Advances in Mechanisms of Anaphylaxis in Wheat Allergy: Utility of Rodent Models
Abstract
:1. Introduction
2. Complexity of Anaphylaxis in Wheat Allergy: Current Status
2.1. Overall Classification of Types of Anaphylaxis in Wheat Allergy
2.2. Role of Host Genetic Susceptibility in the Development of Wheat Allergy and Anaphylaxis
| Genetic Factors | Evidence from the Study | References |
|---|---|---|
| TLR4 | Lower risk of respiratory wheat allergy was associated with TLR4 polymorphism as follows: homozygotes for the −2027 G and −1608 C alleles (n = 381, adults, South Korean bakers study). | [26] |
| IL-4 | Single nucleotide polymorphism at the IL-4 locus (IL-4-C590T) was associated with WDEIA; Chinese study, n = 51, Age 5–77 years. | [29] |
| IL-4R | Single nucleotide polymorphism at IL-4R alpha locus (IL-4RA A1727G) was not associated with WDEIA; Chinese study, n = 51, Age 5–77 years. | [29] |
| Increased positive skin-prick test to wheat flour in bakery workers (n = 373, South Korean study, adults) was associated with polymorphic variant of IL-4Rα (Ile375Val and Gln576Arg polymorphisms). | [30] | |
| Filaggrin gene | A patient (woman age 51) had developed WDEIA upon using detergents containing HWP (Glupearl); however, she had no mutation in filaggrin gene that had been implicated for skin sensitization in Japanese subjects. | [31] |
| In a Japanese family, a mother–daughter pair with the same filaggrin loss-of-function mutation developed WDEIA; the daughter was compound heterozygous for c.441_442delAG (p.Gly149Glufs*4) and c.5368C > T (p.Gln1790Ter), and the mother was heterozygous for c.441_442delAG. | [32] | |
| In a Denmark population (n = 7931, age: 18–69), filaggrin gene loss-of-function mutation was associated with self-reported food allergy, including wheat allergy, but not oral allergy syndrome (OR for wheat allergy 3.59; 95% CI 1.61–8.02). | [33] | |
| HLA-class II variants | HLA class II DPB1*02:01:02 allele was associated with increased risk of WDEIA; Japanese population study, n = 77, adults. | [34] |
| HLA class II (HLA DQ) locus on chromosome 6p21 was associated with wheat allergy (skin, eye, airways symptoms when used soap containing hydrolyzed wheat protein and/or skin, eye, airways, gut, and shock symptoms upon eating wheat products/SPT, IgE, basophil activation positive); Japanese population study, n = 452, adults. | [35] | |
| RBFOX1 | RBFOX1 locus on chromosome 16p 13 locus was associated with wheat allergy; same population as above. | [35] |
| IL-18 | Increased risk of WDEIA was associated with IL-18 gene locus (haplotype AGG); (n = 130, Han Chinese study, adults). | [36] |
| Increased risk of sensitization to wheat among South Korean bakery workers was associated with IL-18 polymorphism (373 adults; South Korean study Genotype 137G/C (GC or CC) and haplotype ht3 [ACC]. | [37] | |
| MBL | Higher levels of blood MBL are associated with increased risk of baker’s asthma in Korean population (n = 273); MBL levels were associated in the MBL2 gene haplotypes. | [38] |
| Family genetics aggregation study | IgE-mediated food allergy trait (including wheat allergy) was associated with estimated heritability of 0.15–0.35; American nuclear family study (n = 581). | [39] |
2.3. Role of Environment Factors in the Development of Wheat Allergy and Anaphylaxis
