Investigating the Impact of Chlorogenic Acid Content and Cellulose Nanoparticles on Sunflower Protein-Based Emulsions and Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of YSF and GSF
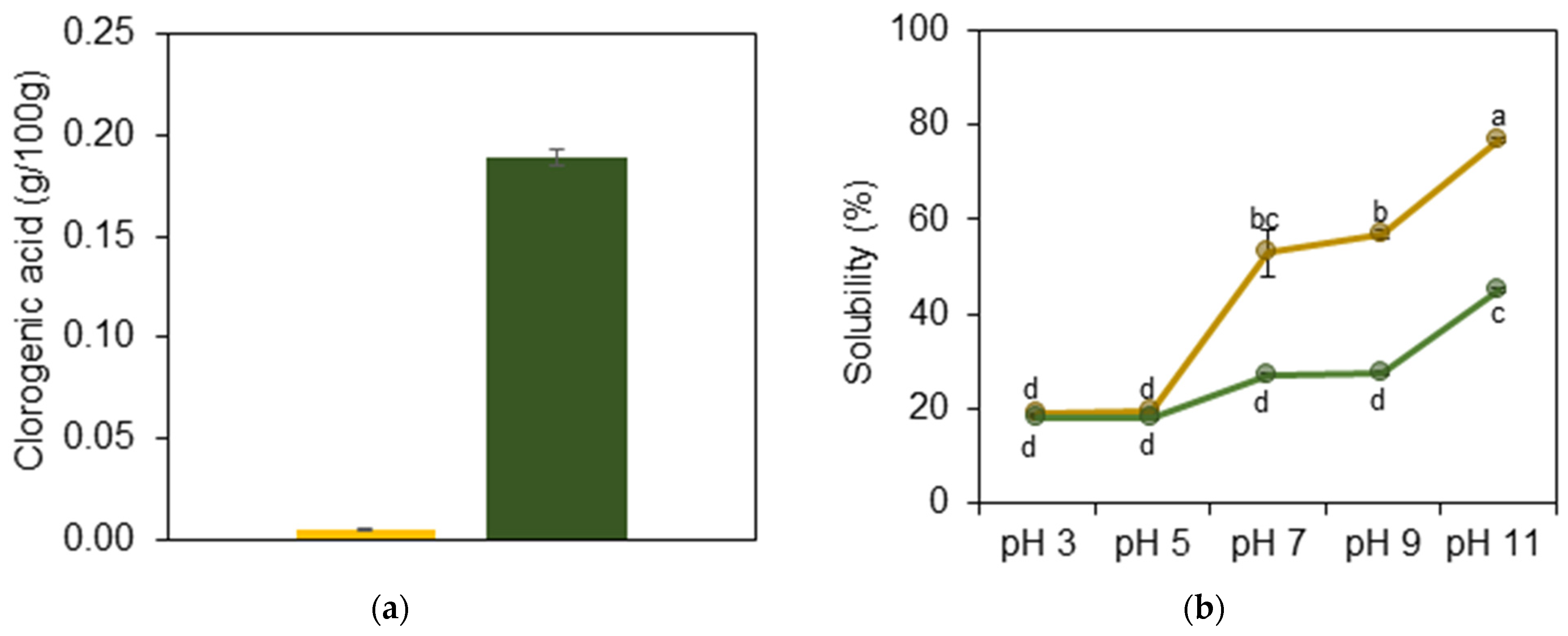
2.2.1. Quantification of Chlorogenic Acid
2.2.2. Solubility
2.2.3. Zeta Potential and Particle Size of Soluble Portion of SF
2.2.4. Particle Size of Insoluble Portion of SF
2.2.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.6. Differential Scanning Calorimetry (DSC)
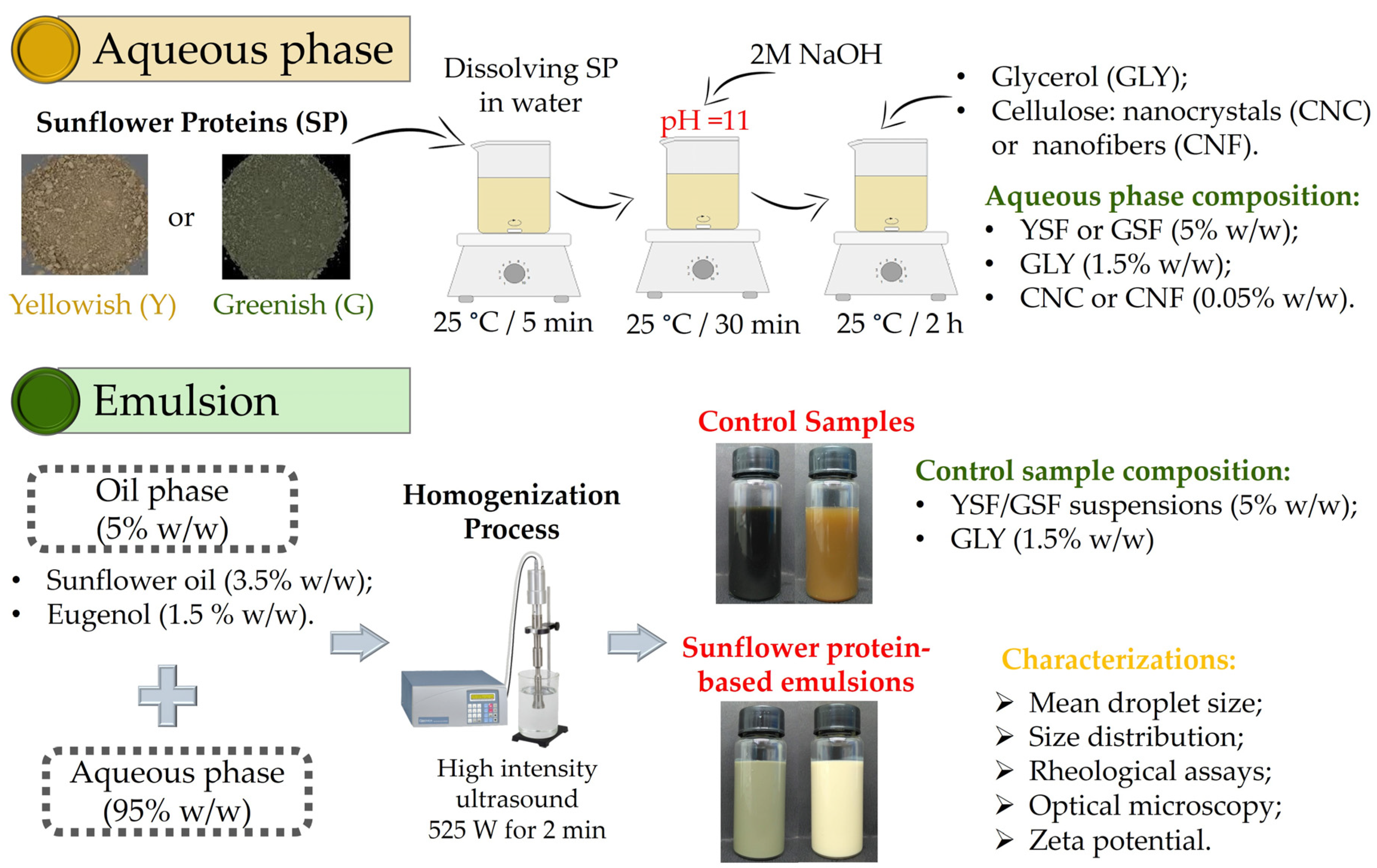
2.3. Emulsion Production
2.4. Characterization of Emulsions
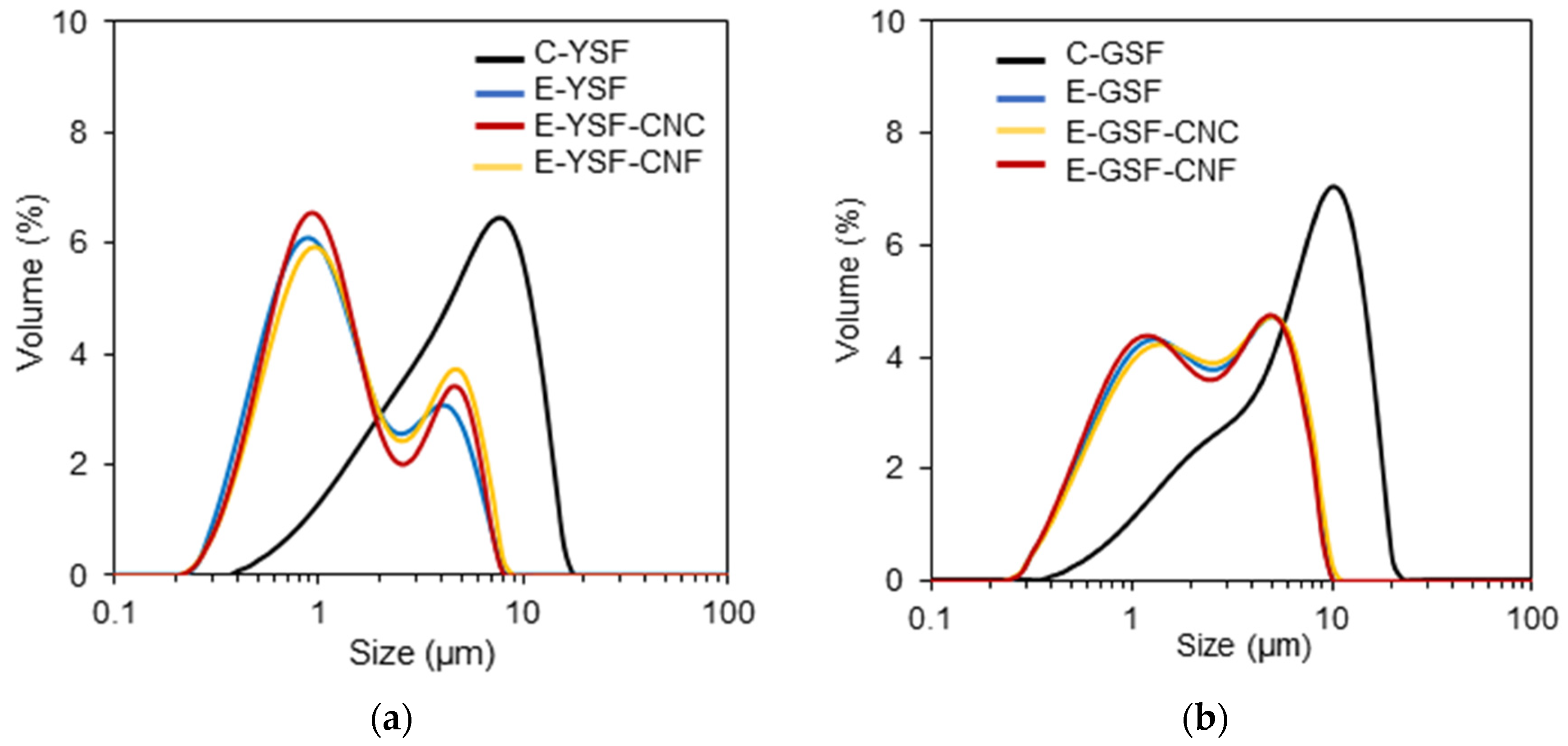
2.4.1. Mean Droplet Size, Size Distribution, and Rheological Assays
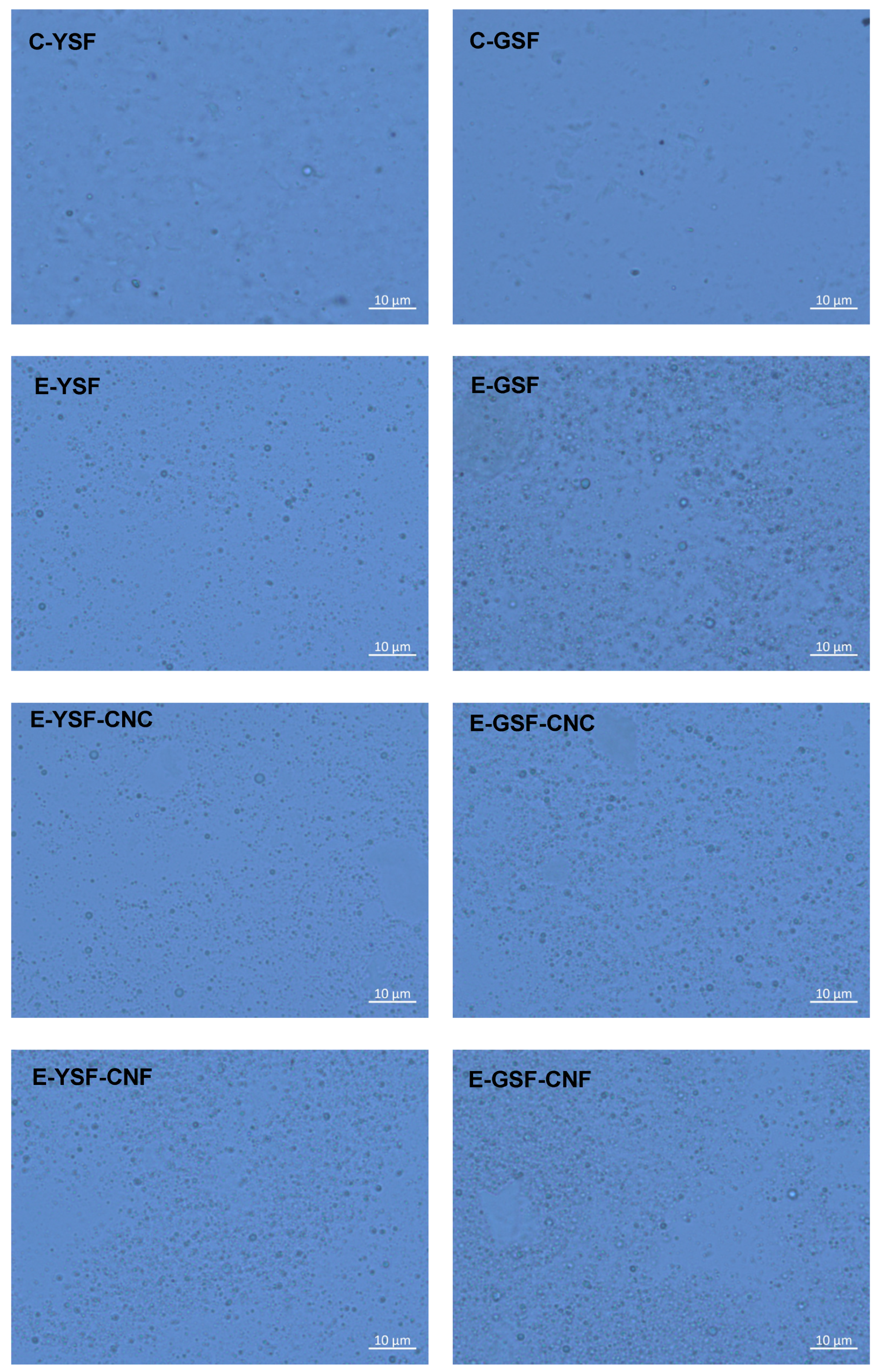
2.4.2. Optical Microscopy and Zeta Potential
2.5. Film Production
2.6. Characterization of Films
2.6.1. Mechanical Properties, Thickness, and Water Vapor Permeability (WVP)
2.6.2. Color and Scanning Electron Microscopy (SEM)
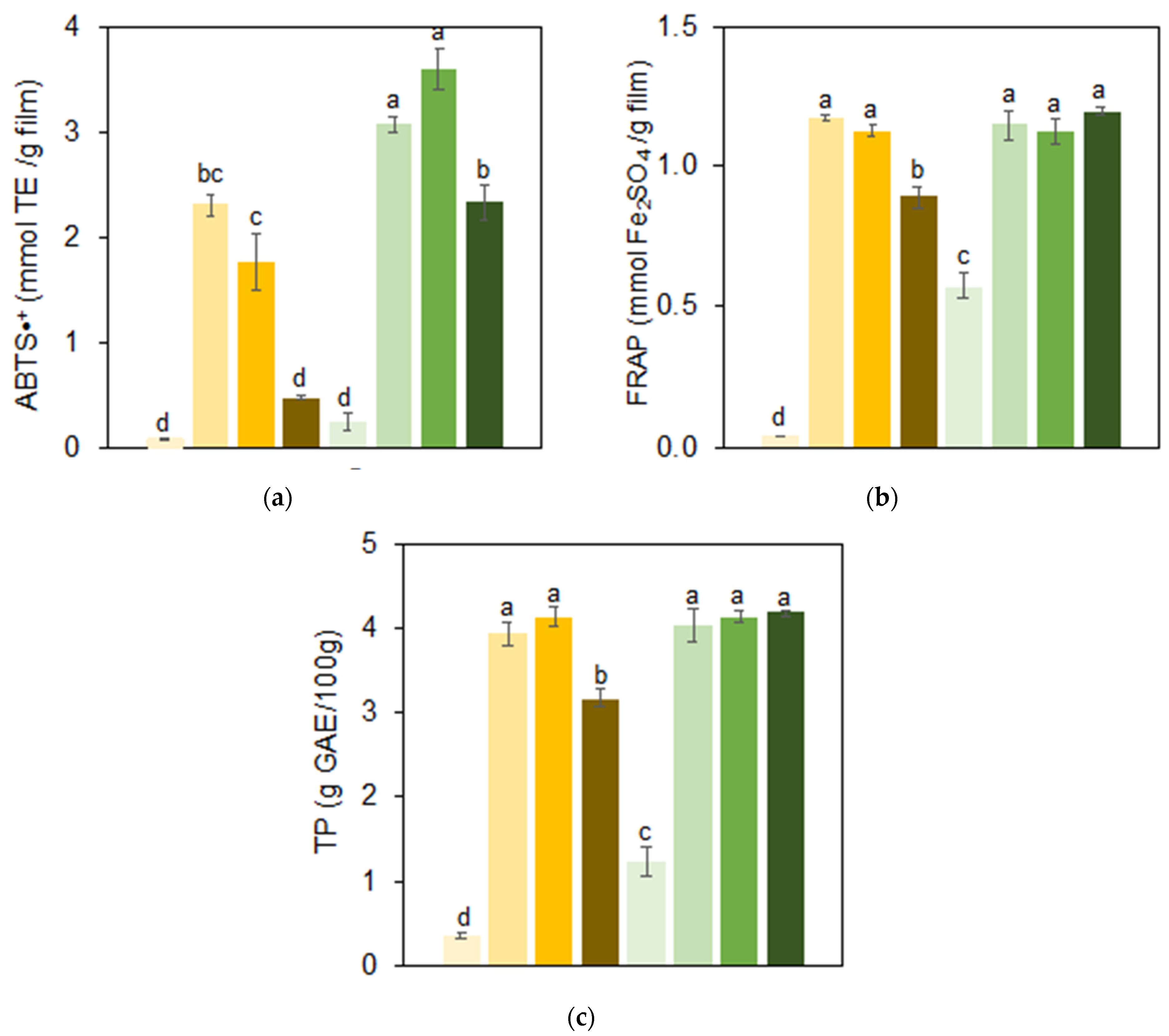
2.6.3. Antioxidant Activity and Total Phenolic Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. YSF and GSF Properties
3.2. Emulsion Properties
3.3. Film Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, D.; Panesar, P.S.; Saini, C.S. Effect of Montmorillonite (MMT) on the Properties of Soybean Meal Protein Isolate-Based Nanocomposite Film Loaded with Debittered Kinnow Peel Powder. Food Res. Int. 2024, 185, 114292. