Novel Strategies for Yuba Quality Improvement: Protein Modification Based on Physical Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Preparation of Wet Yuba
2.2.2. Protein Modification Strategies
2.2.3. Determination of Moisture Content on a Wet Basis
2.2.4. Determination of Moisture Content on a Dry Basis and the Drying Rate
2.2.5. Analysis of the Mechanical Properties of Yuba
2.2.6. Determination of the Color of Yuba
2.2.7. Determination of the Rehydration Ratio of Yuba
2.2.8. Determination of the Microstructure of Yuba
2.2.9. Analysis of Thermal Stability of Yuba
2.2.10. Analysis of Protein Secondary Structure
2.2.11. Analysis of Protein Tertiary Structure
2.2.12. Sensory Evaluation of Yuba
2.3. Statistical Analysis
3. Results and Discussion
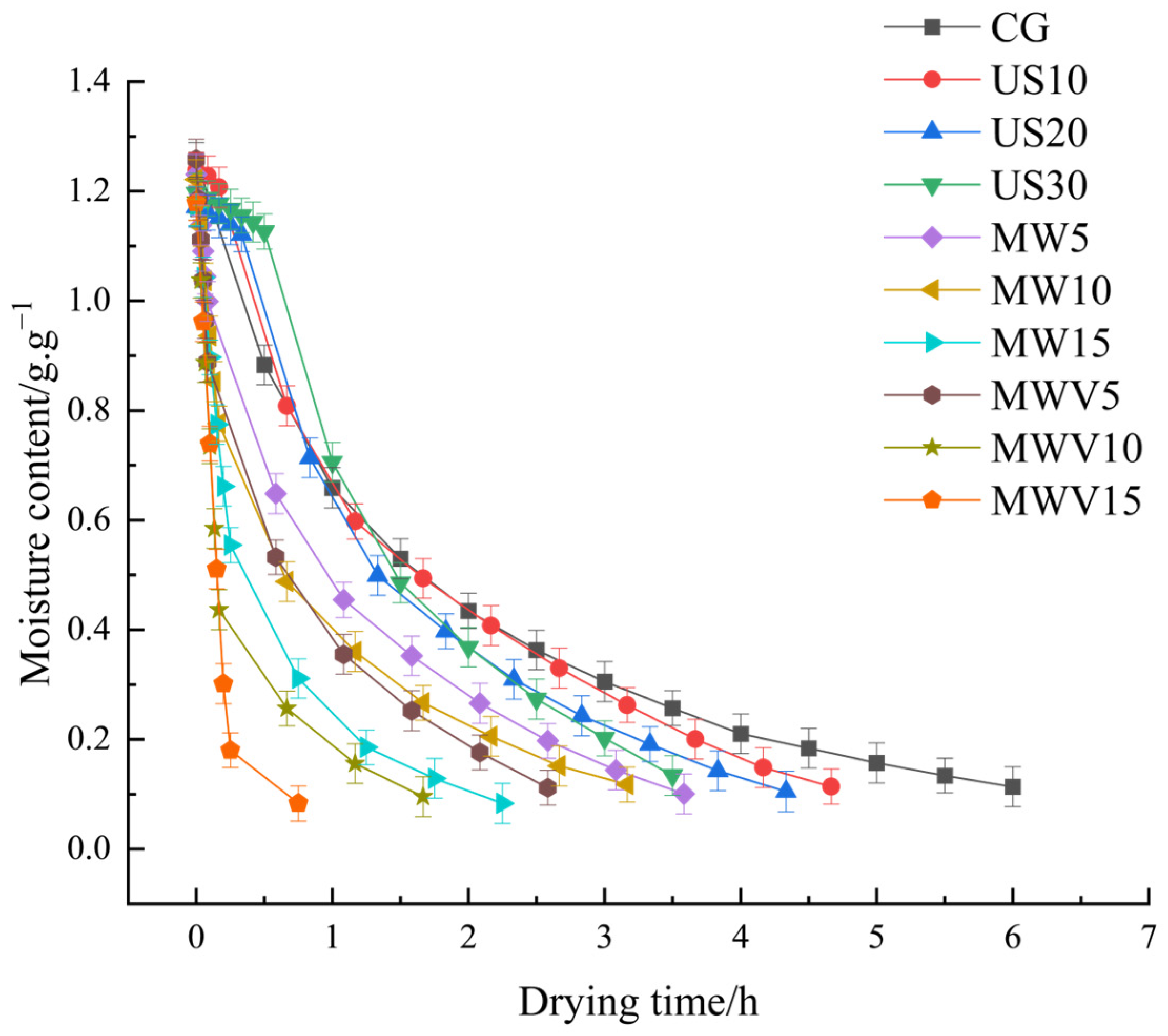
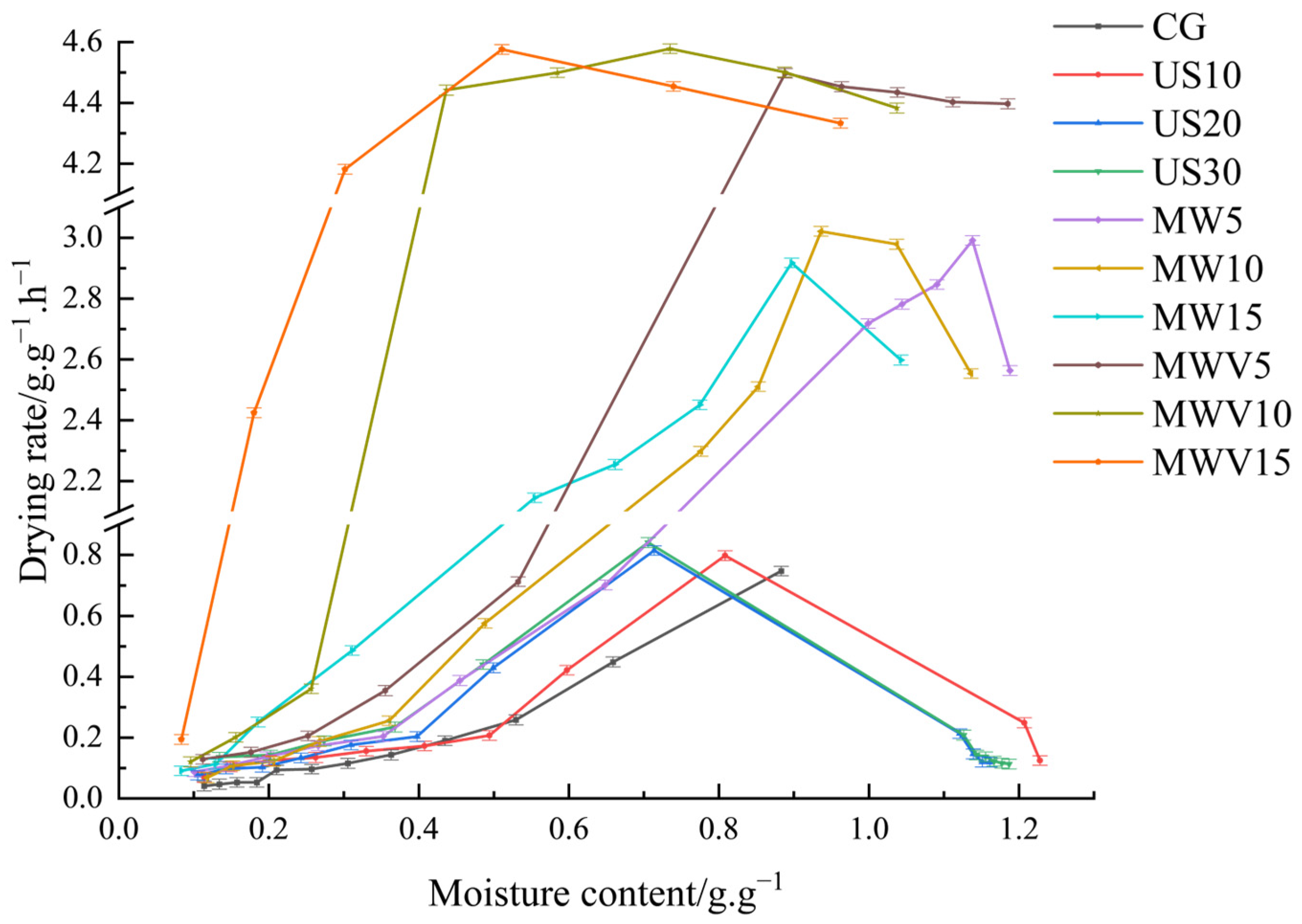
3.1. Effects of Protein Modification Methods on the Drying Characteristics of Yuba
3.2. Effects of Protein Modification Methods on the Mechanical Properties and Color of Yuba
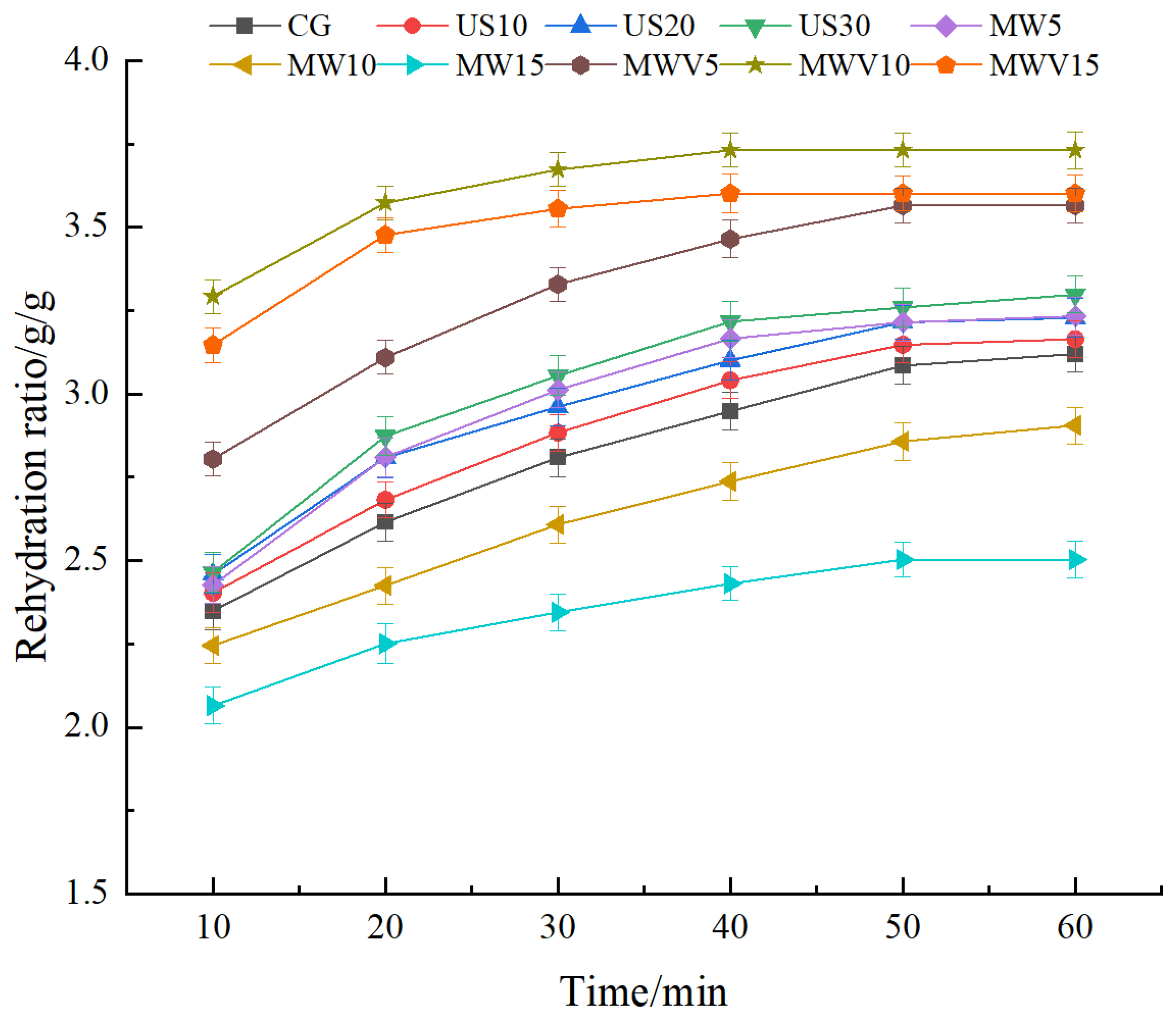
3.3. Effects of Protein Modification Methods on the Rehydration Properties of Yuba
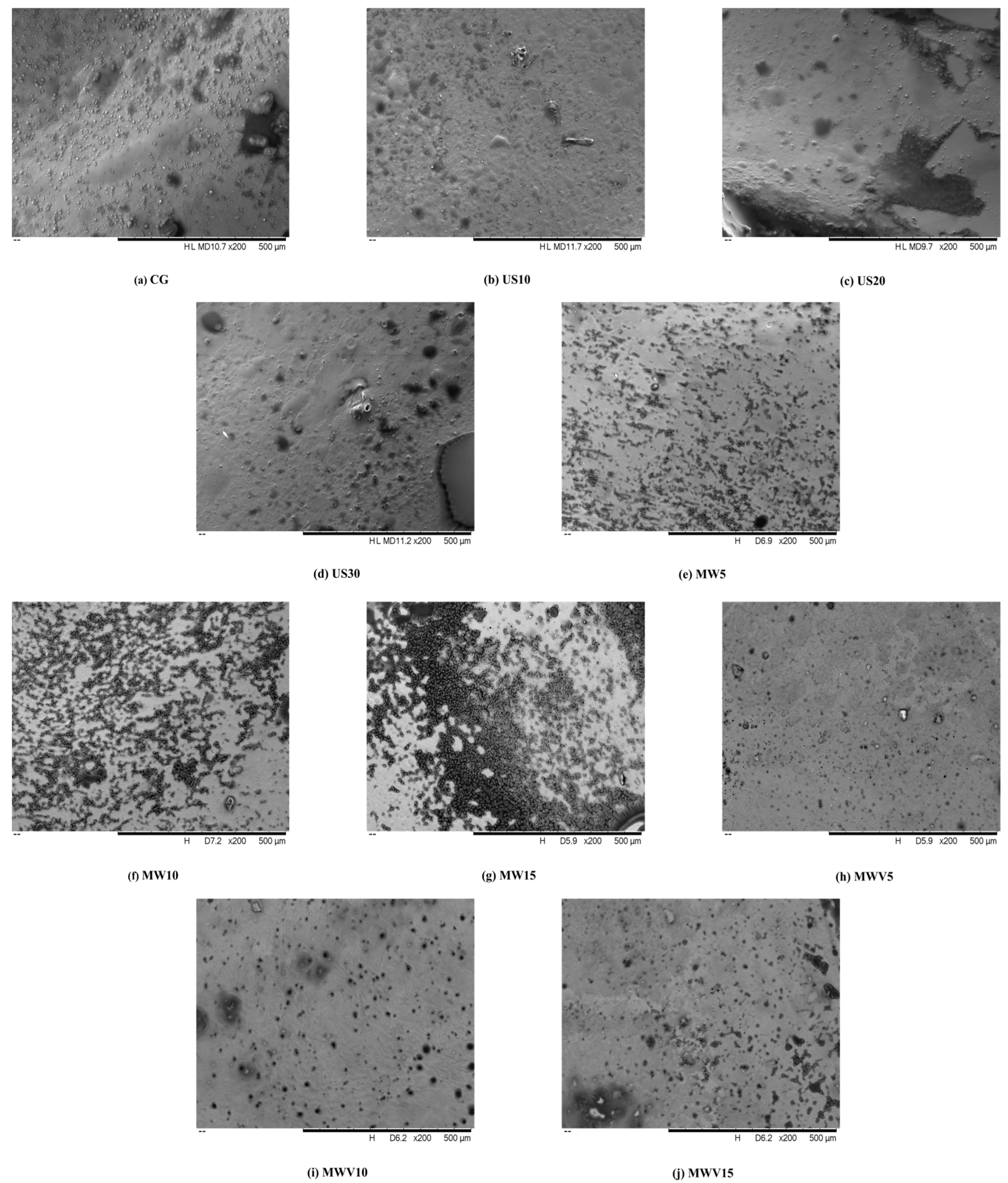
3.4. Effects of Protein Modification Methods on the Microstructure of Yuba
3.5. Effects of Protein Modification Methods on the Thermal Stability of Yuba
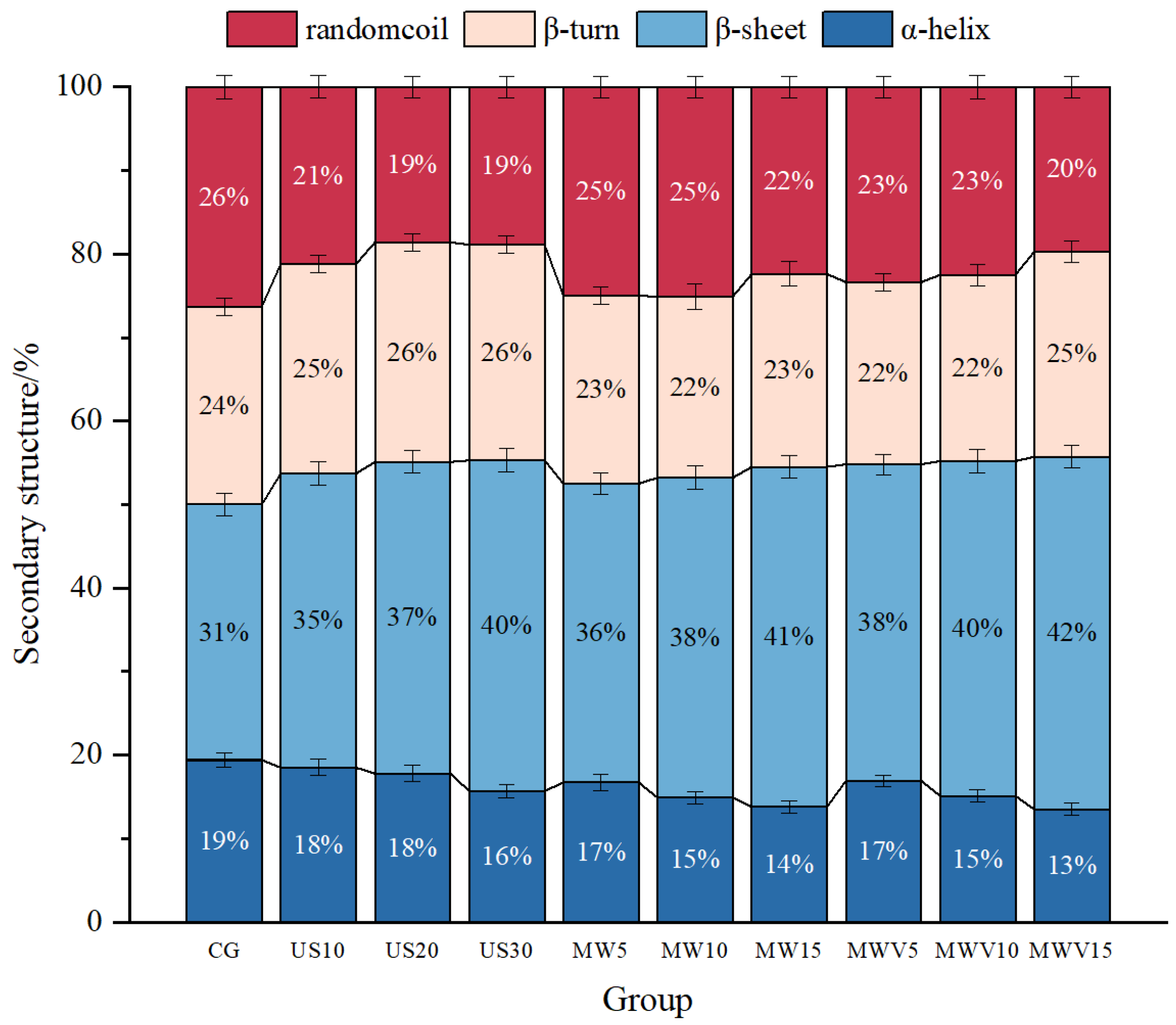
3.6. Effects of Protein Modification Methods on the Secondary Structure of Yuba Protein
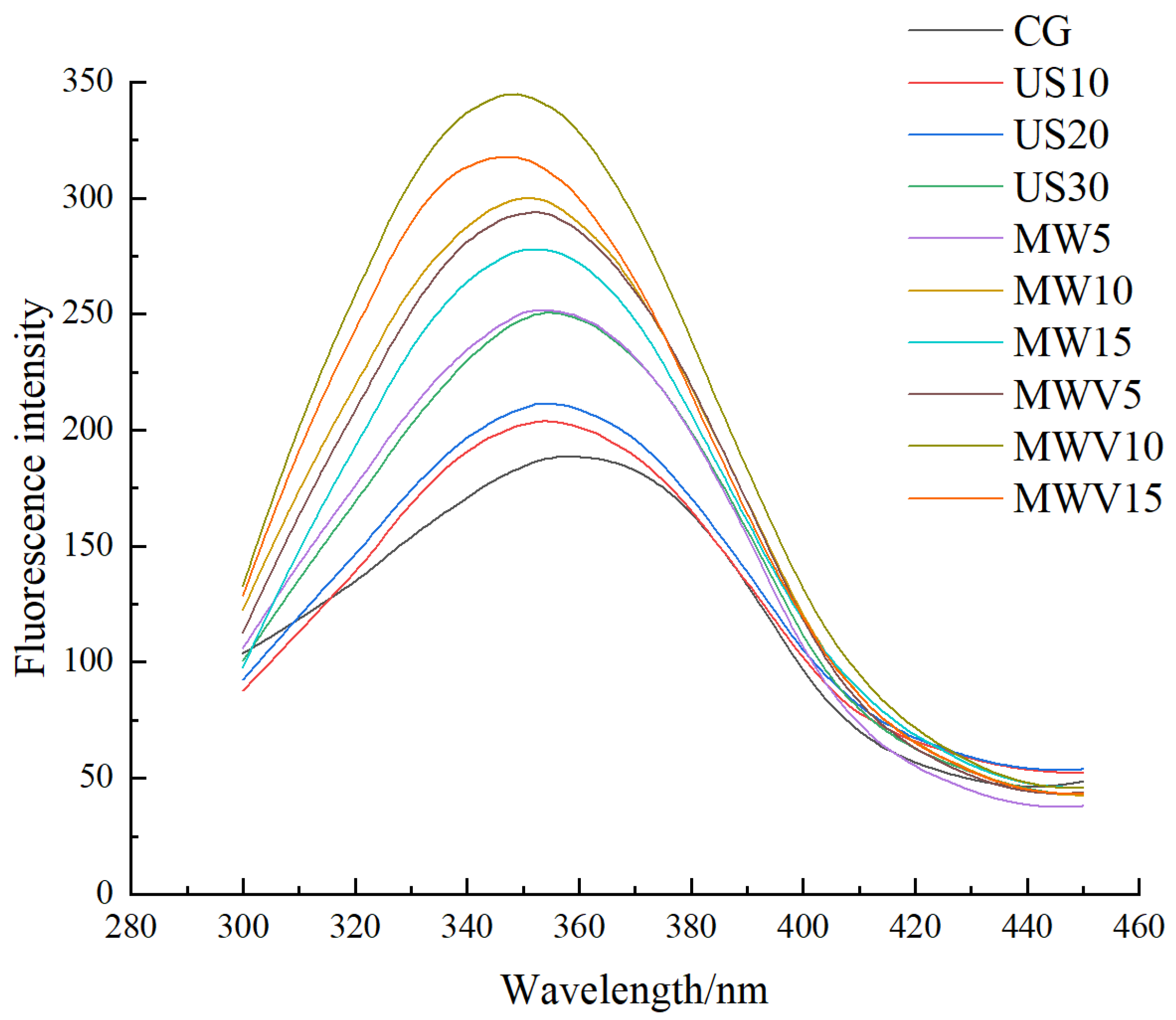
3.7. Effects of Protein Modification Methods on the Tertiary Structure of Yuba Protein
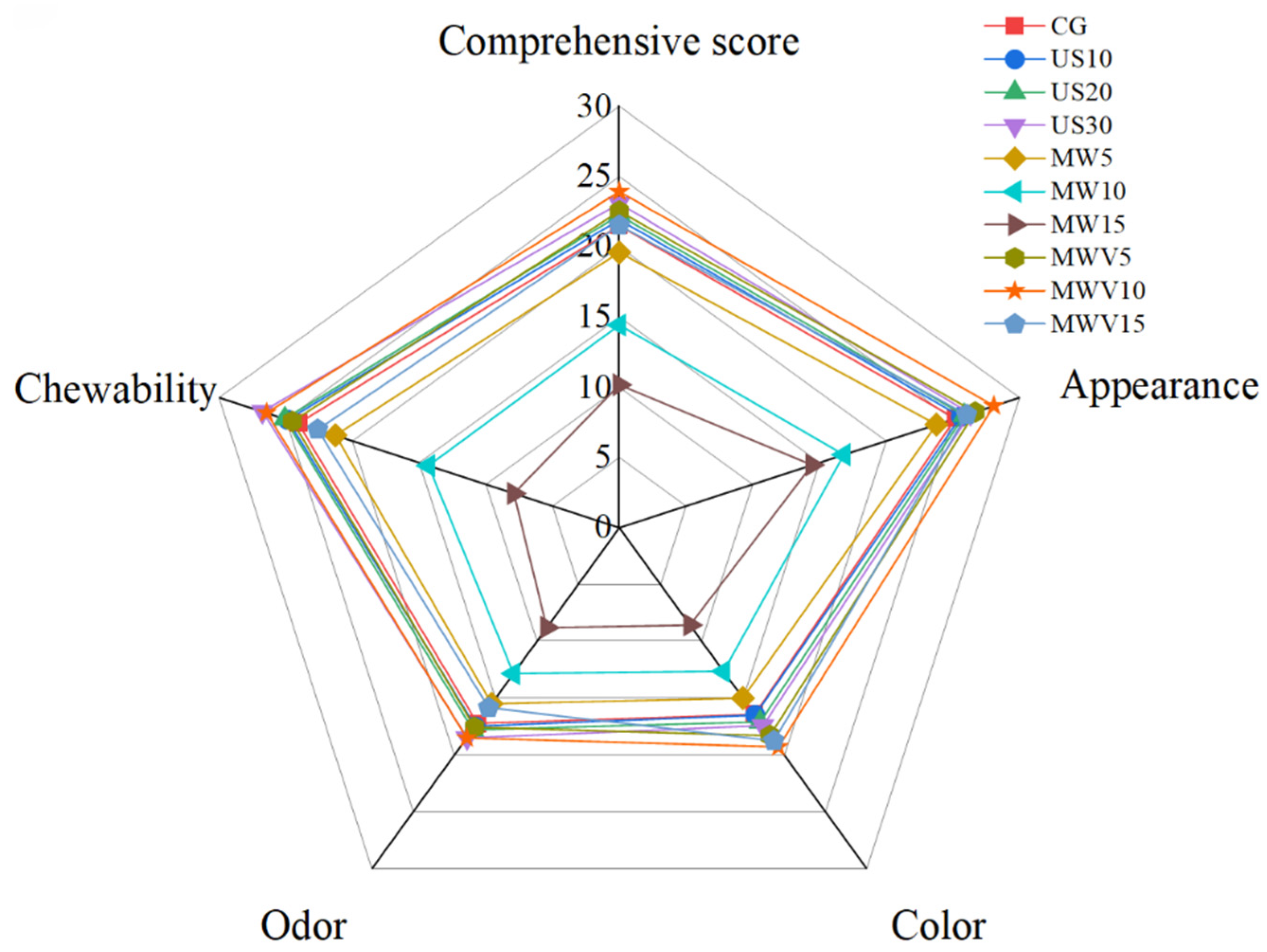
