colorectal) | Inhibits carcinogen activation via CYP450, induces apoptosis via JNK and caspases | Modulates NF-κB and AP-1 pathways | Improves endothelial function, reduces atherosclerosis progression |
| Murashima M. [82] | Broccoli sprouts | Isothiocyanates | 100 g/day | - | - | ↓ oxidative stress markers | Improved cholesterol metabolism, ↓ oxidative stress markers |
| Christiansen B. [83] | Dried broccoli sprouts | Glucosinolates | 10 g/day | - | - | = | = endothelial function in hypertensive individuals |
| Bahadoran Z. [84] | Broccoli sprouts powder | Isothiocyanates | 10 g/day | - | - | ↓ MDA, oxidized LDL, OSI | Favorable effects on oxidative stress status in Type 2 diabetes patients |
| Bahadoran Z. [85] | Broccoli sprouts powder | Isothiocyanates | 10 g/day | - | - | ↓ serum triglycerides, OX-LDL/LDL ratio | Improved lipid profiles, OX-LDL/LDL ratio, and HDL-C in Type 2 diabetes patients |
| Armah C. [80] | High-glucoraphanin broccoli | Glucoraphanin, Isothiocyanates | 400 g HG broccoli/week | ↓ cancer risk | Modulates mitochondrial function | Rebalances TCA cycle and FA oxidation | = effect on CVD biomarkers |
| Armah C. [86] | High-glucoraphanin broccoli | Glucoraphanin, Isothiocyanates | 400 g HG broccoli/week | ↓ cancer risk | LDL ↓ through glucosinolate derivatives (isothiocyanates) | - | HG broccoli ↓ LDL by 5–7% in RCTs |
| Sturm C. [78] | Broccoli, cabbage, rocket (arugula) | Sulforaphane, Allyl isothiocyanate (AITC), Erucin | 50–100 g/day | ↓ cancer risk (colorectal, lung) | Activates Nrf2 pathway, suppresses NF-κB, induces phase II enzymes | ↓ IL-6, TNF-α, CRP, COX-2 | Improves antioxidant response, reduces inflammatory markers |
| Johnson I. [75] | Broccoli, cauliflower, kale, Brussels sprouts | Sulforaphane, Indoles, Isothiocyanates | ~200 g/day cruciferous vegetables | ↓ risk of colorectal and gastric cancers by 8% and 19%, respectively | Modulates phase I and II detoxifying enzymes, induces apoptosis | ↓ IL-6, TNF-α, modulation of immune response | Improves vascular health, reduces risk of atherosclerosis |
| Abellán A. [77] | Broccoli, radish, kale, mustard, pak choi | Sulforaphane, Glucoiberin | 30 g/day of sprouts (broccoli/radish) | ↓ cancer risk (colon, breast) | Inhibits phase-I enzymes, induces phase-II detoxifying enzymes (Nrf2) | ↓ IL-6, TNF-α, CRP by 59% | Modulates oxidative stress, improves lipid profile |
| López-Chillón M. [87] | Broccoli sprouts | Isothiocyanates | 30 g/day | - | - | ↓IL-6 and CRP | ↓ inflammatory markers in overweight subjects |
| Sikorska-Zimny K & Beneduce L [23] | Brassica species (B. nigra, B. oleracea, B. rapa, etc.) | Isothiocyanates (e.g., Sulforaphane), Indoles | 150–200 g/day of Brassica vegetables | ↓ cancer risk (lung, gastrointestinal) | Modulates phase II detoxification enzymes, induces apoptosis | ↓ IL-6, CRP, TNF-α | Protects endothelial function, reduces atherosclerosis progression |
| Langston-Cox A. [88] | Broccoli extract | Sulforaphane | Higher doses needed for efficacy in pregnant women | - | May improve endothelial function and blood pressure | - | Modest ↓ in diastolic blood pressure in women with pregnancy hypertension |
| Na G. [89] | Broccoli, watercress, kale, mustard | Sulforaphane, Allyl isothiocyanate (AITC) | 70 g/day of broccoli sprouts | ↓ cancer risk (breast, liver, bladder) | Modulates tumor microenvironment, inhibits glycolysis, regulates self-renewal signaling of cancer stem cells | ↓ IL-8, modulates gut microbiota | Protects cardiovascular health via angiogenesis regulation |
| Connolly E. [76] | Broccoli, kale, cauliflower, cabbage | Glucoraphanin, Sulforaphane | 300 g/day of cruciferous vegetables | Reduces risk of cardiovascular events | Activates Nrf2, reduces oxidative stress | ↓ markers of inflammation such as F2-isoprostanes | ↓ systolic blood pressure compared to root and squash vegetables |
4.2. Role of Glucosinolates in Chronic Disease Prevention
4.2.1. Oxidative Stress and Anti-Inflammatory Effects
4.2.2. Anti-Cancer Effects of GSLs
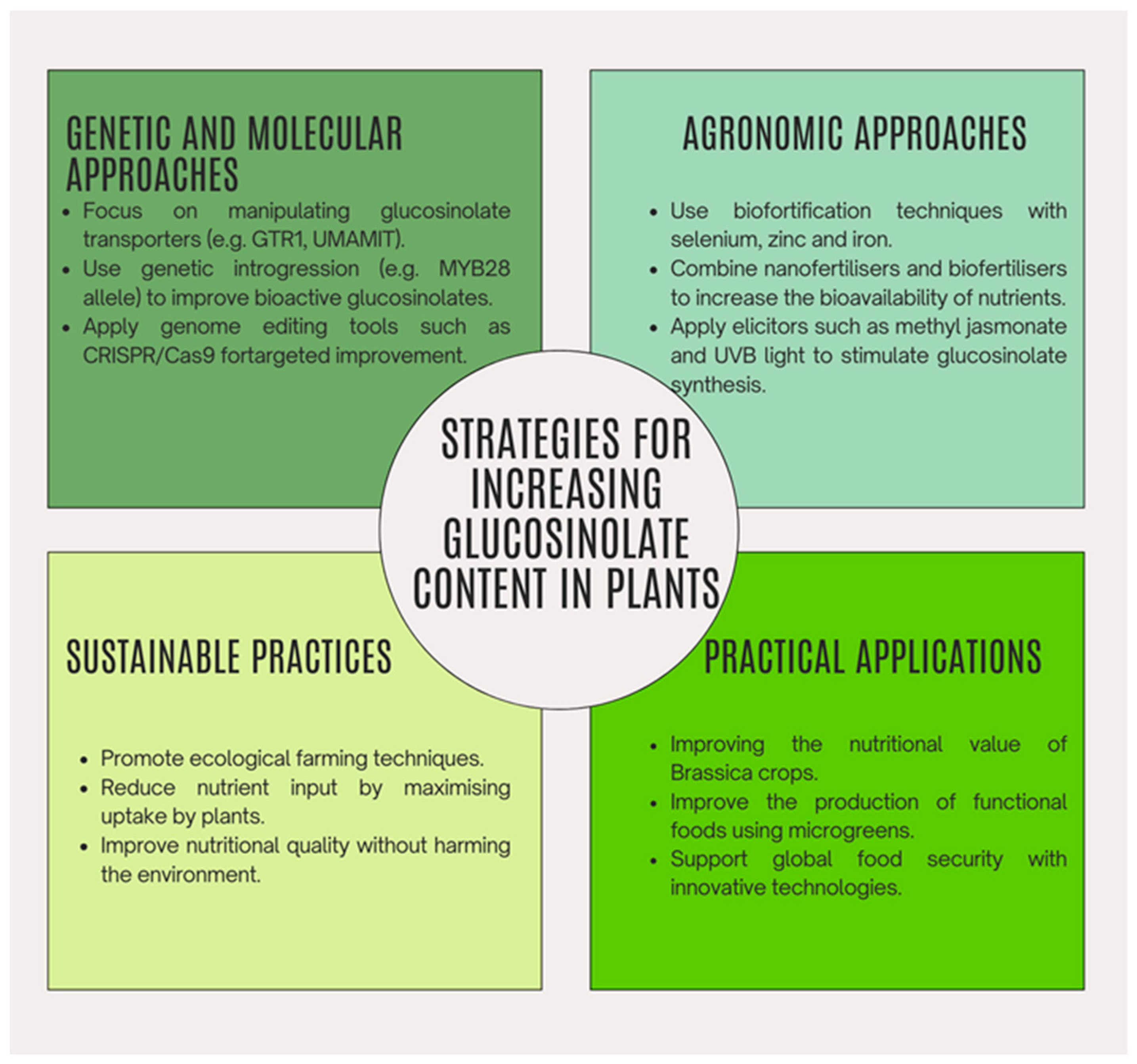
5. Strategies for Increasing Glucosinolate Content in Plants
5.1. Genetic and Molecular Approaches
5.2. Agronomic and Technical Approaches
5.3. Genetic Introgression
5.4. Metabolic Engineering
5.5. Use of Microgreens and Pre-Harvest Light Application
6. Strengths, Limitations, and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AITC | Allyl Isothiocyanate |
| AMPK | Adenosine 5′-Monophosphate-Activated Protein Kinase |
| ARE | Antioxidant-Responsive Element |
| CR | Controlled Release |
| CRP | C-Reactive Protein |
| FA | Fatty Acid |
| GCLC | Glutamate-Cysteine γ-Ligase |
| GSH | Glutathione |
| GSLs | Glucosinolates |
| HCC | hepato cellular carcinoma |
| HDAC | Histone Deacetylase |
| HDL | High-Density Lipoprotein |
| HO-1 | Heme Oxygenase-1 |
| HSP | Heat Shock Protein |
| IL-6 | Interleukin-6 |
| ITC | Isothiocyanate |
| LDL | Low-Density Lipoprotein |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| Nrf2 | Nuclear Factor Erythroid 2–related factor 2 |
| OS | Oxidative Stress |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitric Oxide Species |
| SFN | Sulforaphane |
| SOD | Superoxide Dismutase |
| TCA | Tricarboxylic Acid (Cycle) |
| TNF-α | Tumor Necrosis Factor-Alpha |
References