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of Ultrasound Treatment on the Properties of Nano-Emulsion Films Obtained from Hazelnut Meal Protein and Clove Essential Oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Gosh, T.; Badwaik, L.S. Optimization of Mustard, Soybean and Flaxseed Meal Blend Formulation for Development of Biopolymeric Film and Its Characterization. Sustain. Chem. Pharm. 2023, 33, 101147. [Google Scholar] [CrossRef]
- Rani, R.; Badwaik, L.S. Synergistic Impact of Natural Gums and Crosslinkers on the Properties of Oilseed Meals Based Biopolymeric Films. Int. J. Biol. Macromol. 2024, 265, 130809. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Sunflower Protein Films Incorporated with Clove Essential Oil Have Potential Application for the Preservation of Fish Patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and Quantification of Phenolic Compounds from Sunflower (Helianthus annuus L.) Kernels and Shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed Proteins–Properties and Application as a Food Ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Salgado, P.R.; Molina Ortiz, S.E.; Petruccelli, S.; Mauri, A.N. Sunflower Protein Concentrates and Isolates Prepared from Oil Cakes Have High Water Solubility and Antioxidant Capacity. J. Am. Oil Chem. Soc. 2011, 88, 351–360. [Google Scholar] [CrossRef]
- Sastry, M.C.S.; Rao, M.S.N. Binding of Chlorogenic Acid by the Isolated Polyphenol-Free 11 S Protein of Sunflower (Helianthus annuus) Seed. J. Agric. Food Chem. 1990, 38, 2103–2110. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid.-Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of Chlorogenic Acid in Vegetables and Fruits as an Inhibitor of 8-Hydroxydeoxyguanosine Formation in Vitro and in a Rat Carcinogenesis Model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E.P. Evaluation of the Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The Combination of Ultrasound and Chlorogenic Acid to Inactivate Staphylococcus Aureus under Planktonic, Biofilm, and Food Systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, F.; Zhe, T.; Sun, X.; Zhang, X.; Wan, P.; Na, H.; Zhao, J.; Wang, L. Biopolymer Films Incorporated with Chlorogenic Acid Nanoparticles for Active Food Packaging Application. Food Chem. 2024, 435, 137552. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Oracz, J. Effect of Addition of Green Coffee Extract and Nanoencapsulated Chlorogenic Acids on Aroma of Different Food Products. LWT 2016, 73, 197–204. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Saini, C.S. Polyphenol Removal from Sunflower Seed and Kernel: Effect on Functional and Rheological Properties of Protein Isolates. Food Hydrocoll. 2017, 63, 705–715. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein Extracts from De-Oiled Sunflower Cake: Structural, Physico-Chemical and Functional Properties after Removal of Phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Saricaoglu, B.; Yılmaz, H.; Subaşı, B.G.; Capanoglu, E. Effect of De-Phenolization on Protein-Phenolic Interactions of Sunflower Protein Isolate. Food Res. Int. 2023, 164, 112345. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Molina Ortiz, S.E.; Petruccelli, S.; Mauri, A.N. Biodegradable Sunflower Protein Films Naturally Activated with Antioxidant Compounds. Food Hydrocoll. 2010, 24, 525–533. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Exploration of the Antioxidant and Antimicrobial Capacity of Two Sunflower Protein Concentrate Films with Naturally Present Phenolic Compounds. Food Hydrocoll. 2012, 29, 374–381. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Altunkaya, S.; Berton-Carabin, C.C.; van der Goot, A.J.; Nikiforidis, C.V. Physical Bonding between Sunflower Proteins and Phenols: Impact on Interfacial Properties. Food Hydrocoll. 2017, 73, 326–334. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.P.L.F.; Bertan, L.C.; De Rensis, C.M.V.B.; Bilck, A.P.; Vianna, P.C.B. Whey Protein-Based Films Incorporated with Oregano Essential Oil. Polímeros 2017, 27, 158–164. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa Protein–Chitosan–Sunflower Oil Edible Film: Mechanical, Barrier and Structural Properties. LWT-Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Tessaro, L.; Pereira, A.G.R.; Martelli-Tosi, M.; Sobral, P.J.d.A. Improving