3.8. Effects of Protein Modification Methods on Sensory Evaluation of Yuba
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, L.; Zhu, Y.; Zhu, X.; Liu, L.; Lv, M.; Huang, Y.; Sun, B.; Qu, M. Effect of different frozen storage conditions on Yuba quality. LWT 2024, 205, 116515. [Google Scholar] [CrossRef]
- Wang, J.; Tan, F.; Su, H.; Xie, Y.; Cheng, X.; Xu, X.; Pan, S.; Hu, H. Ultrasound-induced yield increase of yuba (protein-lipid film from soybean milk) and changes in structural, physicochemical, and digestive properties during film-peeling process. J. Food Compos. Anal. 2024, 132, 106341. [Google Scholar] [CrossRef]
- Hu, J.; Xu, H.; Shi, R.; Gantumur, M.A.; Jiang, Z.; Hou, J. Emerging thermal modifying methods in milk protein: A review. Trends Food Sci. Technol. 2024, 146, 104407. [Google Scholar] [CrossRef]
- Liang, P.; Chen, S.; Fang, X.; Wu, J. Recent advance in modification strategies and applications of soy protein gel properties. Compr. Rev. Food Sci. Food Saf. 2023, 23, e13276. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, W.; Chen, B.; Zhu, J.; Wang, L. Enhancing the solubility and foam ability of rice glutelin by heat treatment at pH12: Insight into protein structure. Food Hydrocoll. 2020, 103, 105626. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, N.; Yokoyama, W.; Kim, Y. Effects of moisture content on mechanical properties, transparency, and thermal stability of yuba film. Food Chem. 2018, 243, 202–207. [Google Scholar] [CrossRef]
- Khanal, S.K.; Montalbo, M.; Van Leeuwen, J.; Srinivasan, G.; Grewell, D. Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnol. Bioeng. 2007, 98, 978–985. [Google Scholar] [CrossRef]