- Akram, M.; Jabeen, F.; Riaz, M.; Khan, F.S.; Okushanova, E.; Imran, M.; Shariati, M.A.; Riaz, T.; Egbuna, C.; Ezeofor, N.J. Health Benefits of Glucosinolate Isolated from Cruciferous and Other Vegetables. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 361–371. ISBN 978-0-12-820284-5. [Google Scholar]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Juvik, J. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A Comparative Review of Key Isothiocyanates and Their Health Benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef]
- Prado, N.J.; Ramirez, D.; Mazzei, L.; Parra, M.; Casarotto, M.; Calvo, J.P.; Cuello Carrión, D.; Ponce Zumino, A.Z.; Diez, E.R.; Camargo, A.; et al. Anti-Inflammatory, Antioxidant, Antihypertensive, and Antiarrhythmic Effect of Indole-3-Carbinol, a Phytochemical Derived from Cruciferous Vegetables. Heliyon 2022, 8, e08989. [Google Scholar] [CrossRef]
- Chen, M.; Huang, L.; Lv, Y.; Li, L.; Dong, Q. Sulforaphane protects against oxidative stress induced apoptosis via activating SIRT1 in mouse osteoarthritis. Mol. Med. Rep. 2021, 24, 612. [Google Scholar] [CrossRef]
- Shakour, Z.; Shehab, N.; Gomaa, A.; Wessjohann, L.; Farag, M. Metabolic and Biotransformation Effects on Dietary Glucosinolates, Their Bioavailability, Catabolism and Biological Effects in Different Organisms. Biotechnol. Adv. 2022, 54, 107784. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.Y. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A Systematic Review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Glucosinolates in the human diet. Bioavailability and implications for health. Phytochem. Rev. 2002, 1, 183–188. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Özer, Ş.D.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid. Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellostas, N.; Sorensen, J.; Sorensen, H. Profiling Glucosinolates in Vegetative and Reproductive Tissues of Four Brassica Species of the U-Triangle for Their Biofumigation Potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Nguyen, V.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica Foods: Bioavailability in Food and Significance for Human Health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; De Haro, A. Seasonal Variation in Glucosinolate Content in Brassica oleracea Crops Grown in Northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors Affecting the Glucosinolate Content of Kale (Brassica oleracea Acephala Group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Galanakis, C.M. Glucosinolates: Properties, Recovery, and Applications; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-816493-8. [Google Scholar]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, L. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.; Dinkova-Kostova, A.; Kensler, T. Broccoli or Sulforaphane: Is It the Source or Dose that Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Kamal, R.; Razis, A.; Sukri, N.; Perimal, E.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Kuchernig, J.C.; Burow, M.; Wittstock, U. Evolution of specifier proteins in glucosinolate-containing plants. BMC Evol. Biol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Baskar, V.; Park, S.W.; Nile, S.H. An Update on Potential Perspectives of Glucosinolates on Protection against Microbial Pathogens and Endocrine Dysfunctions in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 2231–2249. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Reid, R.W.; Chebrolu, K.K.; Thomas, A.; Krueger, C.; Jeffery, E.; Jackson, E.; Juvik, J.A. Genetic Analysis of Glucosinolate Variability in Broccoli Florets Using Genome-Anchored Single Nucleotide Polymorphisms. Theor. Appl. Genet. 2015, 128, 1431–1447. [Google Scholar] [CrossRef]
- Cieslik, E.; Leszczynska, T.; Filipiak-Florkiewicz, A.; Sikora, E.; Pisulewski, P. Effects of Some Technological Processes on Glucosinolate Contents in Cruciferous Vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Tarar, A.; Peng, S.; Cheema, S.; Peng, C.-A. Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate. Bioengineering 2022, 9, 470. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshimoto, M.; Murata, Y.; Shimoishi, Y.; Asai, Y.; Park, E.Y.; Sato, K.; Nakamura, Y. Papaya Seed Represents a Rich Source of Biologically Active Isothiocyanate. J. Agric. Food Chem. 2007, 55, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Vicas, S.I.; Teusdea, A.C.; Carbunar, M.; Socaci, S.A.; Socaciu, C. Glucosinolates Profile and Antioxidant Capacity of Romanian Brassica Vegetables Obtained by Organic and Conventional Agricultural Practices. Plant Foods Hum. Nutr. 2013, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Won, Y.K.; Ong, C.N.; Whiteman, M. β-Phenylethyl and 8-Methylsulphinyloctyl Isothiocyanates, Constituents of Watercress, Suppress LPS Induced Production of Nitric Oxide and Prostaglandin E2 in RAW 264.7 Macrophages. Nitric Oxide 2005, 12, 237–243. [Google Scholar] [CrossRef]
- Justen, V.L.; Cohen, J.D.; Gardner, G.; Fritz, V.A. Seasonal Variation in Glucosinolate Accumulation in Turnip Cultivars Grown with Colored Plastic Mulches. HortScience 2011, 46, 1608–1614. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical Composition and Biological Activity of 8 Varieties of Radish (Raphanus sativus L.) Sprouts and Mature Taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–46. ISBN 978-3-319-26479-0. [Google Scholar]