3. Mechanisms of Anaphylaxis in Wheat Allergy
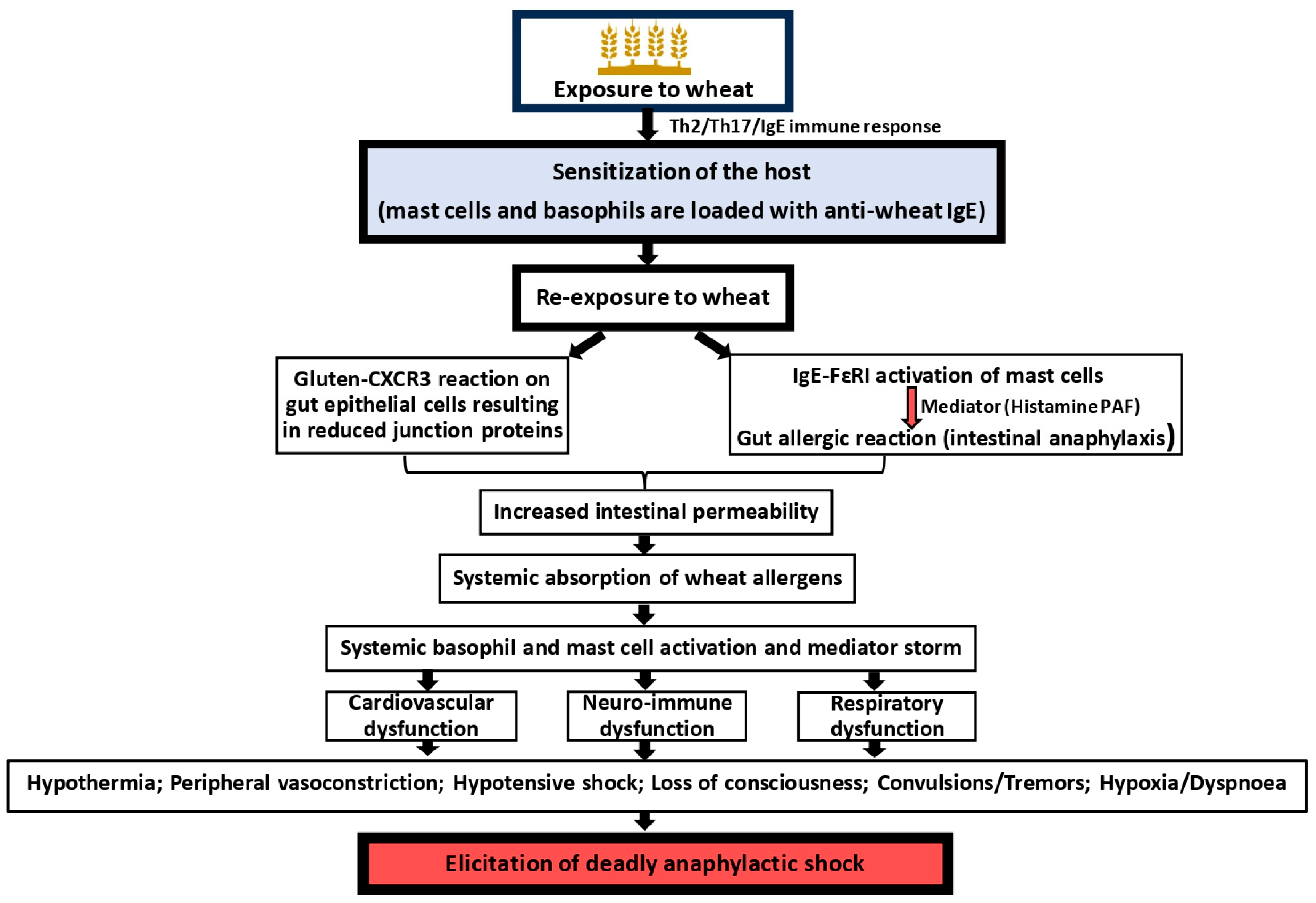
3.1. Mechanism of Classical Anaphylaxis in Wheat Allergy
3.2. Mechanism of Wheat-Dependent Exercise-Induced Anaphylaxis
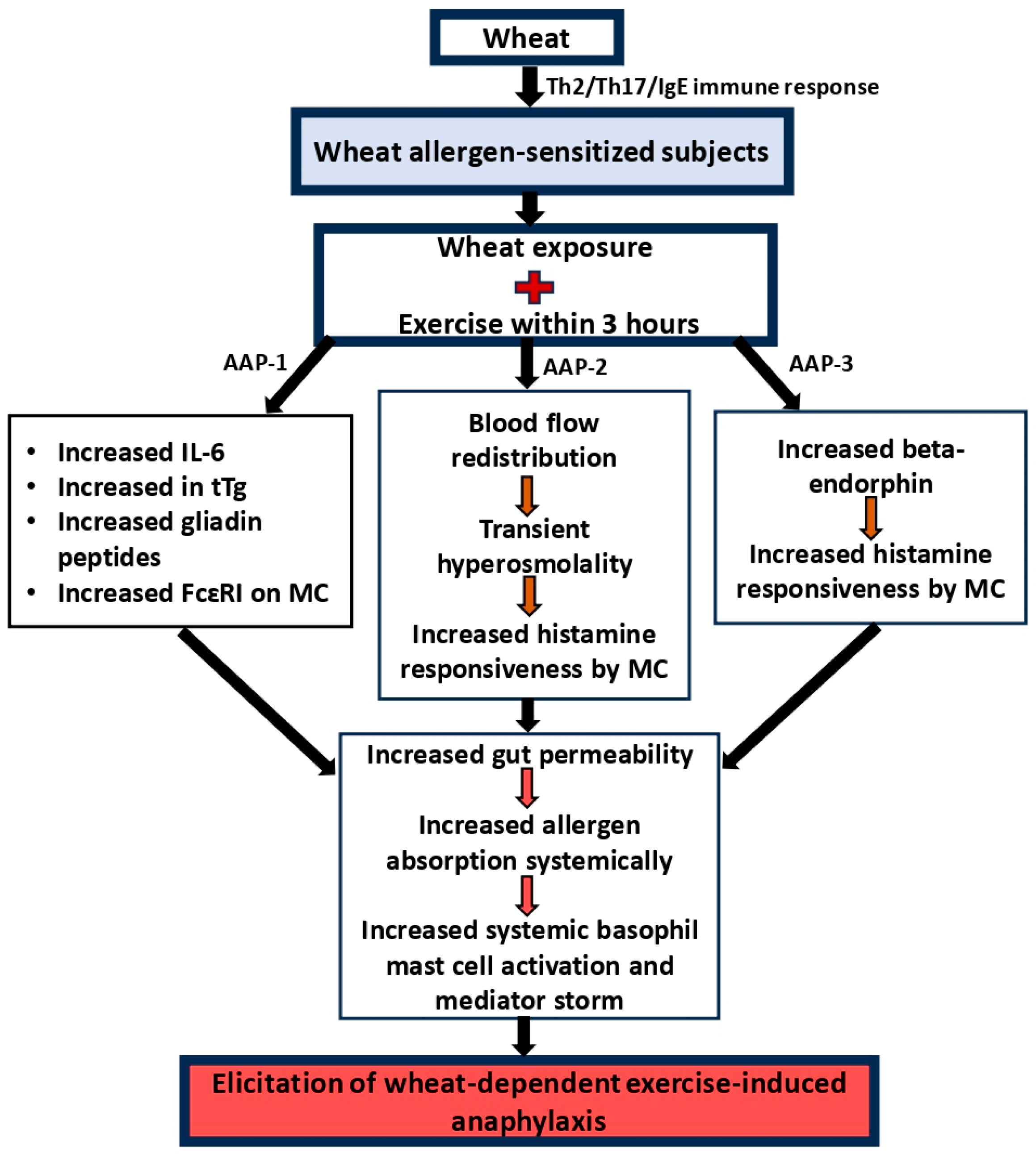
3.3. The Potential Role of Emerging Immune Mediators or Pathways That Have Not Been Extensively Covered in the Current Literature
3.4. Mechanism of Other Cofactor-Induced Anaphylaxis
4. Utility of Rodent Models in Elucidating Mechanisms of Anaphylaxis in Wheat Allergy
4.1. Rodent Models of Classical Anaphylaxis in Wheat Allergy
4.1.1. Adjuvant-Based Models
4.1.2. Adjuvant-Free Models
4.2. Rodent Models of WDEIA
4.3. Lessons Learnt from the Rodent Models and Potential Utility to Advance the Field
- All three species of laboratory rodents (rats, guinea pigs, and mice) can be used to develop models simulating the two critical aspects of human disease—namely, sensitization, as demonstrated by induction of wheat-specific IgE antibodies, and elicitation of systemic anaphylaxis, as demonstrated by clinical disease and/or disease markers, such as hypothermic response.
- Similar to humans, both gluten (gliadin and glutenin), as well as non-gluten proteins, elicit sensitization and anaphylaxis in rodents.
- In the reported rodent models, the symptoms of anaphylaxis vary broadly from mild to moderate, severe, and life-threatening reactions; this spectrum of variation is also noted in humans.
- In rodent models, sensitization is typically quantified by measuring wheat-specific IgE antibodies; there are no reports of developing skin testing in rodents, in contrast to the skin-prick test commonly carried out in humans to determine sensitization. However, in dog models of wheat food allergy, such tests are routinely performed [91]. Therefore, it may be possible to develop such a test in rodents in the future.
- In rodent models, identified immune mediators associated with anaphylaxis include not only a selected set of Th2/Th17 cytokines and chemokines but also histamine, PAF, and mMCP-1. There is ample scope to expand the mediator analysis to include novel targets for potential diagnosis and therapy.
- There are two mouse models and one guinea pig model of WDEIA. In a mouse model, it was demonstrated that exercise leads to lesion formation in the intestine associated with increased gut permeability and leakage of glutenin allergen into portal circulation and appearance in the liver. However, in these studies, appropriate controls were not used. Therefore, it remains to be clarified whether leakage of allergens to the liver from the gut is caused by exercise or whether such leakage happens in classical wheat-induced anaphylaxis. The guinea pig mouse model provides another useful model to study mechanisms of WDEIA, for which, currently, there is very limited information in the literature [84].
- There is strong direct evidence from rodent models that exposure to wheat proteins (both glutens and non-glutens) via undamaged skin can clinically sensitize the host to subsequent life-threatening systemic anaphylaxis caused by wheat proteins. These findings have further bolstered the case for wheat anaphylaxis as an occupational public health issue in the food industry (e.g., baking) where such exposures must be closely monitored, prevented, and managed.