- Dongyan, Z.; Dan, H.; Xiyang, Z.; Hangyi, Z.; Guiliang, G.; Xiaohong, T.; Lijun, L. Drying performance and energy consumption of Camelliaoleifera seeds under microwave-vacuum drying. Food Sci. Biotechnol. 2023, 32, 969–977. [Google Scholar]
- Reis, C.G.; Lemos, M.R.; Borges, L.J.; Duarte, A.P.E. Microwave and microwave-vacuum drying as alternatives to convective drying in barley malt processing. Innov. Food Sci. Emerg. Technol. 2021, 73, 102770. [Google Scholar]
- Kim, N.; Seo, E.; Kim, Y. Physical, mechanical and water barrier properties of yuba films incorporated with various types of additives. J. Sci. Food Agric. 2019, 99, 2808–2817. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, Y.; Zhu, X.; Liu, L.; Lv, M.; Huang, Y.; Sun, B.; Qu, M. Effect of freeze-thaw cycles on the quality of Yuba with different protein-lipid ratios on its protein-lipid network system. Food Chem. 2025, 465, 142096. [Google Scholar] [CrossRef]
- Cai, W.-Q.; Zou, B.-W.; Na, X.-K.; Ren, C.; Zheng, X.-H.; Xu, X.-B.; Du, M.; Zhu, B.; Wu, C. Structure of yuba films at the air/liquid interface as effected by the interfacial adsorption behavior of protein aggregates. Food Chem. 2024, 460, 140818. [Google Scholar] [CrossRef]
- Aiello, G.; Pugliese, R.; Rueller, L.; Bollati, C.; Bartolomei, M.; Li, Y.; Robert, J.; Arnoldi, A.; Lammi, C. Assessment of the physicochemical and conformational changes of ultrasound-driven proteins extracted from soybean okara byproduct. Foods 2021, 10, 562. [Google Scholar] [CrossRef]
- Hui, X.; Yonggang, T.; Guowen, Z.; Xiaojuan, X.; Hui, H.; Wei, Q.; Dandan, R.; Yan, Z. Mechanism of ultrasound and tea polyphenol assisted ultrasound modification of egg white protein gel. Ultrason. Sonochemistry 2021, 81, 105857. [Google Scholar]