- Maldini, M.; Maksoud, S.A.; Natella, F.; Montoro, P.; Petretto, G.L.; Foddai, M.; De Nicola, G.R.; Chessa, M.; Pintore, G. Moringa Oleifera: Study of Phenolics and Glucosinolates by Mass Spectrometry. J. Mass Spectrom. 2014, 49, 900–910. [Google Scholar] [CrossRef]
- Clarke, D. Glucosinolates, Structures and Analysis in Food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Orlando, P.; Nartea, A.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Dludla, P.; Fiorini, R.; Pacetti, D.; Loizzo, M.; Lucci, P.; et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. Var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants 2022, 11, 209. [Google Scholar] [CrossRef]
- Zhi, Q.; Li, Y.; Li, F.; Tian, Y.; Li, F.; Tang, Y.; Yang, Y.; Yin, R.; Ming, J. Polyphenols Extracted from Coreopsis Tinctoria Buds Exhibited a Protective Effect against Acute Liver Damage. J. Funct. Foods 2018, 44, 201–208. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate-Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; López, C.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. In Functional Food Ingredients from Plants; Academic Press: Cambridge, MA, USA, 2019; Volume 90, ISBN 978-0-12-816567-6. [Google Scholar]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 2020, 180, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of glucoraphanin and sulforaphane from high-glucoraphanin broccoli. Mol. Nutr. Food Res. 2018, 62, 1700911. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.; Beresford, S.; Lampe, J. Variation of Glucoraphanin Metabolism in Vivo and Ex Vivo by Human Gut Bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef]
- Winde, I.; Wittstock, U. Insect Herbivore Counteradaptations to the Plant Glucosinolate–Myrosinase System. Phytochemistry 2011, 72, 1566–1575. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Tomkins, B.; Nicolas, M.E.; Premier, R.R.; Bennett, R.N.; Eagling, D.R.; Taylor, P.W.J. The Effect of Post-Harvest and Packaging Treatments on Glucoraphanin Concentration in Broccoli (Brassica oleracea Var. Italica). J. Agric. Food Chem. 2002, 50, 7386–7391. [Google Scholar] [CrossRef]
- Badełek, E.; Kosson, R.; Adamicki, F. The Effect of Storage in Controlled Atmosphere on the Quality and Health-Promoting Components of Broccoli (Brassica oleracea bar. Italica). J. Fruit Ornam. Plant Res. 2012, 77, 89–100. [Google Scholar] [CrossRef]
- Tiwari, U.; Sheehy, E.; Rai, D.; Gaffney, M.; Evans, P.; Cummins, E. Quantitative Human Exposure Model to Assess the Level of Glucosinolates upon Thermal Processing of Cruciferous Vegetables. LWT-Food Sci. Technol. 2015, 63, 253–261. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from Brassica Vegetables Effects of Processing, Cooking, Mastication, and Digestion. Mol. Nutr. Food Res. 2018, 62, 1701069. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Massih, R.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F. Glucosinolates, a Natural Chemical Arsenal: More to Tell than the Myrosinase Story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Renz, M.; Andernach, L.; Kaufmann, M.; Rohn, S.; Hanschen, F. Degradation of Glucosinolates and Formation of Isothiocyanates, Nitriles, Amines, and N,N′-dialk(en)yl thioureas during Domestic Boiling of Red Cabbage. Food Chem. 2023, 435, 137550. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Kim, G.-H. Effects of Various Heating Methods on Glucosinolate, Carotenoid and Tocopherol Concentrations in Broccoli. Int. J. Food Sci. Nutr. 2013, 64, 103–111. [Google Scholar] [CrossRef]
- Wu, W.; Chen, J.; Yu, D.; Chen, S.; Ye, X.; Zhang, Z. Analysis of Processing Effects on Glucosinolate Profiles in Red Cabbage by LC-MS/MS in Multiple Reaction Monitoring Mode. Molecules 2021, 26, 5171. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of Thermal and Non-Thermal Processing of Cruciferous Vegetables on Glucosinolates and Its Derived Forms. J. Food Sci. Technol. 2018, 55, 1973–1981. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Drabińska, N. Changes in Glucosinolates and Their Breakdown Products during the Fermentation of Cabbage and Prolonged Storage of Sauerkraut: Focus on Sauerkraut Juice. Food Chem. 2021, 365, 130498. [Google Scholar] [CrossRef]
- Frandsen, H.; Markedal, K.; Martín-Belloso, O.; Sánchez-Vega, R.; Soliva-Fortuny, R.; Sorensen, H.; Sorensen, S.; Sorensen, J. Effects of Novel Processing Techniques on Glucosinolates and Membrane Associated Myrosinases in Broccoli. Pol. J. Food Nutr. Sci. 2014, 64, 17–25. [Google Scholar] [CrossRef]
- Westphal, A.; Riedl, K.M.; Cooperstone, J.L.; Kamat, S.; Balasubramaniam, V.M.; Schwartz, S.J.; Böhm, V. High-Pressure Processing of Broccoli Sprouts: Influence on Bioactivation of Glucosinolates to Isothiocyanates. J. Agric. Food Chem. 2017, 65, 8578–8585. [Google Scholar] [CrossRef]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Moreno, D.A.; Ferreres, F. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; Rosa, E. Glucosinolate Composition of Brassica is Affected by Postharvest, Food Processing and Myrosinase Activity. J. Food Process. Preserv. 2012, 36, 214–224. [Google Scholar] [CrossRef]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 4769–4776. [Google Scholar] [CrossRef]
- Verkerk, R.; Dekker, M. Glucosinolates and Myrosinase Activity in Red Cabbage (Brassica oleracea L. Var. Capitata f. rubra DC.) after Various Microwave Treatments. J. Agric. Food Chem. 2004, 52, 7318–7323. [Google Scholar] [CrossRef]
