Benefits of Essential Oil-Enriched Chitosan on Beef: From Appearance and Odour Improvement to Protection Against Blowfly Oviposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils Purchase
2.2. Chitosan and Essential Oil-Enriched Chitosan Solutions Preparation
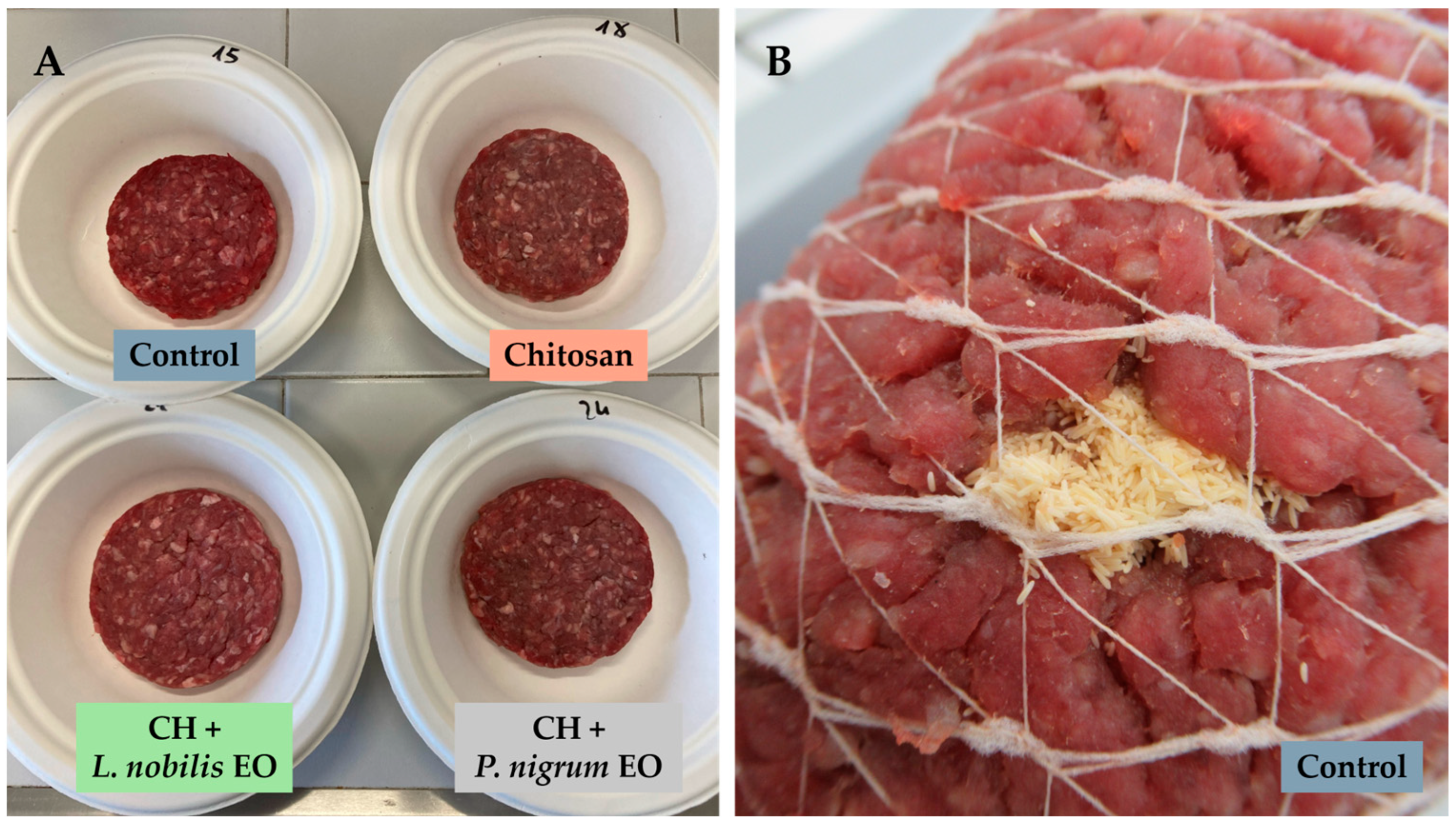
2.3. Preparation and Treatment of Beef Patties
2.4. Proximate Composition of Beef Patties
2.5. Evaluation of Colour on Beef Patties
2.6. Evaluation of Secondary Oxidation Products in Beef Patties
2.7. Solid Phase Microextraction-Gas Chromatography/Mass Spectrometry (SPME-GC/MS) Analysis of Beef Patties
2.8. Evaluation of Calliphora Vomitoria Oviposition Deterrence on Beef Loaves
2.9. Statistical Data Analyses
- yijk = variable,
- μ = mean,
- Ci = fixed effect of the ith treatment (control, CH, CH+ L. nobilis EO, CH+ P. nigrum EO),
- Tj = fixed effect of the jth time of storage (T0, T1, T2),
- εijk = random error.