- Jing, S.; Artur, C.-P. Effect of ultrasound on protein functionality. Ultrason. Sonochemistry 2021, 76, 105653. [Google Scholar]
- Zhiyu, L.; Qian, S.; Yimei, Z.; Jianyi, W.; Yuting, T.; Baodong, Z.; Zebin, G. Effect of two-step microwave heating on the gelation properties of golden threadfin bream (Nemipterus virgatus) myosin. Food Chem. 2020, 328, 127104. [Google Scholar]
- Bi, J.; Zhang, J.; Chen, Z.; Li, Y.; Obadi, M.; Liu, W.; Qin, R.; Zhang, L.; He, H. Quality variation analysis and rehydration kinetics modeling of yuba subjecting to three different drying process. Food Chem. X 2024, 24, 101987. [Google Scholar] [CrossRef]
- Kiana, P.; Mehran, S.; Elahe, A. Ultrasound-assisted activation amylase in the presence of calcium ion and effect on liquefaction process of dual frequency ultrasonicated potato starch. J. Food Meas. Charact. 2023, 17, 3435–3449. [Google Scholar]
- Xiu, H.; Rong, L.; Shasha, C.; Siqi, W.; Lijing, Y.; Haitao, W.; Huihui, W.; Mingqian, T. Effects of microwave vacuum drying on the moisture migration, microstructure, and rehydration of sea cucumber. J. Food Sci. 2021, 86, 2499–2512. [Google Scholar]
- Li, P.; Jin, Y.; Sheng, L. Impact of microwave assisted phosphorylation on the physicochemistry and rehydration behaviour of egg white powder. Food Hydrocoll. 2020, 100, 105380. [Google Scholar] [CrossRef]
- Jie, Z.; Denglin, L.; Jinle, X.; Wei, X.; Baocheng, X.; Peiyan, L.; Jihong, H. Structural Variations of Wheat Proteins Under Ultrasound Treatment. J. Cereal Sci. 2021, 99, 103219. [Google Scholar]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef] [PubMed]