- Nandasiri, R.; Semenko, B.; Wijekoon, C.; Suh, M. Air-Frying Is a Better Thermal Processing Choice for Improving Antioxidant Properties of Brassica Vegetables. Antioxidants 2023, 12, 490. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Glucosinolates and Vitamin C Content in Edible Parts of Broccoli Florets after Domestic Cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial Facts about Health Benefits of Popular Cruciferous Vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Lund, E. Non-Nutritive Bioactive Constituents of Plants: Dietary Sources and Health Benefits of Glucosinolates. Int. J. Vitam. Nutr. Res. 2003, 73, 135–143. [Google Scholar] [CrossRef]
- Johnson, I. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.; Liu, A.H.; Radavelli-Bagatini, S.; Shafaei, A.; Boyce, M.C.; Wood, L.G.; McCahon, L.; Koch, H.; Sim, M.; Hill, C.R.; et al. Cruciferous Vegetables Lower Blood Pressure in Adults with Mildly Elevated Blood Pressure in a Randomized, Controlled, Crossover Trial: The VEgetableS for vaScular hEaLth (VESSEL) Study. BMC Med. 2024, 22, 353. [Google Scholar] [CrossRef] [PubMed]
- Abellán, A.; Domínguez-Perles, R.; Moreno, D.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef]
- Sturm, C.; Wagner, A. Brassica-Derived Plant Bioactives as Modulators of Chemopreventive and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef]
- Clarke, J.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.; Stevens, J.; Ho, E. Bioavailability and Inter-Conversion of Sulforaphane and Erucin in Human Subjects Consuming Broccoli Sprouts or Broccoli Supplement in a Cross-over Study Design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Armah, C.; Traka, M.; Dainty, J.; Defernez, M.; Janssens, A.; Leung, W.; Doleman, J.; Potter, J.; Mithen, R. A Diet Rich in High-Glucoraphanin Broccoli Interacts with Genotype to Reduce Discordance in Plasma Metabolite Profiles by Modulating Mitochondrial Function. Am. J. Clin. Nutr. 2013, 98, 712–722. [Google Scholar] [CrossRef]
- Keum, Y.; Jeong, W.; Kong, A. Chemoprevention by Isothiocyanates and Their Underlying Molecular Signaling Mechanisms. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2004, 555, 191–202. [Google Scholar] [CrossRef]
- Murashima, M.; Watanabe, S.; Zhuo, X.; Uehara, M.; Kurashige, A. Phase 1 Study of Multiple Biomarkers for Metabolism and Oxidative Stress after One-Week Intake of Broccoli Sprouts. BioFactors 2004, 22, 271–275. [Google Scholar] [CrossRef]
- Christiansen, B.; Muguerza, N.; Petersen, A.; Kveiborg, B.; Madsen, C.; Thomas, H.; Ihlemann, N.; Sorensen, J.; Kober, L.; Sorensen, H.; et al. Ingestion of Broccoli Sprouts Does Not Improve Endothelial Function in Humans with Hypertension. PLoS ONE 2010, 5, e12461. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli Sprouts Reduce Oxidative Stress in Type 2 Diabetes: A Randomized Double-Blind Clinical Trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli Sprouts Powder Could Improve Serum Triglyceride and Oxidized LDL/LDL-Cholesterol Ratio in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Armah, C.; Derdemezis, C.; Traka, M.; Dainty, J.; Doleman, J.; Saha, S.; Leung, W.; Potter, J.; Lovegrove, J.; Mithen, R. Diet Rich in High Glucoraphanin Broccoli Reduces Plasma LDL Cholesterol: Evidence from Randomised Controlled Trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.; Villaño, D. Effects of Long-Term Consumption of Broccoli Sprouts on Inflammatory Markers in Overweight Subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Anderson, D.; Creek, D.; Palmer, K.; Marshall, S.; Wallace, E. Sulforaphane Bioavailability and Effects on Blood Pressure in Women with Pregnancy Hypertension. Reprod. Sci. 2021, 28, 1489–1497. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Wojtasinska, A.; Kucmierz, J.; Tokarek, J.; Dybiec, J.; Rodzen, A.; Mlynarska, E.; Rysz, J.; Franczyk, B. New Insights into Cardiovascular Diseases Treatment Based on Molecular Targets. Int. J. Mol. Sci. 2023, 24, 16735. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef]
- Shah, S.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response 2019, 17, 1559325819852243. [Google Scholar] [CrossRef]
- Connolly, E.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.; Bondonno, C.; Lewis, J.; Hodgson, J.; Blekkenhorst, L. Glucosinolates from Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Kala, C.; Ali, S.; Ahmad, N.; Gilani, S.; Khan, N. Isothiocyanates: A Review. Res. J. Pharmacogn. 2018, 5, 71–89. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.; Kostov, R. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Corssac, G.; Campos-Carraro, C.; Hickmann, A.; Araujo, A.; Fernandes, R.; Belló-Klein, A. Sulforaphane Effects on Oxidative Stress Parameters in Culture of Adult Cardiomyocytes. Biomed. Pharmacother. 2018, 104, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Hrelia, S.; Malaguti, M. Neuroprotective Effects of Glucosinolates. In Glucosinolates; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-25749-5. [Google Scholar]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-Derived Isothiocyanates Impact Mitochondrial Function in Fungal Cells and Elicit an Oxidative Stress Response Necessary for Growth Recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.; El-Naga, R.; Lotfy, R.; Al-Gendy, A.; El-Demerdash, E. Anti-Fibrotic Potential of a Matthiola Arabica Isothiocyanates Rich Fraction: Impact on Oxidative Stress, Inflammatory and Fibrosis Markers. Die Pharm. 2017, 72, 614–624. [Google Scholar] [CrossRef]