3. Results
3.1. Chemical Composition and Colour Parameters of Beef Patties
3.2. Secondary Oxidation Products in Beef Patties
3.3. Volatile Organic Compounds (VOCs) Released from Beef Patties
3.4. Oviposition Deterrence Exerted on Calliphora vomitoria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | Chitosan |
| EO | Chitosan |
| MDA | Malonyldialdehyde |
| PVC | Polyvinylidene chlorid |
| SPME-GC/MS | Solid phase microextraction-gas chromatography/mass spectrometry |
| TBA | Thiobarbituric acid |
| TBARS | Thiobarbituric acid-reacting substances |
| VOC | Volatile organic compound |
References
- OECD/FAO Organisation for Economic Co-Operation and Development and the Food and Agriculture Organisation. OECD-FAO Agricultural Outlook 2024–2033; OECD Publishing: Paris, France; FAO: Rome, Italy, 2024; p. 335. [Google Scholar] [CrossRef]
- Karwowska, M.; Łaba, S.; Szczepański, K. Food loss and waste in meat sector—Why the consumption stage generates the most losses? Sustainability 2021, 13, 6227. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, B. Why does animal-based food loss and waste matter? Anim. Front. 2020, 10, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, W.; Li, M.; Zhang, J.; Ji, L.; Zhao, Z.; Zhang, R.; Cai, D.; Chen, L. Microbial diversity of meat products under spoilage and its controlling approaches. Front. Nutr. 2022, 9, 1078201. [Google Scholar] [CrossRef]
- Niederegger, S.; Wartenberg, N.; Spiess, R.; Mall, G. Influence of food substrates on the development of the blowflies Calliphora vicina and Calliphora vomitoria (Diptera, Calliphoridae). Parasitol. Res. 2013, 112, 2847–2853. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food (21 C.F.R. Part 117). 2023. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-117 (accessed on 2 March 2025).