- De Mendonça, K.S.; Corrêa, J.L.G.; de Jesus Junqueira, J.R.; de Carvalho, E.E.N.; Silveira, P.G.; Uemura, J.H.S. Peruvian carrot chips obtained by microwave and microwave-vacuum drying. LWT 2023, 187, 187. [Google Scholar] [CrossRef]
- Futian, Y.; Fang, W.; Janine, M.; Xiao, H. Ultrasound-assisted air-jet spinning of silk fibroin-soy protein nanofiber composite biomaterials. Ultrason. Sonochemistry 2023, 94, 106341. [Google Scholar]
- Chen, L.; Zhang, S.B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef]
- Roy, T.; Singh, A.; Sari, T.P.; Gupta, R. Microwave-assisted enzymatic hydrolysis: A sustainable approach for enhanced structural and functional properties of broken rice protein. Process Biochem. 2024, 136, 301–310. [Google Scholar] [CrossRef]
- Eze, O.F.; Chatzifragkou, A.; Charalampopoulos, D. Properties of protein isolates extracted by ultrasonication from soybean residue (okara). Food Chem. 2021, 368, 130837. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H.; et al. Intervention of transglutaminase in surimi gel under microwave irradiation. Food Chem. 2018, 268, 378–385. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Feng, X.; Li, S.; Zhou, L.; Wang, J.; Tang, X. Structures and properties of chicken myofibrillar protein gel induced by microwave heating. Int. J. Food Sci. Technol. 2020, 55, 2691–2699. [Google Scholar] [CrossRef]
- Asif, M.N.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Hailu, G.G. Physicochemical and Functional Properties of Moringa Seed Protein Treated with Ultrasound. ACS Omega 2024, 9, 4102–4110. [Google Scholar] [CrossRef]