- Clemente, M.; Miguel, M.; Felipe, K.; Gribner, C.; Moura, P.; Rigoni, A.; Parisotto, E.; Piltz, M.; Valdameri, G.; Henneberg, R.; et al. Biomarkers of Oxidative Stress and Inflammation in People Witha Physical Disability Treated with a Standardized Extract of Nasturtium Officinale: A Randomized, Double-Blind, and Placebo-Controlled Trial. Phytother. Res. 2020, 34, 2756–2765. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.; Wu, J.; Xu, L.; Zhang, M.; Li, Z.; Wang, D. Allyl Isothiocyanate Treatment Alleviates Chronic Obstructive Pulmonary Disease through the Nrf2-Notch1 Signaling and Upregulation of MRP1. Life Sci. 2020, 243, 117291. [Google Scholar] [CrossRef]
- Lee, Y.; Seon, M.; Cho, H.; Kim, J.; Park, J. Benzyl Isothiocyanate Exhibits Anti-Inflammatory Effects in Murine Macrophages and in Mouse Skin. J. Mol. Med. 2009, 87, 1251–1261. [Google Scholar] [CrossRef]
- Huang, C.; Lin, A.; Liu, C.; Tsai, C.; Chang, I.; Chen, H.; Lii, C. Isothiocyanates Protect against Oxidized LDL-Induced Endothelial Dysfunction by Upregulating Nrf2-Dependent Antioxidation and Suppressing NFκB Activation. Mol. Nutr. Food Res. 2013, 57, 1918–1930. [Google Scholar] [CrossRef]
- Mahn, A.; Castillo, A. Potential of Sulforaphane as a Natural Immune System Enhancer: A Review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef]
- Patel, V.; Dial, K.; Wu, J.; Gauthier, A.; Wu, W.; Lin, M.; Espey, M.; Thomas, D.; Ashby, C.; Mantell, L. Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1. Int. J. Mol. Sci. 2020, 21, 977. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dodd, G.; Kunsch, C. Sulforaphane Inhibits TNF-α-Induced Activation of P38 MAP Kinase and VCAM-1 and MCP-1 Expression in Endothelial Cells. Inflamm. Res. 2009, 58, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsieh, C.; Weng, Y.; Chuang, S.; Hsieh, C.; Wung, B. Sulforaphane Inhibition of Monocyte Adhesion via the Suppression of ICAM-1 and NF-κB Is Dependent upon Glutathione Depletion in Endothelial Cells. Vasc. Pharmacol. 2008, 48, 54–61. [Google Scholar] [CrossRef]
- Hung, C.; Huang, H.; Wang, C.; Liu, K.; Lii, C. Sulforaphane Inhibits TNF-α-Induced Adhesion Molecule Expression Through the Rho A/ROCK/NF-κB Signaling Pathway. J. Med. Food 2014, 17, 1095–1102. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane Protects from Myocardial Ischemia-Reperfusion Damage through the Balanced Activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Q.; Sun, Y.; Wang, X.; Lv, L.; Zhang, L.; Liu, J.; Zhao, S.; Wang, X. Sulforaphane Protection against the Development of Doxorubicin-Induced Chronic Heart Failure Is Associated with Nrf2 Upregulation. Cardiovasc. Ther. 2017, 35, 12277. [Google Scholar] [CrossRef]
- Kee, H.; Kim, G.; Kim, I.; Jeong, M. Sulforaphane Suppresses Cardiac Hypertrophy by Inhibiting GATA4/GATA6 Expression and MAPK Signaling Pathways. Mol. Nutr. Food Res. 2015, 59, 221–230. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, D.; Chen, D.; Zhang, Q.; Dong, H.; Wu, W.; Lu, H.; Wu, G. Sulforaphane, a Natural Isothiocyanate Compound, Improves Cardiac Function and Remodeling by Inhibiting Oxidative Stress and Inflammation in a Rabbit Model of Chronic Heart Failure. Med. Sci. Monit. 2018, 24, 1473–1483. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; Di Cesare Mannelli, L.; Manzo, O.L.; Bucci, M.; et al. Erucin Exhibits Vasorelaxing Effects and Antihypertensive Activity by H2 S-releasing Properties. Br. J. Pharmacol. 2020, 177, 824–835. [Google Scholar] [CrossRef]
- Gladine, C.; Combe, N.; Vaysse, C.; Pereira, B.; Huertas, A.; Salvati, S.; Rossignol-Castera, A.; Cano, N.; Chardigny, J. Optimized Rapeseed Oil Enriched with Healthy Micronutrients: A Relevant Nutritional Approach to Prevent Cardiovascular Diseases. Results of the Optim’Oils Randomized Intervention Trial. J. Nutr. Biochem. 2013, 24, 544–549. [Google Scholar] [CrossRef]
- Heggen, E.; Granlund, L.; Pedersen, J.; Holme, I.; Ceglarek, U.; Thiery, J.; Kirkhus, B.; Tonstad, S. Plant Sterols from Rapeseed and Tall Oils: Effects on Lipids, Fat-Soluble Vitamins and Plant Sterol Concentrations. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Chiu, C.; Hsieh, P. Bioactive Compounds and Bioactivities of Brassica oleracea L. Var. Italica Sprouts and Microgreens: An. Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, J.; Ullah, S.; Ahmad, I. Sulforaphane and Its Protective Role in Prostate Cancer: A Mechanistic Approach. Int. J. Mol. Sci. 2023, 24, 6979. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Mao, X.; Du, M. Metabolism, Absorption, and Anti-Cancer Effects of Sulforaphane: An Update. Crit. Rev. Food Sci. Nutr. 2020, 62, 3437–3452. [Google Scholar] [CrossRef]
- Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Freire-Garabal, M. Glucosinolate-degradation products as co-adjuvant therapy on prostate cancer in vitro. Int. J. Mol. Sci. 2019, 20, 4977. [Google Scholar] [CrossRef]
- Singh, K.; Hahm, E.; Alumkal, J.; Foley, L.; Hitchens, T.; Shiva, S.; Parikh, R.; Jacobs, B.; Singh, S. Reversal of the Warburg Phenomenon in Chemoprevention of Prostate Cancer by Sulforaphane. Carcinogenesis 2019, 40, 1545–1556. [Google Scholar] [CrossRef]