- Prado e Castro, C.; Paulo Sousa, J.; Arnaldos, M.I.; Gaspar, J.; García, M.D. Blowflies (Diptera: Calliphoridae) activity in sun exposed and shaded carrion in Portugal. Ann. Soc. Entomol. Fr. 2011, 47, 128–139. [Google Scholar] [CrossRef]
- Saari, S.; Näreaho, A.; Nikander, S.E. Chapter 8—Insecta. In Canine Parasites and Parasitic Diseases, 1st ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 159–185. [Google Scholar] [CrossRef]
- Pava-Ripoll, M.; Pearson, R.E.G.; Miller, A.K.; Ziobro, G.C. Prevalence and relative risk of Cronobacter spp., Salmonella spp., and Listeria monocytogenes associated with the body surfaces and guts of individual filth flies. App. Environ. Microbiol. 2012, 78, 7891–7902. [Google Scholar] [CrossRef]
- Royden, A.; Wedley, A.; Merga, J.Y.; Rushton, S.; Hald, B.; Humphrey, T.; Williams, N.J. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol. Infect. 2016, 144, 3326–3334. [Google Scholar] [CrossRef]
- Greco, S.; Brandmayr, P.; Bonacci, T. Synanthropy and temporal variability of Calliphoridae living in Cosenza (Calabria, Southern Italy). J. Insect Sci. 2014, 14, 216. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Piwowarska, D.; Festinger, N.; Chruściel, J.J. Risks associated with the presence of polyvinyl chloride in the environment and methods for its disposal and utilization. Materials 2023, 17, 173. [Google Scholar] [CrossRef]
- Fernando, S.S.; Jo, C.; Mudannayake, D.C.; Jayasena, D.D. An overview of the potential application of chitosan in meat and meat products. Carbohydr. Polym. 2024, 324, 121477. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, G.; Cosentino, M.; Sakurada, T. Physiology, taxonomy, and morphology of aromatic plants: A botanical perspective. In Aromatherapy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 16. [Google Scholar]
- Saeed, K.; Pasha, I.; Jahangir Chughtai, M.F.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of essential oils in food industry: Challenges and innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Abenaim, L.; Conti, B. Chitosan as a control tool for insect pest management: A Review. Insects 2023, 14, 949. [Google Scholar] [CrossRef]
- Farina, P.; Ascrizzi, R.; Bedini, S.; Castagna, A.; Flamini, G.; Macaluso, M.; Mannucci, A.; Pieracci, Y.; Ranieri, A.; Sciampagna, M.C.; et al. Chitosan and essential oils combined for beef meat protection against the oviposition of Calliphora vomitoria, water loss, lipid peroxidation, and colour changes. Foods 2022, 11, 3994. [Google Scholar] [CrossRef]
- CABI—Centre for Agriculture and Bioscience International Calliphora vomitoria (Bluebottle, Fly). Available online: https://www.cabi.org/isc/datasheet/10954#REF-DDB-151457 (accessed on 6 February 2025).
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Parichanon, P.; Farina, P.; Vicente, I.; Cesarini, M.; Hotaj, E.; Sarrocco, S.; Pellegrini, E.; Conti, B. Chitosan/mandarin essential oil-based films on citrus fruits for the control of the medfly attack and to prevent the occurrence of grey and blue mould in post-harvest. J. Stored Prod. 2024, 108, 102380. [Google Scholar] [CrossRef]
- Conte, G.; Serra, A.; Casarosa, L.; Ciucci, F.; Cappucci, A.; Bulleri, E.; Corrales-Retana, L.; Buccioni, A.; Mele, M. Effect of linseed supplementation on total longissimus muscle lipid composition and shelf-life of beef from young Maremmana bulls. Front. Vet. Sci. 2019, 5, 326. [Google Scholar] [CrossRef]
- CIE—Commission Internationale de l’Eclairage. Colorimetry, 2nd ed.; Publication CIE No. 15.2.; Commission Internationale de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- MacDougall, D.B. The chemistry of colour and appearance. Food Chem. 1986, 21, 283–299. [Google Scholar] [CrossRef]
- Serra, A.; Conte, G.; Cappucci, A.; Casarosa, L.; Mele, M. Cholesterol and fatty acids oxidation in meat from three muscles of Massese suckling lambs slaughtered at different weights. Ital. J. Anim. Sci. 2014, 13, 3275. [Google Scholar] [CrossRef]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Buccioni, A.; Rodriguez-Estrada, M.T.; Conte, G.; Cappucci, A.; Mele, M. Fatty acid composition, oxidation status and volatile organic compounds in “Colonnata” lard from Large White or Cinta Senese pigs as affected by curing time. Meat Sci. 2014, 97, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.N.; Sun, B.G.; Tian, D.T.; Qu, W.Y. Analysis of volatile compounds in traditional smoke-cured bacon(CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef]
- Povolo, M.; Contarini, G.; Mele, M.; Secchiari, P. Study on the influence of pasture on volatile fraction of ewes’ dairy products by solid-phase microextraction and gas chromatography-mass spectrometry. J. Dairy Sci. 2007, 90, 556–569. [Google Scholar] [CrossRef]
- Ujvari, B.; Wallman, J.F.; Madsen, T.; Whelan, M.; Hulbert, A.J. Experimental studies of blowfly (Calliphora stygia) longevity: A little dietary fat is beneficial but too much is detrimental. Comp. Biochem. Phys. A 2009, 154, 383–388. [Google Scholar] [CrossRef]
- Szpila, K. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of forensic importance—Adult flies. In Forensic Entomology, an Introduction, 2nd ed.; Willey-Blackwell: West Sussex, UK, 2012; pp. 77–81 + plates 5.1–5.9. [Google Scholar]
- SIR—Servizio Idrologico Della Regione Toscana (Regional Hydrological Service of Tuscany Database). ID TOS01000544, Pisa (Fac. Agraria). Available online: http://www.sir.toscana.it/consistenza-rete (accessed on 6 February 2025).