- Mijalković, J.; Šekuljica, N.; Tanasković, S.J.; Petrović, P.; Balanč, B.; Korićanac, M.; Conić, A.; Bakrač, J.; Đorđević, V.; Bugarski, B.; et al. Ultrasound as Green Technology for the Valorization of Pumpkin Leaves: Intensification of Protein Recovery. Molecules 2024, 29, 4027. [Google Scholar] [CrossRef] [PubMed]
| Group | Modification Method |
|---|---|
| CG | Hot air drying at 60 °C until the moisture content is about 10% |
| US10 | After 100 W ultrasonic treatment for 10 min, hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| US20 | After 100 W ultrasonic treatment for 20 min, hot air drying at 60 °C on a wet basis moisture content of about 10% |
| US30 | After 100 W ultrasonic treatment for 30 min, hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MW5 | Microwave power 3 W/g for 5 min, then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MW10 | Microwave power 3 W/g for 10 min, then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MW15 | Microwave power 3 W/g for 15 min, then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MWV5 | Microwave power 3 W/g and vacuum degree −0.1 MPa treatment for 5 min and then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MWV10 | Microwave power 3 W/g and vacuum degree −0.1 MPa treatment for 10 min and then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| MWV15 | Microwave power 3 W/g and vacuum degree −0.1 MPa treatment for 15 min and then hot air drying at 60 °C on a wet basis with moisture content of about 10% |
| Types | Points | Evaluation Standards |
|---|---|---|