- Ahmed, Z.; Li, X.; Li, F.; Cheaito, H.; Patel, K.; Mosallam, E.; Elbargeesy, G.; Dou, Q. Computational and Biochemical Studies of Isothiocyanates as Inhibitors of Proteasomal Cysteine Deubiquitinases in Human Cancer Cells. J. Cell. Biochem. 2018, 119, 9006–9016. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.; Blaheta, R. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef]
- Singh, K.; Kim, S.; Hahm, E.; Pore, S.; Jacobs, B.; Singh, S. Prostate Cancer Chemoprevention by Sulforaphane in a Preclinical Mouse Model Is Associated with Inhibition of Fatty Acid Metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Z.; Khor, T.; Shu, L.; Kong, A. Sulforaphane Enhances Nrf2 Expression in Prostate Cancer TRAMP C1 Cells through Epigenetic Regulation. Biochem. Pharmacol. 2013, 85, 1398–1404. [Google Scholar] [CrossRef]
- Negele, L.; Schneider, B.; Ristl, R.; Stulnig, T.; Willfort-Ehringer, A.; Helk, O.; Widhalm, K. Effect of a Low-Fat Diet Enriched Either with Rapeseed Oil or Sunflower Oil on Plasma Lipoproteins in Children and Adolescents with Familial Hypercholesterolaemia. Results of a Pilot Study. Eur. J. Clin. Nutr. 2015, 69, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Aquilano, K.; Ciriolo, M. Punctum on Two Different Transcription Factors Regulated by PGC-1α: Nuclear Factor Erythroid-Derived 2-like 2 and Nuclear Respiratory Factor 2. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 4137–4146. [Google Scholar] [CrossRef]
- Ali, M.; Khan, N.; Kaleem, N.; Ahmad, W.; Alharethi, S.; Alharbi, B.; Alhassan, H.; Al-Enazi, M.; Razis, A.; Modu, B.; et al. Anticancer Properties of Sulforaphane: Current Insights at the Molecular Level. Front. Oncol. 2023, 13, 1168321. [Google Scholar] [CrossRef]
- Traka, M.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.; Bernuzzi, F.; et al. Transcriptional Changes in Prostate of Men on Active Surveillance after a 12-Mo Glucoraphanin-Rich Broccoli Intervention-Results from the Effect of Sulforaphane on Prostate Cancer Prevention (ESCAPE) Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Garzotto, M.; Davis, E.; Mori, M.; Stoller, W.; Farris, P.; Wong, C.; Beaver, L.; Thomas, G.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer-Int. J. 2019, 72, 74–87. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, X.; Zhan, J.; Shen, M.; Li, R. Sulforaphane Inhibits the Growth of Prostate Cancer by Regulating the microRNA-3919/DJ-1 Axis. Front. Oncol. 2024, 14, 1361152. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence Are Accompanied by DNA Hypomethylation and Changes in microRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef]
- Jackson, S.; Singletary, K. Sulforaphane: A Naturally Occurring Mammary Carcinoma Mitotic Inhibitor, Which Disrupts Tubulin Polymerization. Carcinogenesis 2004, 25, 219–227. [Google Scholar] [CrossRef]
- Jackson, S.; Singletary, K. Sulforaphane Inhibits Human MCF-7 Mammary Cancer Cell Mitotic Progression and Tubulin Polymerization. J. Nutr. 2004, 134, 2229–2236. [Google Scholar] [CrossRef]
- Alhazmi, N.; Pai, C.; Albaqami, A.; Wang, H.; Zhao, X.; Chen, M.; Hu, P.; Guo, S.; Starost, K.; Hajihassani, O.; et al. The Promyelocytic Leukemia Protein Isoform PML1 Is an Oncoprotein and a Direct Target of the Antioxidant Sulforaphane (SFN). Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118707. [Google Scholar] [CrossRef]
- Pawlik, A.; Slominska-Wojewódzka, M.; Herman-Antosiewicz, A. Sensitization of Estrogen Receptor-Positive Breast Cancer Cell Lines to 4-Hydroxytamoxifen by Isothiocyanates Present in Cruciferous Plants. Eur. J. Nutr. 2016, 55, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.; Udayakumar, N.; Lewis, K.; Tollefsbol, T. A Novel Combination of Withaferin A and Sulforaphane Inhibits Epigenetic Machinery, Cellular Viability and Induces Apoptosis of Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1092. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mohsin, J.; Prabhu, S.; Begum, S.; Nusri, Q.; Harish, G.; Javed, E.; Khan, M.; Sharma, C. Sulforaphane Inhibits Growth of Human Breast Cancer Cells and Augments the Therapeutic Index of the Chemotherapeutic Drug, Gemcitabine. Asian Pac. J. Cancer Prev. 2013, 14, 5855–5860. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Sulphoraphane, a Naturally Occurring Isothiocyanate Induces Apoptosis in Breast Cancer Cells by Targeting Heat Shock Proteins. Biochem. Biophys. Res. Commun. 2012, 427, 80–85. [Google Scholar] [CrossRef]
- Kanematsu, S.; Uehara, N.; Miki, H.; Yoshizawa, K.; Kawanaka, A.; Yuri, T.; Tsubura, A. Autophagy Inhibition Enhances Sulforaphane-Induced Apoptosis in Human Breast Cancer Cells. Anticancer Res. 2010, 30, 3381–3390. [Google Scholar]
- Bagheri, M.; Fazli, M.; Saeednia, S.; Kharanagh, M.; Ahmadiankia, N. Sulforaphane Modulates Cell Migration and Expression of β-Catenin and Epithelial Mesenchymal Transition Markers in Breast Cancer Cells. Iran. J. Public Health 2020, 49, 77–85. [Google Scholar]
- Atwell, L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.; Naik, A.; Oh, K.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Zhang, Z.; Atwell, L.; Farris, P.; Ho, E.; Shannon, J. Associations between Cruciferous Vegetable Intake and Selected Biomarkers among Women Scheduled for Breast Biopsies. Public Health Nutr. 2016, 19, 1288–1295. [Google Scholar] [CrossRef]