- There are no rodent models reported for wheat-dependent alcohol, drug, or infection-induced systemic anaphylaxis at present; clearly, rodent models are needed in this area.
- Rodent models provide ample opportunity to elucidate the role of genetic and environmental factors in determining anaphylaxis in wheat allergy; however, they have not been explored so far—they therefore constitute areas for further research.
- There is growing evidence that the food and industrial processing of wheat proteins can influence its allergenic properties; therefore, rodent models can be employed to determine the impact of processing technology on the anaphylaxis-eliciting properties of wheat proteins.
- There is growing interest in using rodent models to test novel genetically modified wheats for food safety assessment; currently, this has been carried out using rats and guinea pigs, but mouse models offer improved opportunities for this application [8].
4.4. Limitations of Rodent Models and Challenges in Translating Findings from Rodent Models to Human Clinical Settings
- In humans, specific mechanisms of sensitization to wheat are thought to occur upon oral ingestion of wheat-containing foods, although wheat dust inhaled in bakery settings and skin exposure to gluten via cosmetics (soaps, detergents, shampoos, etc.) is also reported [26,30,31,37,38,96]. In contrast, rodent models generally use sensitization methods that are artificial (for example, IP injections) (Table 3, Table 4 and Table 5).
- In humans, sensitization to wheat proteins occurs after exposure to a complex mixture of proteins as they exist in the food matrix. In contrast, purified wheat proteins [glutens (gliadins, glutenin) and non-glutens (albumin and globulins)] are used in most rodent models (Table 3, Table 4 and Table 5).
- Most rodent models have used adjuvants such as alum and detergents to elicit detectable sensitization to wheat proteins (Table 3, Table 4 and Table 5); although the role of detergents in causing sensitization to gluten in the context of cosmetic exposure is plausible, alum adjuvant is not expected to be involved in human sensitization to wheat proteins [31,35,82].
- Human wheat anaphylaxis is reported after oral exposure to wheat-containing foods [3,13]. In contrast, in rodent models, except for studies conducted by Gao et al. (2022, 2023), Tanaka et al. (2011), Jorgensen et al. (2023), Kohno et al. (2016), and Kozai et al. (2006), where oral wheat protein challenges were carried out to elicit systemic anaphylaxis, all other studies used intraperitoneal or intravenous challenge to elicit anaphylaxis (Table 3, Table 4 and Table 5) [78,80,81,83,88,89]. Unlike in humans, where IgE primarily causes wheat anaphylaxis upon oral exposure to wheat allergens, in mouse models, anaphylaxis upon IP challenge with wheat allergens involves both IgE- and IgG1-mediated activation mechanisms [17].
- All rodent models used inbred strains of animals that are expected to be genetically identical for each type of strain. Therefore, results from such studies must be interpreted carefully for translation to humans, where the population is outbred in nature. There is ample opportunity to develop outbred rodent models to simulate human wheat anaphylaxis. Such efforts are already in place for other human diseases, including asthma, obesity, diabetes, and cardiovascular diseases [97,98,99,100].
4.5. Current Efforts and Future Directions to Refine the Rodent Models to Better Mimic the Full Spectrum of Human Anaphylactic Reactions and the Influence of Cofactors
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WDEIA | Wheat-dependent exercise-induced anaphylaxis |
| WAO | Wheat allergy organization |
| TLR4 | Toll-like receptor 4 |
| LPS | Lipopolysaccharide |
| IL-4 | Interleukin 4 |
| IL-4R | Interleukin-4 receptor |
| HWP | Hydrolyzed wheat protein |
| HLA | Human leukocyte antigen |
| IL-18 | Interleukin-18 |
| MBL | Mannose-binding lectin |
| RBFOX1 | RNA-binding fox-1 homolog 1 |
| aOR | Adjusted odds ratio |
| PPI | Proton pump inhibitor |
| TNP-ova | Trinitrophenyl-ovalbumin |
| Th2, Th17 | T helper2, T helper17 |
| CXCR3 | C-X-C motif chemokines receptor 3 |
| PAF | Platelet-activating factor |
| AAPs | Anaphylaxis activation pathways |
| ATI | Anti-trypsin inhibitor |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PAMP | Pathogen-associated molecular pattern |
| PRR | Pattern recognition receptors |
| LOAELs | Lowest observable adverse effect levels |
| NOAELs | No observable adverse effect levels |
| WIA | Wheat-induced anaphylaxis |
| IP | Intraperitoneal |
| HSR | Hypothermic shock response |
| TDE | Transdermal exposure |
| mMCP-1 | Murine mucosal mast cell protease-1 |
| SSP | Salt-soluble protein |
| IV | Intravenous |
| SC | Subcutaneous |
| V/P | Vehicle application over the skin/protein injection IP |
| P/P | Protein application over the skin/protein injection IP |
| SIgE | Specific IgE antibodies |
| TIgE | Total IgE antibodies |
References
- Levy, A.A.; Feldman, M. Evolution and Origin of Bread Wheat. Plant Cell 2022, 34, 2549–2567. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food Allergy across the Globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Spergel, J.M. Food Allergy: Review, Classification and Diagnosis. Allergol. Int. 2009, 58, 457–466. [Google Scholar] [CrossRef]
- Health Canada Wheat & Triticale—Priority Food Allergens. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/reports-publications/food-safety/wheat-priority-food-allergen.html (accessed on 26 January 2025).
- European Food Safety Authority Allergens|EFSA. Available online: https://www.efsa.europa.eu/en/safe2eat/allergens (accessed on 26 January 2025).
- Food Standards Australia New Zealand Allergen Labelling for Consumers. Available online: https://www.foodstandards.gov.au/consumer/labelling/allergen-labelling (accessed on 26 January 2025).
- U.S. Food and Drug Administration Food Allergies. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/food-allergies (accessed on 23 January 2025).