| Appearance | 30 | Even branches, flat surface, and complete structure, 21–30 points; partially broken branches, rough surface, and damaged structure, 11–20 points; rough and incomplete surface and damaged structure, 0–10 points |
| Color | 20 | The color is bright and light yellow with natural oily luster, 16–20 points; the color is darker and basically dull, 11–15 points; the color is dark yellow or yellowish brown, and the color is dull, 0–10 points |
| Odor | 20 | Fresh and rich soybean aroma, no odor, 16–20 points; light soybean aroma, slight odor, 11–15 points; no soybean aroma, strong odor, 0–10 points |
| Chewability | 30 | Good chewability and good taste, 21–30 points; poor chewability and chewing is more laborious, 11–20 points; poor chewability and chewing is laborious, 0–10 points |
| Group | Tensile Strength/MPa | Elongation/% | L* | a* | b* |
|---|---|---|---|---|---|
| CG | 3.54 ± 0.19 | 12.61 ± 0.61 | 66.74 ± 0.43 | −1.64 ± 0.08 | 7.27 ± 1.03 |
| US10 | 3.72 ± 0.26 | 13.29 ± 0.41 | 67.08 ± 0.45 | −2.15 ± 0.26 | 6.70 ± 0.80 |
| US20 | 3.83 ± 0.26 | 13.17 ± 0.36 | 68.01 ± 0.55 | −2.16 ± 0.10 | 7.45 ± 0.63 |
| US30 | 3.91 ± 0.29 | 12.82 ± 0.67 | 69.05 ± 1.32 | −2.70 ± 0.21 | 6.01 ± 0.78 |
| MW5 | 4.29 ± 0.28 | 10.90 ± 0.76 | 60.65 ± 0.58 | 2.31 ± 0.20 | 7.35 ± 0.14 |
| MW10 | 4.84 ± 0.26 | 7.92 ± 0.40 | 60.44 ± 0.57 | 2.24 ± 0.33 | 8.24 ± 0.41 |
| MW15 | 5.22 ± 0.23 | 6.63 ± 0.55 | 58.66 ± 0.41 | 1.94 ± 0.30 | 10.51 ± 0.79 |
| MWV5 | 3.92 ± 0.39 | 11.48 ± 0.35 | 66.59 ± 0.95 | −1.91 ± 0.27 | 6.00 ± 0.17 |
| MWV10 | 4.21 ± 0.25 | 10.41 ± 0.59 | 64.98 ± 0.62 | −1.92 ± 0.42 | 5.75 ± 0.60 |
| MWV15 | 4.43 ± 0.30 | 8.47 ± 0.57 | 63.87 ± 0.68 | −2.60 ± 0.35 | 7.55 ± 1.33 |