- Yan, L.; Yan, Y. Therapeutic Potential of Sulforaphane in Liver Diseases: A Review. Front. Pharmacol. 2023, 14, 1256029. [Google Scholar] [CrossRef]
- Liu, P.; Atkinson, S.; Akbareian, S.; Zhou, Z.; Munsterberg, A.; Robinson, S.; Bao, Y. Sulforaphane Exerts Anti-Angiogenesis Effects against Hepatocellular Carcinoma through Inhibition of STAT3/HIF-1α/VEGF Signalling. Sci. Rep. 2017, 7, 12651. [Google Scholar] [CrossRef]
- Zou, X.; Qu, Z.; Fang, Y.; Shi, X.; Ji, Y. Endoplasmic Reticulum Stress Mediates Sulforaphane-Induced Apoptosis of HepG2 Human Hepatocellular Carcinoma Cells. Mol. Med. Rep. 2017, 15, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.; Kang, S.; Kim, K.; Kim, M.; Choi, Y.; Kim, G. Sulforaphane Decreases Viability and Telomerase Activity in Hepatocellular Carcinoma Hep3B Cells through the Reactive Oxygen Species-Dependent Pathway. Cancer Lett. 2010, 295, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, J.; Hou, B.; Deng, C.; Wu, H.; Shen, L. Sulforaphane Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma Cells via the Reactive Oxygen Species-Dependent Pathway. Oncol. Rep. 2016, 35, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wallig, M.; Jeffery, E. Dietary Broccoli Lessens Development of Fatty Liver and Liver Cancer in Mice Given Diethylnitrosamine and Fed a Western or Control Diet. J. Nutr. 2016, 146, 542–550. [Google Scholar] [CrossRef]
- Rudolf, E.; Cervinka, M. Sulforaphane Induces Cytotoxicity and Lysosome- and Mitochondria-Dependent Cell Death in Colon Cancer Cells with Deleted P53. Toxicol. Vitr. 2011, 25, 1302–1309. [Google Scholar] [CrossRef]
- Liu, K.; Shih, T.; Kuo, C.; Ma, Y.; Yang, J.; Wu, P.; Huang, Y.; Lai, K.; Chung, J. Sulforaphane Induces Cell Death Through G2/M Phase Arrest and Triggers Apoptosis in HCT 116 Human Colon Cancer Cells. Am. J. Chin. Med. 2016, 44, 1289–1310. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Zhu, J.; Xie, C.; Li, X.; Wu, J.; Geng, S.; Han, H.; Zhong, C. TAp63α Targeting of Lgr5 Mediates Colorectal Cancer Stem Cell Properties and Sulforaphane Inhibition. Oncogenesis 2020, 9, 89. [Google Scholar] [CrossRef]
- Coutinho, L.; Tortelli, T.J.; Rangel, M. Sulforaphane: An Emergent Anti-Cancer Stem Cell Agent. Front. Oncol. 2023, 13, 1089115. [Google Scholar] [CrossRef]
- Nambiar, D.M.; Kumari, J.; Augustine, R.; Kumar, P.; Bajpai, P.K.; Bisht, N.C. GTR1 and GTR2 transporters differentially regulate tissue-specific glucosinolate contents and defence responses in the oilseed crop Brassica juncea. Plant Cell Environ. 2021, 44, 2729–2743. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sanden, N.C.H.; Hansen, L.L.; Belew, Z.M.; Madsen, S.R.; Meyer, L.; Jørgensen, M.E.; Hunziker, P.; Veres, D.; Crocoll, C.; et al. Export of defensive glucosinolates is key for their accumulation in seeds. Nature 2023, 617, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, D.; Stylianidis, G.; Manta, V.; Karousis, E.; Tzanaki, A.; Dimitriadi, D.; Bouzas, E.; Siyiannis, V.; Constantinou-Kokotou, V.; Chorianopoulou, S.N.; et al. Floret Biofortification of Broccoli Using Amino Acids Coupled with Selenium under Different Surfactants: A Case Study of Cultivating Functional Foods. Plants 2023, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E. Selenium Biofortification in Radish Enhances Nutritional Quality via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics, and Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.; García-Sánchez, C.; Sánchez-Arellano, O.; Bojorquez-Rodríguez, E.; Jacobo-Velázquez, D. Biofortification of Broccoli Microgreens (Brassica oleracea Var. Italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers. Foods 2023, 12, 3826. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.; Ruiz, J. Zinc Biofortification Improves Phytochemicals and Amino-Acidic Profile in Brassica oleracea Cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef]
- Ramchiary, N.; Bisht, N.; Gupta, V.; Mukhopadhyay, A.; Arumugam, N.; Sodhi, Y.; Pental, D.; Pradhan, A. QTL Analysis Reveals Context-Dependent Loci for Seed Glucosinolate Trait in the Oilseed Brassica juncea: Importance of Recurrent Selection Backcross Scheme for the Identification of “true” QTL. Theor. Appl. Genet. 2007, 116, 77–85. [Google Scholar] [CrossRef]
- Neequaye, M.; Steuernagel, B.; Saha, S.; Trick, M.; Troncoso-Rey, P.; van den Bosch, F.; Traka, M.; Ostergaard, L.; Mithen, R. Characterisation of the Introgression of Brassica villosa Genome into Broccoli to Enhance Methionine-Derived Glucosinolates and Associated Health Benefits. Front. Plant Sci. 2022, 13, 855707. [Google Scholar] [CrossRef]
- Yi, G.; Robin, A.; Yang, K.; Park, J.; Hwang, B.; Nou, I. Exogenous Methyl Jasmonate and Salicylic Acid Induce Subspecies-Specific Patterns of Glucosinolate Accumulation and Gene Expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef]
- Qin, H.; King, G.; Borpatragohain, P.; Zou, J. Developing Multifunctional Crops by Engineering Brassicaceae Glucosinolate Pathways. Plant Commun. 2023, 4, 100565. [Google Scholar] [CrossRef]