- Jorgensen, R.; Devarahalli, S.S.; Shah, Y.; Gao, H.; Arul Arasan, T.S.; Ng, P.K.W.; Gangur, V. Advances in Gluten Hypersensitivity: Novel Dietary-Based Therapeutics in Research and Development. Int. J. Mol. Sci. 2024, 25, 4399. [Google Scholar] [CrossRef]
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of Sensitization Reported and Objectively Assessed Food Hypersensitivity amongst Six-Year-Old Children: A Population-Based Study. Pediatr. Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Higgins, B.; Arshad, S.H.; Dean, T. Prevalence and Cumulative Incidence of Food Hypersensitivity in the First 3 Years of Life. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 354–359. [Google Scholar] [CrossRef]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of Self-Reported Food Allergy in American Adults and Use of Food Labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat Allergy: Diagnosis and Management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Bao, C.; Chen, O.; Sheng, H.; Zhang, J.; Luo, Y.; Hayes, B.W.; Liang, H.; Liedtke, W.; Ji, R.R.; Abraham, S.N. A Mast Cell–Thermoregulatory Neuron Circuit Axis Regulates Hypothermia in Anaphylaxis. Sci. Immunol. 2023, 8, eadc9417. [Google Scholar] [CrossRef]
- Watson, C.T.; Cohain, A.T.; Griffin, R.S.; Chun, Y.; Grishin, A.; Hacyznska, H.; Hoffman, G.E.; Beckmann, N.D.; Shah, H.; Dawson, P.; et al. Integrative Transcriptomic Analysis Reveals Key Drivers of Acute Peanut Allergic Reactions. Nat. Commun. 2017, 8, 1943. [Google Scholar] [CrossRef]
- Cabanillas, B. Gluten-Related Disorders: Celiac Disease, Wheat Allergy, and Nonceliac Gluten Sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Rothenberg, M.E.; Brandt, E.B.; Morris, S.C.; Strait, R.T. Molecular Mechanisms of Anaphylaxis: Lessons from Studies with Murine Models. J. Allergy Clin. Immunol. 2005, 115, 449–457. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Leung, D.Y.M. Advances in Allergic Skin Disease, Anaphylaxis, and Hypersensitivity Reactions to Foods, Drugs, and Insects in 2014. J. Allergy Clin. Immunol. 2015, 135, 357–367. [Google Scholar] [CrossRef]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Carr, S.; Custovic, A.; Durham, S.; Ebisawa, M.; Geller, M.; Gonzalez-Estrada, A.; et al. Updated Grading System for Systemic Allergic Reactions: Joint Statement of the World Allergy Organization Anaphylaxis Committee and Allergen Immunotherapy Committee. World Allergy Organ. J. 2024, 17, 100876. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Jones, S.M.; Kim, E.H.; Sicherer, S.H.; Shreffler, W.; Lanser, B.J.; Atri, N.; Babineau, D.C.; Adelman, D.C.; Iqbal, A.; et al. Updating the CoFAR Grading Scale for Systemic Allergic Reactions in Food Allergy. J. Allergy Clin. Immunol. 2022, 149, 2166–2170.e1. [Google Scholar] [CrossRef]
- Rutkowski, K.; Dua, S.; Nasser, S. Anaphylaxis: Current State of Knowledge for the Modern Physician. Postgrad. Med. J. 2012, 88, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lieberman, J.A.; Wallace, D.V.; Waserman, S.; Golden, D.B.K. Anaphylaxis in Practice: A Guide to the 2023 Practice Parameter Update. J. Allergy Clin. Immunol. Pract. 2024, 12, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, R.; Arul Arasan, T.S.; Srkalovic, M.B.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway. Int. J. Mol. Sci. 2024, 25, 7324. [Google Scholar] [CrossRef] [PubMed]
- Shin, M. Food Allergies and Food-Induced Anaphylaxis: Role of Cofactors. Clin. Exp. Pediatr. 2021, 64, 393–399. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, S.H.; Kim, J.H.; Choi, H.; Son, J.K.; Hur, G.Y.; Park, H.S. Effect of Toll-like Receptor 4 Gene Polymorphisms on Work-Related Respiratory Symptoms and Sensitization to Wheat Flour in Bakery Workers. Ann. Allergy Asthma Immunol. 2011, 107, 57–64. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Lin, T.H.; Su, H.H.; Kang, H.Y.; Chang, T.H. The Interactive Roles of Lipopolysaccharides and DsRNA/Viruses on Respiratory Epithelial Cells and Dendritic Cells in Allergic Respiratory Disorders: The Hygiene Hypothesis. Int. J. Mol. Sci. 2017, 18, 2219. [Google Scholar] [CrossRef]
- Cai, P.P.; Yin, J. Association between Single Nucleotide Polymorphisms and Wheat-Dependent Exercise-Induced Anaphylaxis in Chinese Population. Chin. Med. J. 2013, 126, 1159–1165. [Google Scholar] [CrossRef]
- Hur, G.Y.; Ye, Y.M.; Koh, D.H.; Kim, S.H.; Park, H.S. IL-4 Receptor a Polymorphisms May Be a Susceptible Factor for Work-Related Respiratory Symptoms in Bakery Workers. Allergy Asthma Immunol. Res. 2013, 5, 371–376. [Google Scholar] [CrossRef]
- Iga, N.; Tanizaki, H.; Endo, Y.; Egawa, G.; Fujisawa, A.; Tanioka, M.; Miyachi, Y.; Kabashima, K. Hydrolyzed Wheat Protein-Containing Facial Soap-Induced Wheat-Dependent Exercise-Induced Anaphylaxis in a Patient without Filaggrin Mutations. J. Dermatol. 2013, 40, 494–495. [Google Scholar] [CrossRef]
- Mizuno, O.; Nomura, T.; Ohguchi, Y.; Suzuki, S.; Nomura, Y.; Hamade, Y.; Hoshina, D.; Sandilands, A.; Akiyama, M.; McLean, W.H.I.; et al. Loss-of-Function Mutations in the Gene Encoding Filaggrin Underlie a Japanese Family with Food-Dependent Exercise-Induced Anaphylaxis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 805–808. [Google Scholar] [CrossRef]
- Linneberg, A.; Fenger, R.V.; Husemoen, L.L.N.; Thuesen, B.H.; Skaaby, T.; Gonzalez-Quintela, A.; Vidal, C.; Carlsen, B.C.; Johansen, J.D.; Menné, T.; et al. Association between Loss-of-Function Mutations in the Filaggrin Gene and Self-Reported Food Allergy and Alcohol Sensitivity. Int. Arch. Allergy Immunol. 2013, 161, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Chinuki, Y.; Hamada, Y.; Fukutomi, Y.; Sugiyama, A.; Kishikawa, R.; Fukunaga, A.; Oda, Y.; Ugajin, T.; Yokozeki, H.; et al. Genome-Wide Association Study Reveals an Association between the HLA-DPB1∗02:01:02 Allele and Wheat-Dependent Exercise-Induced Anaphylaxis. Am. J. Hum. Genet. 2021, 108, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, E.; Akiyama, M.; Yagami, A.; Hirota, T.; Okada, Y.; Kato, Z.; Kishikawa, R.; Fukutomi, Y.; Hide, M.; Morita, E.; et al. HLA-DQ and RBFOX1 as Susceptibility Genes for an Outbreak of Hydrolyzed Wheat Allergy. J. Allergy Clin. Immunol. 2019, 144, 1354–1363. [Google Scholar] [CrossRef]