| Group | Tg (°C) | Tm (°C) | ∆H (J/g) |
|---|---|---|---|
| CG | 81.52 ± 0.66 | 172.33 ± 0.91 | 45.50 ± 0.66 |
| US10 | 82.32 ± 0.78 | 172.77 ± 0.63 | 42.20 ± 0.73 |
| US20 | 83.67 ± 0.71 | 173.39 ± 0.58 | 40.26 ± 0.73 |
| US30 | 85.13 ± 1.12 | 174.63 ± 0.65 | 37.72 ± 0.70 |
| MW5 | 84.34 ± 0.55 | 174.22 ± 1.27 | 38.83 ± 0.83 |
| MW10 | 87.27 ± 0.81 | 178.03 ± 0.79 | 33.77 ± 0.69 |
| MW15 | 89.13 ± 0.64 | 181.57 ± 1.10 | 30.79 ± 0.66 |
| MWV5 | 84.46 ± 0.67 | 173.50 ± 0.86 | 40.68 ± 0.78 |
| MWV10 | 85.74 ± 0.67 | 177.27 ± 0.86 | 36.31 ± 0.59 |
| MWV15 | 87.67 ± 0.74 | 180.37 ± 0.86 | 33.77 ± 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Tian, Y.; Wang, L.; Hu, R.; Zhang, Y.; Li, L.; Cao, W.; Duan, X.; Ren, G. Novel Strategies for Yuba Quality Improvement: Protein Modification Based on Physical Fields. Foods 2025, 14, 1033. https://doi.org/10.3390/foods14061033
Liu W, Tian Y, Wang L, Hu R, Zhang Y, Li L, Cao W, Duan X, Ren G. Novel Strategies for Yuba Quality Improvement: Protein Modification Based on Physical Fields. Foods. 2025; 14(6):1033. https://doi.org/10.3390/foods14061033
Chicago/Turabian StyleLiu, Wenchao, You Tian, Lijuan Wang, Rui Hu, Yan Zhang, Linlin Li, Weiwei Cao, Xu Duan, and Guangyue Ren. 2025. "Novel Strategies for Yuba Quality Improvement: Protein Modification Based on Physical Fields" Foods 14, no. 6: 1033. https://doi.org/10.3390/foods14061033
APA StyleLiu, W., Tian, Y., Wang, L., Hu, R., Zhang, Y., Li, L., Cao, W., Duan, X., & Ren, G. (2025). Novel Strategies for Yuba Quality Improvement: Protein Modification Based on Physical Fields. Foods, 14(6), 1033. https://doi.org/10.3390/foods14061033