- Sohn, S.; Thamilarasan, S.; Pandian, S.; Oh, Y.; Ryu, T.; Lee, G.; Shin, E. Interspecific Hybridization of Transgenic Brassica napus and Brassica rapa-An Overview. Genes 2022, 13, 1442. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Miles, A.; Strackhouse, T.; Cook, L.; Leng, S.; Patel, S.; Klinger, K.; Rudrabhatla, S.; Potlakayala, S. Methods of Crop Improvement and Applications towards Fortifying Food Security. Front. Genome Ed. 2023, 5, 1171969. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR Transporters Are Essential for Translocation of Glucosinolate Defence Compounds to Seeds. Nature 2012, 488, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pujante, P.; Sabater-Jara, A.; Belchí-Navarro, S.; Pedreño, M.; Almagro, L. Increased Glucosinolate Production in Brassica oleracea Var. Italica Cell Cultures Due to Coronatine Activated Genes Involved in Glucosinolate Biosynthesis. J. Agric. Food Chem. 2019, 67, 102–111. [Google Scholar] [CrossRef]
- Qian, H.; Sun, B.; Miao, H.; Cai, C.; Xu, C.; Wang, Q. Variation of Glucosinolates and Quinone Reductase Activity among Different Varieties of Chinese Kale and Improvement of Glucoraphanin by Metabolic Engineering. Food Chem. 2015, 168, 321–326. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Wang, J.; Zhang, F.; Sun, B.; Wang, Q. Improvement of Glucosinolates by Metabolic Engineering in Brassica Crops. Abiotech 2021, 2, 314–329. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens-A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Ebert, A. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment Reveals an Elevated Level of Glucosinolate Content in CaCl2 Treated Broccoli Microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Yang, T.; Luo, Y.; Chen, P. Preharvest UVB Application Increases Glucosinolate Contents and Enhances Postharvest Quality of Broccoli Microgreens. Molecules 2021, 26, 3247. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; He, X.; He, R.; Li, Y.; Liu, X.; Liu, H. Effects of Pre-Harvest Supplemental UV-A Light on Growth and Quality of Chinese Kale. Molecules 2022, 27, 7763. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Park, S.; Kim, J.; Park, Y.; Kim, M.; Nguyen, B.; Lee, S. Metabolic Profiling of Primary and Secondary Metabolites in Kohlrabi (Brassica oleracea Var. gongylodes) Sprouts Exposed to Different Light-Emitting Diodes. Plants 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Li, F.; Lei, X.; Ge, L.; Li, H.; Zhao, N.; Ming, J. The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods 2024, 13, 3507. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhao, Y.; Peng, R.; Zhang, Z.; Xu, Z.; Simal-Gandara, J.; Yang, H.; Deng, J. Bioactive Sulforaphane from Cruciferous Vegetables: Advances in Biosynthesis, Metabolism, Bioavailability, Delivery, Health Benefits, and Applications. Crit. Rev. Food Sci. Nutr. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rani, H.; Kaur, G.; Kumar, S.; Sheikh, S.; Samota, M.K. Comprehensive Overview of Glucosinolates in Crucifers: Occurrence, Roles, Metabolism, and Transport Mechanisms—A Review. Phytochem. Rev. 2024, 1–28. [Google Scholar] [CrossRef]
| Glucosinolate Metabolite | Precursor Glucosinolate | Chemical Structure 1 | Primary Source |
|---|---|---|---|
| Sulforaphane (SFN) | Glucoraphanin | 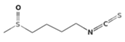 | Broccoli, broccoli sprouts [30,31] |
| Allyl isothiocyanate (AITC) | Sinigrin |  | Mustard, wasabi [32] |
| Benzyl isothiocyanate (BITC) | Glucotropaeolin | 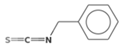 | Papaya seeds, mustard [33] |
| Phenethyl-isothiocyanate (PEITC) | Gluconasturtiin | 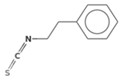 | Watercress [24] |
| Erucin (ER) | Glucoerucin | 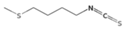 | Arugula, broccoli [30] |
| 3,3′-Diindolylmethane (DIM) | Indole-3-carbinol |  | Cabbage, broccoli [30] |
| Goitrin | Progoitrin | 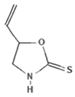 | Mustard greens, turnips [34] |
| Sulforaphane nitrile | Glucoraphanin | 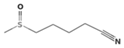 | Broccoli, cabbage [30,35] |
| Iberin nitrile | Glucoiberin | 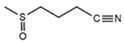 | Broccoli, watercress [36,37] |
| Raphasatin | Glucoraphasatin | 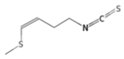 | Radish, daikon [37] |
| Epithionitrile compounds | Sinigrin, Gluconapin | 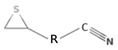 R: alkyl group | Various crucifers [38] |
| Thiocyanates | Various GSL (e.g., benzyl, allyl GSL) | 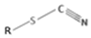 | Rare in dietary plants [38] |
| Moringin (4-(α-L-rhamnopyranosyloxy)-benzyl ITC) | Glucomoringin | 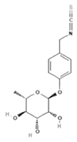 | Moringa seeds, leaves [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldelli, S.; Lombardo, M.; D’Amato, A.; Karav, S.; Tripodi, G.; Aiello, G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods 2025, 14, 912. https://doi.org/10.3390/foods14060912
Baldelli S, Lombardo M, D’Amato A, Karav S, Tripodi G, Aiello G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods. 2025; 14(6):912. https://doi.org/10.3390/foods14060912
Chicago/Turabian StyleBaldelli, Sara, Mauro Lombardo, Alfonsina D’Amato, Sercan Karav, Gianluca Tripodi, and Gilda Aiello. 2025. "Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention" Foods 14, no. 6: 912. https://doi.org/10.3390/foods14060912
APA StyleBaldelli, S., Lombardo, M., D’Amato, A., Karav, S., Tripodi, G., & Aiello, G. (2025). Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods, 14(6), 912. https://doi.org/10.3390/foods14060912