- Gao, X.; Wen, L.; Li, H.; Wang, R.; Yin, J. Genetic Variation at the Interleukin-18 Locus Is Associated with Wheat-Dependent Exercise-Induced Anaphylaxis in the Han Chinese Population. Gene 2020, 737, 144462. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hur, G.Y.; Jin, H.J.; Choi, H.; Park, H.S. Effect of Interleukin-18 Gene Polymorphisms on Sensitization to Wheat Flour in Bakery Workers. J. Korean Med. Sci. 2012, 27, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Yoon, M.K.; Kim, S.H.; Park, H.S. Association of MBL with Work-Related Respiratory Symptoms in Bakery Workers. Allergy Asthma Immunol. Res. 2017, 9, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Kumar, R.; Pongracic, J.; Liu, X.; Story, R.; Yu, Y.; Caruso, D.; Costello, J.; Schroeder, A.; Fang, Y.; et al. Familial Aggregation of Food Allergy and Sensitization to Food Allergens: A Family-Based Study. Clin. Exp. Allergy 2009, 39, 101–109. [Google Scholar] [CrossRef]
- Vecchione, A.; Devlin, J.C.; Tasker, C.; Raman Ramnarayan, V.; Haase, P.; Conde, E.; Srivastava, D.; Atwal, G.S.; Bruhns, P.; Murphy, A.J.; et al. IgE Plasma Cells Are Transcriptionally and Functionally Distinct from Other Isotypes. Sci. Immunol. 2024, 9, eadm8964. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Katona, I.M.; Urban, J.F.; Holmes, J.; Ohara, J.; Tung, A.S.; Sample, J.V.; Paul, W.E. IL-4 Is Required to Generate and Sustain in Vivo IgE Responses. J. Immunol. 1988, 141, 2335–2341. [Google Scholar] [CrossRef]
- Katona, I.M.; Urban, J.F.; Finkelman, F.D. The Role of L3T4+ and Lyt-2+ T Cells in the IgE Response and Immunity to Nippostrongylus Brasiliensis. J. Immunol. 1988, 140, 3206–3211. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the Frontline: Role in Skin Barrier Function and Disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Li, J.; Fung, I.; Glessner, J.T.; Pandey, R.; Wei, Z.; Bakay, M.; Mentch, F.D.; Pellegrino, R.; Wang, T.; Kim, C.; et al. Copy Number Variations in CTNNA3 and RBFOX1 Associate with Pediatric Food Allergy. J. Immunol. 2015, 195, 1599–1607. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 Cytokine in Immunity, Inflammation, and Autoimmunity: Biological Role in Induction, Regulation, and Treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef] [PubMed]
- Okabe, H.; Hashimoto, K.; Yamada, M.; Ono, T.; Yaginuma, K.; Kume, Y.; Chishiki, M.; Sato, A.; Ogata, Y.; Imaizumi, K.; et al. Associations between Fetal or Infancy Pet Exposure and Food Allergies: The Japan Environment and Children’s Study. PLoS ONE 2023, 18, e0282725. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A Prospective Microbiome-Wide Association Study of Food Sensitization and Food Allergy in Early Childhood. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 145–152. [Google Scholar] [CrossRef]
- Baek, J.H.; Shin, Y.H.; Chung, I.H.; Kim, H.J.; Yoo, E.G.; Yoon, J.W.; Jee, H.M.; Chang, Y.E.; Han, M.Y. The Link between Serum Vitamin d Level, Sensitization to Food Allergens, and the Severity of Atopic Dermatitis in Infancy. J. Pediatr. 2014, 165, 849–854.e1. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Bakos, N.; Schöll, I.; Kundi, M.; Roth-Walter, F.; Szalai, K.; Riemer, A.B.; Ankersmit, H.J.; Scheiner, O.; Boltz-Nitulescu, G.; et al. Anti-ulcer Drugs Promote IgE Formation toward Dietary Antigens in Adult Patients. FASEB J. 2005, 19, 1–16. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Q.; Zhu, R. Vitamin D and Allergic Diseases. Front. Immunol. 2024, 15, 1420883. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. [Google Scholar] [CrossRef]
- Downs, S.H.; Marks, G.B.; Mitakakis, T.Z.; Lëuppi, J.D.; Car, N.G.; Peat, J.K. Having Lived on a Farm and Protection against Allergic Diseases in Australia. Clin. Exp. Allergy 2001, 31, 570–575. [Google Scholar] [CrossRef]
- Illi, S.; Depner, M.; Genuneit, J.; Horak, E.; Loss, G.; Strunz-Lehner, C.; Büchele, G.; Boznanski, A.; Danielewicz, H.; Cullinan, P.; et al. Protection from Childhood Asthma and Allergy in Alpine Farm Environments—The GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 2012, 129, 1470–1477.e6. [Google Scholar] [CrossRef]
- Orivuori, L.; Mustonen, K.; Roduit, C.; Braun-Fahrländer, C.; Dalphin, J.C.; Genuneit, J.; Lauener, R.; Pfefferle, P.; Riedler, J.; Weber, J.; et al. Immunoglobulin A and Immunoglobulin G Antibodies against β-Lactoglobulin and Gliadin at Age 1 Associate with Immunoglobulin E Sensitization at Age 6. Pediatr. Allergy Immunol. 2014, 25, 329–337. [Google Scholar] [CrossRef]
- Sampson, H.A. Update on Food Allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef]
- Tanabe, S. Analysis of Food Allergen Structures and Development of Foods for Allergic Patients. Biosci. Biotechnol. Biochem. 2008, 72, 649–659. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, H.; Jorgensen, R.; Salloum, J.; Jian, D.I.; Ng, P.K.W.; Gangur, V. Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. Int. J. Mol. Sci. 2020, 21, 3205. [Google Scholar] [CrossRef]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef]
- Juhász, A.; Nye-Wood, M.G.; Tanner, G.J.; Colgrave, M.L. Digestibility of Wheat Alpha-Amylase/Trypsin Inhibitors Using a Caricain Digestive Supplement. Front. Nutr. 2022, 9, 977206. [Google Scholar] [CrossRef]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204.e3. [Google Scholar] [CrossRef]
- Ahrens, R.; Osterfeld, H.; Wu, D.; Chen, C.Y.; Arumugam, M.; Groschwitz, K.; Strait, R.; Wang, Y.H.; Finkelman, F.D.; Hogan, S.P. Intestinal Mast Cell Levels Control Severity of Oral Antigen-Induced Anaphylaxis in Mice. Am. J. Pathol. 2012, 180, 1535–1546. [Google Scholar] [CrossRef]
- Nguyen, S.M.T.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond. Int. J. Mol. Sci. 2021, 22, 7785. [Google Scholar] [CrossRef]