- Lanari, M.C.; Schaefer, D.M.; Scheller, K.K. Dietary vitamin E supplementation and discoloration of pork bone and muscle following modified atmosphere packaging. Meat Sci. 1995, 41, 237–250. [Google Scholar] [CrossRef]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.-D.; Le, Q.-T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef]
- Rmili, R.; Ramdani, M.; Ghazi, Z.; Saidi, N.; El Mahi, B. Composition comparison of essential oils extracted by hydrodistil-lation and microwave-assisted hydrodistillation from Piper nigrum L. J. Mater. Environ. Sci. 2014, 5, 1560–1567. [Google Scholar]
- Corlett, M.T.; Pethick, D.W.; Kelman, K.R.; Jacob, R.H.; Gardner, G.E. Consumer perceptions of meat redness were strongly influenced by storage and display times. Foods 2021, 10, 540. [Google Scholar] [CrossRef]
- Jo, C.; Lee, J.W.; Lee, K.H.; Byun, M.W. Quality properties of pork sausage prepared with water-soluble chitosan oligomer. Meat Sci. 2001, 59, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Combined chitosan-thyme treatments with modified atmosphere packaging on a ready-to-cook poultry product. J. Food Prot. 2010, 73, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Mc Carthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Assessment of the antioxidant potential of natural food and plant extracts in fresh and previously frozen pork patties. Meat Sci. 2001, 57, 177–184. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Monteschio, J.D.O.; Valero, M.V.; Carvalho, C.B.; de Abreu Filho, B.A.; Madrona, G.S.; Do Prado, I.N. Effect of edible and active coating (with rosemary and oregano essential oils) on beef characteristics and consumer acceptability. PLoS ONE 2016, 11, e0160535. [Google Scholar] [CrossRef]
- Farokhzad, P.; Dastgerdi, A.A.; Nimavard, J.T. The effect of chitosan and rosemary essential oil on the quality characteristics of chicken burgers during storage. J. Food Process. Preserv. 2023, 2023, 8381828. [Google Scholar] [CrossRef]
- Lauriano Souza, V.G.; Pires, J.R.A.; Torrico Vieira, É.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocol. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan and mint mixture: A new preservative for meat and meat products. Food Chem. 2008, 107, 845–852. [Google Scholar] [CrossRef]
- Ni, Q.; Amalfitano, N.; Biasioli, F.; Gallo, L.; Tagliapietra, F.; Bittante, G. Bibliometric review on the volatile organic compounds in meat. Foods 2022, 11, 3574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Hayat, K.; Duhoranimana, E.; Zhang, X.; Xia, S.; Yu, J.; Xing, F. Characterization of odor-active compounds of chicken broth and improved flavor by thermal modulation in electrical stewpots. Food Res. Int. 2018, 109, 72–81. [Google Scholar] [CrossRef]
- Santos, M.D.; Matos, G.; Casal, S.; Delgadillo, I.; Saraiva, J.A. Quality evolution of raw meat under hyperbaric storage—Fatty acids, volatile organic compounds and lipid oxidation profiles. Food Biosci. 2021, 42, 101108. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, J.; Zhang, N.; Wang, S.; Sun, B. Effects of cooking methods on aroma formation in pork: A comprehensive review. Food Chem. X 2023, 20, 100884. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.J.; Jung, Y.; Kim, D.; Jo, C.; Nam, K.C.; Lee, J.H.; Choo, H.J.; Jang, A. Identification and comparison of aroma and taste-related compounds from breast meat of three breeds of Korean native chickens. Poult. Sci. 2024, 103, 103462. [Google Scholar] [CrossRef]
- TGSC—The Good Scents Company Information System. 2021. Available online: https://www.thegoodscentscompany.com/index.html (accessed on 6 February 2025).