- Srisuwatchari, W.; Kanchanaphoomi, K.; Nawiboonwong, J.; Thongngarm, T.; Sompornrattanaphan, M. Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments. Foods 2023, 12, 3768. [Google Scholar] [CrossRef]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-Induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Miyazaki, A.; Adachi, H.; Oshima, S.; Taniguchi, K.; Hasegawa, A.; Kurabayashi, M. Blood flow redistribution during exercise contributes to exercise tolerance in patients with chronic heart failure. Circ. J. Off. J. Jpn. Circ. Soc. 2007, 71, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Kindermann, W. Fl-Endorphin, Adrenocorticotropic Hormone, Cortisol and Catecholamines during Aerobic and Anaerobic Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Asaumi, T.; Manabe, T.; Yanagida, N.; Sato, S.; Ebisawa, M. Wheat-Dependent Exercise-Induced Anaphylaxis. Curr. Treat. Options Allergy 2017, 4, 291–302. [Google Scholar] [CrossRef]
- Ansley, L.; Bonini, M.; Delgado, L.; Del Giacco, S.; Du Toit, G.; Khaitov, M.; Kurowski, M.; Hull, J.H.; Moreira, A.; Robson-Ansley, P.J. Pathophysiological Mechanisms of Exercise-Induced Anaphylaxis: An EAACI Position Statement. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1212–1221. [Google Scholar] [CrossRef]
- Srisuwatchari, W.; Sompornrattanaphan, M.; Jirapongsananuruk, O.; Visitsunthorn, N.; Pacharn, P. Exercise-Food Challenge Test in Patients with Wheat-Dependent Exercise-Induced Anaphylaxis. Asian Pac. J. Allergy Immunol. 2024, 42, 43–49. [Google Scholar] [CrossRef]
- Gabler, A.M.; Gebhard, J.; Eberlein, B.; Biedermann, T.; Scherf, K.A.; Brockow, K. The Basophil Activation Test Differentiates between Patients with Wheat-Dependent Exercise-Induced Anaphylaxis and Control Subjects Using Gluten and Isolated Gluten Protein Types. Clin. Transl. Allergy 2021, 11, e12050. [Google Scholar] [CrossRef]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-Dependent Exercise-Induced Anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Zhou, Y.; Dial, E.J.; Raphael, R.M. NSAID Injury to the Gastrointestinal Tract: Evidence That NSAIDs Interact with Phospholipids to Weaken the Hydrophobic Surface Barrier and Induce the Formation of Unstable Pores in Membranes. J. Pharm. Pharmacol. 2010, 58, 1421–1428. [Google Scholar] [CrossRef]
- Kawano, T.; Matsuse, H.; Kondo, Y.; Machida, I.; Saeki, S.; Tomari, S.; Mitsuta, K.; Obase, Y.; Fukushima, C.; Shimoda, T.; et al. Acetaldehyde induces histamine release from human airway mast cells to cause bronchoconstriction. Int. Arch. Allergy Immunol. 2004, 134, 233–239. [Google Scholar] [CrossRef]
- Shamim, A.; Abdul Aziz, M.; Saeed, F.; Kumari, R.; Mary Joseph, A.; Ponnachan, P.; Kishore, U.; Masmoudi, K. Revisiting Surfactant Protein D: An Immune Surveillance Molecule Bridging Innate and Adaptive Immunity. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef]
- Xia, P.; Wu, Y.; Lian, S.; Yan, L.; Meng, X.; Duan, Q.; Zhu, G. Research Progress on Toll-like Receptor Signal Transduction and Its Roles in Antimicrobial Immune Responses. Appl. Microbiol. Biotechnol. 2021, 105, 5341–5355. [Google Scholar] [CrossRef]
- Dearman, R.J.; Kimber, I. Animal Models of Protein Allergenicity: Potential Benefits, Pitfalls and Challenges. Clin. Exp. Allergy 2009, 39, 458–468. [Google Scholar] [CrossRef]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Nagisetty, S.; Ng, P.K.W.; Gangur, V. Creating Hypo-/Nonallergenic Wheat Products Using Processing Methods: Fact or Fiction? Compr. Rev. Food Sci. Food Saf. 2021, 20, 6089–6115. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Ng, P.K.W.; Gangur, V. An Adjuvant-Free Mouse Model Using Skin Sensitization Without Tape-Stripping Followed by Oral Elicitation of Anaphylaxis: A Novel Pre-Clinical Tool for Testing Intrinsic Wheat Allergenicity. Front. Allergy 2022, 3, 926576. [Google Scholar] [CrossRef]
- Jorgensen, R.; Gao, H.; Arul Arasan, T.S.; Van Antwerp, C.; Sundar, V.; Ng, P.K.W.; Gangur, V. Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis. Int. J. Mol. Sci. 2023, 24, 17247. [Google Scholar] [CrossRef]
- Jorgensen, R.; Gao, H.; Chandra, S.; Sundar, V.; Loy, J.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Chronic Application of Alcohol-Soluble Gluten Extract over Undamaged Skin Causes Clinical Sensitization for Life-Threatening Anaphylaxis via Activation of Systemic Th2 Immune Responses in Mice. Front. Allergy 2023, 4, 1214051. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nagano, T.; Yano, H.; Haruma, K.; Kato, Y. Exercise-Independent Wheat-Induced Anaphylaxis Caused by ω-5 Gliadin in Mice. Int. Arch. Allergy Immunol. 2011, 156, 434–442. [Google Scholar] [CrossRef]
- Adachi, R.; Nakamura, R.; Sakai, S.; Fukutomi, Y.; Teshima, R. Sensitization to Acid-Hydrolyzed Wheat Protein by Transdermal Administration to BALB/c Mice, and Comparison with Gluten. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a Hypoallergenic Wheat Line Lacking ω-5 Gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse Model of Anaphylaxis and Atopic Dermatitis to Salt-Soluble Wheat Protein Extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef]
- Yamada, Y.; Yokooji, T.; Ninomiya, N.; Taogoshi, T.; Morita, E.; Matsuo, H. Evaluation of the Allergenicity of Ω5-Gliadin-Deficient Hokushin Wheat (1BS-18) in a Wheat Allergy Rat Model. Biochem. Biophys. Rep. 2019, 20, 100702. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yokooji, T.; Kunimoto, K.; Inoguchi, K.; Ogino, R.; Taogoshi, T.; Morita, E.; Matsuo, H. Hypoallergenic Wheat Line (1BS-18H) Lacking Ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy. Foods 2022, 11, 2181. [Google Scholar] [CrossRef]
- Fu, W.; Chen, C.; Xie, Q.; Gu, S.; Tao, S.; Xue, W. Pediococcus acidilactici Strain Alleviates Gluten-Induced Food Allergy and Regulates Gut Microbiota in Mice. Front. Cell. Infect. Microbiol. 2022, 12, 845142. [Google Scholar] [CrossRef]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Chandra, S.; Othman, A.; Olson, E.; Ng, P.K.W.; Gangur, V. Intrinsic Allergenicity Potential of Salt-Soluble Protein Extracts from the Diploid, Tetraploid and Hexaploid Wheats: Validation Using an Adjuvant-Free Mouse Model. Int. J. Mol. Sci. 2023, 24, 5453. [Google Scholar] [CrossRef] [PubMed]
- Kozai, H.; Yano, H.; Matsuda, T.; Kato, Y. Wheat-Dependent Exercise-Induced Anaphylaxis in Mice Is Caused by Gliadin and Glutenin Treatments. Immunol. Lett. 2006, 102, 83–90. [Google Scholar] [CrossRef]
- Tanaka, M.; Nagano, T.; Yano, H.; Matsuda, T.; Ikeda, T.M.; Haruma, K.; Kato, Y. Impact of (0-5 Gliadin on Wheat-Dependent Exercise-Induced Anaphylaxis in Mice. Biosci. Biotechnol. Biochem. 2011, 75, 313–317. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Frick, O.L. The Dog as a Model for Food Allergy. In Proceedings of the Annals of the New York Academy of Sciences. Ann. N. Y. Acad. Sci. 2002, 964, 173–183. [Google Scholar] [CrossRef]
- Fernandez-Bravo, S.; Palacio-Garcia, L.; Requena-Robledo, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Esteban, V. Anaphylaxis: Mediators, Biomarkers, and Microenvironments. J. Investig. Allergol. Clin. Immunol. 2022, 32, 419–439. [Google Scholar] [CrossRef]
- Pampura, A.; Esakova, N.; Zimin, S.; Filippova, E. Anaphylaxis Biomarkers: Present and Future. Eur. Ann. Allergy Clin. Immunol. 2024, 56, 243–251. [Google Scholar] [CrossRef]
- Beck, S.C.; Wilding, T.; Buka, R.J.; Baretto, R.L.; Huissoon, A.P.; Krishna, M.T. Biomarkers in Human Anaphylaxis: A Critical Appraisal of Current Evidence and Perspectives. Front. Immunol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Dass, C.; Ten Eyck, P.; Ballas, Z.; Lee, S. Characterization of Serum Biomarkers during Anaphylaxis in Emergency Department Patients. J. Allergy Clin. Immunol. Pract. 2020, 8, 3213–3215.e1. [Google Scholar] [CrossRef] [PubMed]
- Chinuki, Y.; Morita, E. Wheat-Dependent Exercise-Induced Anaphylaxis Sensitized with Hydrolyzed Wheat Protein in Soap. Allergol. Int. 2012, 61, 529–537. [Google Scholar] [CrossRef]
- Williams, K.R.; Bright, H.A.T.K.; Fryer, A.D.; Jacoby, D.B.; Nie, Z. Maternal High-Fat Diet Programs Offspring Airway Hyperinnervation and Hyperresponsiveness. JCI Insight 2025, 10, e181070. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Curtis, A.M.; Jen, A.; Thomson, J.A.; Clegg, D.O.; Jiang, P.; Coon, J.J.; Overmyer, K.A.; Toh, H. Plasma Metabolomics Supports Non-Fasted Sampling for Metabolic Profiling across a Spectrum of Glucose Tolerance in the Nile Rat Model for Type 2 Diabetes. Lab. Anim. 2023, 52, 269–277. [Google Scholar] [CrossRef]
- Sweet, M.G.; Iglesias-Carres, L.; Ellsworth, P.N.; Carter, J.D.; Nielsen, D.M.; Aylor, D.L.; Tessem, J.S.; Neilson, A.P. Phenotype Variability in Diet-Induced Obesity and Response to (−)-Epigallocatechin Gallate Supplementation in a Diversity Outbred Mouse Cohort: A Model for Exploring Gene x Diet Interactions for Dietary Bioactives. Nutr. Res. 2025, 133, 78–93. [Google Scholar] [CrossRef]
- Guo, Y.J.; Yao, J.J.; Guo, Z.Z.; Ding, M.; Zhang, K.L.; Shen, Q.H.; Li, Y.; Yu, S.F.; Wan, T.; Xu, F.P.; et al. HBB Contributes to Individualized Aconitine-Induced Cardiotoxicity in Mice via Interfering with ABHD5/AMPK/HDAC4 Axis. Acta Pharmacol. Sin. 2024, 45, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, R.; Nagao, M.; Hiraguchi, Y.; Hosoki, K.; Matsuda, T.; Kouno, K.; Morita, E.; Fujisawa, T. Antigen-Induced Expression of CD203c on Basophils Predicts IgE-Mediated Wheat Allergy. Allergol. Int. 2009, 58, 193–199. [Google Scholar] [CrossRef]
- Faihs, V.; Kugler, C.; Schmalhofer, V.; Scherf, K.A.; Lexhaller, B.; Mortz, C.G.; Bindslev-Jensen, C.; Biedermann, T.; Brockow, K. Wheat-Dependent Exercise-Induced Anaphylaxis: Subtypes, Diagnosis, and Management. JDDG-J. Ger. Soc. Dermatol. 2023, 21, 1131–1135. [Google Scholar] [CrossRef]
- Brockow, K.; Kneissl, D.; Valentini, L.; Zelger, O.; Grosber, M.; Kugler, C.; Werich, M.; Darsow, U.; Matsuo, H.; Morita, E.; et al. Using a Gluten Oral Food Challenge Protocol to Improve Diagnosis of Wheat-Dependent Exercise-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 977–984.e4. [Google Scholar] [CrossRef]
- Morita, E.; Chinuki, Y.; Kohno, K.; Matsuo, H. Cofactors of Wheat-Dependent Exercise-Induced Anaphylaxis Increase Gastrointestinal Gliadin Absorption by an Inhibition of Prostaglandin Production. Clin. Exp. Allergy 2023, 53, 359–361. [Google Scholar] [CrossRef]
- Matsukura, S.; Aihara, M.; Sugawara, M.; Kunimi, Y.; Matsuki, M.; Inoue, Y.; Kambara, T.; Ikezawa, Z. Two Cases of Wheat-Dependent Anaphylaxis Induced by Aspirin Administration but Not by Exercise. Clin. Exp. Dermatol. 2010, 35, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Motomura, C.; Matsuzaki, H.; Ono, R.; Iwata, M.; Okabe, K.; Akamine, Y.; Wakatsuki, M.; Murakami, Y.; Taba, N.; Odajima, H. Aspirin Is an Enhancing Factor for Food-Dependent Exercise-Induced Anaphylaxis in Children. Clin. Exp. Allergy 2017, 47, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Horikawa, T.; Ashida, M.; Kamo, T.; Nishioka, E.; Ichihashi, M. Aspirin Enhances the Induction of Type I Allergic Symptoms When Combined with Food and Exercise in Patients with Food-Dependent Exercise-Induced Anaphylaxis. Br. J. Dermatol. 2001, 145, 336–339. [Google Scholar] [CrossRef] [PubMed]
| Environmental Factors | Evidence from the Study | References |
|---|---|---|
| Exposure to cats | Exposure to cats during pregnancy reduced the risk of wheat allergy in children until the age of 3 years (aORs [95% CIs] 0.54 [0.34–0.85]); Exposure to cats during early infancy reduced the risk of wheat allergy until the age of 3 years (0.63 [0.42–0.92]); Japanese study | [46] |
| Gut microbiome | food (milk, egg, peanut, soy, wheat, and walnut) sensitization in adults was associated with reduction in the following genera: Haemophilus, Dialister, Dorea, and Clostridium sensitization (n = 85 total food sensitized, wheat sensitization n = 33); Food (including wheat) allergy in children was associated with a reduction in the following genera Citrobacter, Oscillospira, Lactococcus, and Dorea; wheat allergic children (n = 3) among food allergic subjects (n = 14); United States study, adults, pregnant women, infants. | [47] |
| Vitamin D deficiency | Vitamin D deficiency during childhood increased the risk of sensitization (specific IgE antibody) to wheat (OR 4.2; 95% CI 1.1–15.8); South Korean study. | [48] |