- Farina, P.; Conti, B. Liabilities of essential oils as insect repellents. Curr. Opin. Environ. Sci. Health 2024, 40, 100564. [Google Scholar] [CrossRef]
- Mansur, A.R.; Seo, D.H.; Song, E.-J.; Song, N.-E.; Hwang, S.H.; Yoo, M.; Nam, T.G. Identifying potential spoilage markers in beef stored in chilled air or vacuum packaging by HS-SPME-GC-TOF/MS coupled with multivariate analysis. LWT 2019, 112, 108256. [Google Scholar] [CrossRef]
- Längkvist, M.; Coradeschi, S.; Loutfi, A.; Rayappan, J. Fast classification of meat spoilage markers using nanostructured ZnO thin films and unsupervised feature learning. Sensors 2013, 13, 1578–1592. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasit. Vectors 2017, 10, 80. [Google Scholar] [CrossRef]
- Bedini, S.; Farina, P.; Napoli, E.; Flamini, G.; Ascrizzi, R.; Verzera, A.; Conti, B.; Zappalà, L. Bioactivity of different chemotypes of oregano essential oil against the blowfly Calliphora vomitoria vector of foodborne pathogens. Insects 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Parichanon, P.; Ascrizzi, R.; Tani, C.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Flamini, G.; Pieracci, Y.; Venturi, F.; Conti, B. The protective combined effect of chitosan and essential oil coatings on cheese and cured meat against the oviposition of Piophila casei. Food Biosci. 2023, 56, 103132. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Bedini, S.; Tani, C.; Giannotti, P.; Lombardi, T.; Conti, B.; Fraternale, D. Ferulago campestris essential oil as active ingredient in chitosan seed-coating: Chemical analyses, allelopathic effects, and protective activity against the common bean pest Acanthoscelides obtectus. Agronomy 2021, 11, 1578. [Google Scholar] [CrossRef]
- Djebbi, T.; Ascrizzi, R.; Bedini, S.; Farina, P.; Sanmartin, C.; Ben Jemâ, J.M.; Bozzini, M.F.; Flamini, G.; Conti, B. Physicochemical and repellent properties of chitosan films loaded with essential oils for producing an active packaging effective against the food pest Sitophilus oryzae. J. Stored Prod. Res. 2024, 106, 102297. [Google Scholar] [CrossRef]
- Ojeda-Piedra, S.A.; Zambrano-Zaragoza, M.L.; González-Reza, R.M.; García-Betanzos, C.I.; Real-Sandoval, S.A.; Quintanar-Guerrero, D. Nano-encapsulated essential oils as a preservation strategy for meat and meat products storage. Molecules 2022, 27, 8187. [Google Scholar] [CrossRef]
- Kulawik, P.; Tadesse, E.E.; Szymkowiak, A.; Tkaczewska, J.; Zając, M.; Guzik, P.; Janik, M.; Tadele, W.; Szram, R.; Grzebieniarz, W.; et al. Effect of multilayer nano-/mini-furcellaran/chitosan emulsions with oregano essential oil on quality, oxidation and consumer perception of pork loins stored at 4 °C and −20 °C. Int. J. Biol. Macromol. 2025, 303, 140491. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Jin, X.; Hu, J.; Tao, Y.; Lu, J.; Xia, X.; Tan, M.; Du, J.; Wang, H. Structurally robust chitosan-based active packaging film by Pickering emulsion containing tree essential oil for pork preservation. Food Chem. 2025, 466, 142246. [Google Scholar] [CrossRef]