| Use of antacids/antiulcer medications | Antacids (H2R blocker or PPI) treatment for 3 months increased sensitization (IgE) to food allergens, including wheat (Total n = 152, Hungarian study, adults) | [49] |
| Model | Anaphylaxis Severity | Mechanism | Suggestions for Improvement |
|---|---|---|---|
| B10 female mice and ICR mice; Sensitization: IP injection of gliadin with alum adjuvant. Elicitation: oral high dose gliadin, high dose ω5-gliadin for 30 min to induce WIA [81] | Mild HSR (<2 °C drop in temperature in 30 min). | SIgE, elevation of proteins in the portal blood after oral challenge at 1 h | Study cytokines, histamine, and other mediators |
| Female BALB/c mice; Sensitization: TDE (tape stripping); gluten + adjuvant Elicitation: IP challenge, gluten to induce HSR * [82] | Severe; HSR: (more than 3 °C, 30 min); anaphylactic score: 3 vs. 0 in control; | Histamine, Th1/Th2 cytokines, IgE, IgG1 | Study mMCP-1, chemokines, and other immune markers |
| Male Kud: Hartley guinea pigs; Sensitization: fasting 16 h + intragastric administration with salicylic acid and, 1 h later, gluten solution. Repeated for 9 days; Elicitation: IP injection of gliadin to elicit classical anaphylaxis reaction [83] | Classical systemic anaphylaxis (Severe: 4–6 in IP group) | Unknown | Study: (1) time to exhaustion; (2) antibodies: SIgE/SIgG1; (3) mediators (histamine, cytokines, etc.) |
| Female Balb/c mice; Sensitization: IP; SSP+ Alum. Elicitation: IP, SSP to elicit HSR [57,84] | Modest severity; HSR: (3 °C, 30 min;) | SIgE, TIgE, mMCP-1, spleen cytokine, chemokine, adhesion molecule. | Study histamine |
| Male Bn rats; Sensitization: IP, ω5-gliadin + Alum. Elicitation: IV, ω5-gliadin or gluten extract to elicit HSR [85] | Very mild; HSR: (0.4 °C, 30 min) | Unknown | Study antibodies, mediators (histamine, cytokines, etc.) |
| Female BN rat; Sensitization: TCI gluten ω5-gliadin + alum, SC. Elicitation: IV, TCI gluten or ω5-gliadin to elicit HSR [86] | Very mild; HSR: (approx. 0.8–1.4 °C, 30 min) | SIgE and SIgG1 | Study mediators (histamine, cytokines, etc.) |
| Female Balb/c mice; Sensitization: IP, peptin + trypsin digested gluten + alum. Elicitation: IP, gluten to elicit HSR ** [87] | Modest Severity; HSR: (2.5–3 °C, 30–60 min); Clinical scores: 2–3 by 60 min | SIgE, increased mast cell number in duodenum. Reduced Th1 cytokine (spleen) | Study other cytokines, histamine, and other mediators |
| Model | Anaphylaxis Severity | Mechanism | Suggestions for Improvement |
|---|---|---|---|
| Female Balb/c mice; sensitization: TDE, SSP; Challenge: Oral, SSP to elicit HSR [78,88] | Severe; HSR: (around 3.5°, 30 min) | TIgE, SIgE, SIgG1, mMCP-1, spleen biomarkers | Study histamine, IP challenge |
| Female Balb/c mice; Sensitization: TDE; Gliadin; Challenge: IP/oral Gliadin to elicit HSR * [80] | Life threatening; HSR: (8 °C, 30 min); Clinical symptoms scores 4 | TIgE SIgE, mMCP-1, spleen biomarkers | Study histamine, oral challenge |
| Female Balb/c mice; Sensitization: TDE; Glutenin; Challenge: IP Glutenin to elicit HSR * [79] | Life threatening; HSR: (8 °C, 30 min); Clinical symptom score: 4 vs. 0 in control | TIgE SIgE, mMCP-1, spleen biomarkers | Study histamine, oral challenge |
| Model | Anaphylaxis Severity | Mechanism | Suggestions for Improvement |
|---|---|---|---|
| Female B10.A mice; Sensitization: IP, protein (salt-soluble protein, gliadin, and glutenin) + alum; Elicitation: Oral protein + treadmill to induce WDEIA [89] | Treadmill exhaustion time: gliadin and glutenin 35–50 min vs. control 150 min vs. control (v/v) 200 min | SIgE; Poor response to SSP and good response to gliadin and glutenin. Mucosal lesions in small intestine, leakage of proteins into blood and liver after challenge | Controls missing for V/P and P/P without exercise; Therefore, unclear if this model is truly WDEIA or just WIA; Study mediators |
| B10 female mice and ICR mice; Sensitization: IP injection of gliadin with alum adjuvant. Elicitation: oral gliadin + treadmill for 30 min to induce WDEIA [90] | Mild HSR (1.5 degrees drop in temperature in 30 min). Treadmill exhaustion test: the mice were exhausted by 3 h and remained so up to 9 h (revolutions stay <400 up to 9 h post-challenge vs. around 1000 in control) control—unsensitized mice orally challenged with vehicle (acetic acid 0.1 M). | SIgE | Controls missing for V/P and P/P without exercise; Therefore, unclear if this model is truly WDEIA or just WIA; Study cytokines, histamine, and other mediators |
| Male Kud: Hartley guinea pigs; Sensitization: fasting 16 h + intragastric administration with salicylic acid and, 1 h later, gluten solution. Repeated for 9 days; Elicitation: oral gluten + treadmill for 30 min to elicit WDEIA [83] | WDEIA clinical symptom scores * (Mild: 1–1.4 in oral + exercise group) | Unknown | Controls missing for V/P and P/P without exercise; Therefore, unclear if this model is truly WDEIA or just WIA; Study: (1) time to exhaustion: (2) antibodies: SIgE/SIgG1; (3) Mediators (histamine, cytokines, etc.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arul Arasan, T.S.; Jorgensen, R.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Advances in Mechanisms of Anaphylaxis in Wheat Allergy: Utility of Rodent Models. Foods 2025, 14, 883. https://doi.org/10.3390/foods14050883
Arul Arasan TS, Jorgensen R, Van Antwerp C, Ng PKW, Gangur V. Advances in Mechanisms of Anaphylaxis in Wheat Allergy: Utility of Rodent Models. Foods. 2025; 14(5):883. https://doi.org/10.3390/foods14050883
Chicago/Turabian StyleArul Arasan, Tamil Selvan, Rick Jorgensen, Chris Van Antwerp, Perry K. W. Ng, and Venu Gangur. 2025. "Advances in Mechanisms of Anaphylaxis in Wheat Allergy: Utility of Rodent Models" Foods 14, no. 5: 883. https://doi.org/10.3390/foods14050883
APA StyleArul Arasan, T. S., Jorgensen, R., Van Antwerp, C., Ng, P. K. W., & Gangur, V. (2025). Advances in Mechanisms of Anaphylaxis in Wheat Allergy: Utility of Rodent Models. Foods, 14(5), 883. https://doi.org/10.3390/foods14050883