| Control | Chitosan | CH+ L. nobilis EO | CH+ P. nigrum EO | SE | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | Treat | Time | Treat × Time | ||
| Moisture | 72.46 | 73.47 | 72.13 | 73.82 | 74.29 | 73.42 | 72.79 | 74.06 | 72.74 | 71.62 | 73.38 | 73.81 | 1.56 | ns | ns | ns |
| Protein | 22.77 | 24.47 | 22.13 | 21.76 | 21.60 | 21.91 | 23.93 | 22.08 | 21.72 | 22.32 | 21.44 | 22.64 | 0.85 | ns | ns | ns |
| Lipid | 2.66 | 2.38 | 2.02 | 1.87 | 1.72 | 1.92 | 2.82 | 2.07 | 1.83 | 2.21 | 1.54 | 2.53 | 0.15 | ns | ns | ns |
| Ash | 1.15 | 1.17 | 1.13 | 1.17 | 1.22 | 1.10 | 1.08 | 1.09 | 1.03 | 1.11 | 1.14 | 1.13 | 0.07 | ns | ns | ns |
| L* | 44.85 β | 46.37 β | 44.03 β | 49.72 α | 50.19 α | 49.32 α | 48.69 α | 49.96 α | 47.64 α | 46.52 β | 47.28 β | 48.71 β | 1.76 | * | ns | ns |
| a* | 17.67 α | 15.40 α | 14.89 α | 11.66 β | 10.50 β | 10.81 β | 13.90 β | 12.98 β | 11.62 β | 12.22 β | 11.34 β | 7.54 β | 0.67 | *** | *** | ns |
| b* | 17.71 a | 16.88 a | 16.75 a | 15.60 a | 15.57 a | 14.74 b | 17.38 a | 16.55 a | 14.69 b | 15.44 a | 15.66 a | 13.35 b | 0.54 | *** | *** | * |
| H* | 45.14 β | 47.75 β | 48.41 β | 53.74 α | 56.23 α | 53.48 α | 51.54 α | 51.92 α | 51.71 α | 51.67 α | 54.21 α | 60.59 α | 1.51 | *** | * | * |
| C* | 25.03 α | 22.90 α | 22.45 α | 19.54 β | 18.82 β | 18.33 β | 22.29 β | 21.08 β | 18.79 β | 19.70 β | 19.41 β | 15.38 β | 0.69 | *** | *** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, P.; Tognocchi, M.; Conte, G.; Casarosa, L.; Trusendi, F.; Conti, B. Benefits of Essential Oil-Enriched Chitosan on Beef: From Appearance and Odour Improvement to Protection Against Blowfly Oviposition. Foods 2025, 14, 897. https://doi.org/10.3390/foods14050897
Farina P, Tognocchi M, Conte G, Casarosa L, Trusendi F, Conti B. Benefits of Essential Oil-Enriched Chitosan on Beef: From Appearance and Odour Improvement to Protection Against Blowfly Oviposition. Foods. 2025; 14(5):897. https://doi.org/10.3390/foods14050897
Chicago/Turabian StyleFarina, Priscilla, Monica Tognocchi, Giuseppe Conte, Laura Casarosa, Francesca Trusendi, and Barbara Conti. 2025. "Benefits of Essential Oil-Enriched Chitosan on Beef: From Appearance and Odour Improvement to Protection Against Blowfly Oviposition" Foods 14, no. 5: 897. https://doi.org/10.3390/foods14050897
APA StyleFarina, P., Tognocchi, M., Conte, G., Casarosa, L., Trusendi, F., & Conti, B. (2025). Benefits of Essential Oil-Enriched Chitosan on Beef: From Appearance and Odour Improvement to Protection Against Blowfly Oviposition. Foods, 14(5), 897. https://doi.org/10.3390/foods14050897