the Properties of Gelatin-Based Films by Incorporation of “Pitanga” Leaf Extract and Crystalline Nanocellulose. Foods 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Olsen, C.W.; Bilbao-Sáinz, C.; McHugh, T.H. Mechanical and Water Barrier Properties of Isolated Soy Protein Composite Edible Films as Affected by Carvacrol and Cinnamaldehyde Micro and Nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Cangussu, L.B.; Cunha, R.L.; de Oliveira, L.S.; Franca, A.S. Stabilization Mechanisms of O/W Emulsions by Cellulose Nanocrystals and Sunflower Protein. Food Res. Int. 2022, 152, 110930. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P. Comparative Evaluation of the Antioxidant Efficacy of Encapsulated and Un-Encapsulated Eugenol-Rich Clove Extracts in Soybean Oil: Shelf-Life and Frying Stability of Soybean Oil. J. Food Eng. 2013, 117, 545–550. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Zhong, Q. Eugenol Improves Physical and Chemical Stabilities of Nanoemulsions Loaded with β-Carotene. Food Chem. 2016, 194, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Eugenol-Loaded Chitosan Nanoparticles: II. Application in Bio-Based Plastics for Active Packaging. Carbohydr. Polym. 2013, 96, 586–592. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-Loaded LLDPE Films with Antioxidant Activity by Supercritical Carbon Dioxide Impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-Chitosan Nanoemulsions by Ultrasound-Mediated Emulsification: Formulation, Characterization and Antimicrobial Activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; He, Z.; Han, C.; Liu, S.; Li, Y. Influence of Surfactant and Oil Composition on the Stability and Antibacterial Activity of Eugenol Nanoemulsions. LWT-Food Sci. Technol. 2015, 62, 39–47. [Google Scholar] [CrossRef]
- Majeed, H.; Antoniou, J.; Hategekimana, J.; Sharif, H.R.; Haider, J.; Liu, F.; Ali, B.; Rong, L.; Ma, J.; Zhong, F. Influence of Carrier Oil Type, Particle Size on in Vitro Lipid Digestion and Eugenol Release in Emulsion and Nanoemulsions. Food Hydrocoll. 2016, 52, 415–422. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Álvarez Igarzabal, C.I. Preparation and Characterization of Soy Protein Films Reinforced with Cellulose Nanofibers Obtained from Soybean By-Products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of Cellulose Nanocrystals from Sugarcane Bagasse on Whey Protein Isolate-Based Films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy Protein-Based Films Incorporated with Cellulose Nanocrystals and Pine Needle Extract for Active Packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Ghadiri Alamdari, N.; Salmasi, S.; Almasi, H. Tomato Seed Mucilage as a New Source of Biodegradable Film-Forming Material: Effect of Glycerol and Cellulose Nanofibers on the Characteristics of Resultant Films. Food Bioprocess Technol. 2021, 14, 2380–2400. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Cellulose Nanofibers from Banana Peels as a Pickering Emulsifier: High-Energy Emulsification Processes. Carbohydr. Polym. 2018, 194, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. The Stabilizing Effect of Cellulose Crystals in O/W Emulsions Obtained by Ultrasound Process. Food Res. Int. 2020, 128, 108746. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.R.; Gomes, A.; Furtado, G.D.F.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Modulating in Vitro Digestibility of Pickering Emulsions Stabilized by Food-Grade Polysaccharides Particles. Carbohydr. Polym. 2020, 227, 115344. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.A.; Nypelö, T.E.; Rojas, O.J. Cellulose Nanofibrils for One-Step Stabilization of Multiple Emulsions (W/O/W) Based on Soybean Oil. J. Colloid. Interface Sci. 2015, 445, 166–173. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Chemical and Enzymatic Treatment. LWT-Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Chevalier, R.C.; Gomes, A.; Cunha, R.L. Role of Aqueous Phase Composition and Hydrophilic Emulsifier Type on the Stability of W/O/W Emulsions. Food Res. Int. 2022, 156, 111123. [Google Scholar] [CrossRef] [PubMed]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Enzymatic Treatment: Study of Process Conditions. Ind. Crops Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Belguidoum, K.; Amira-Guebailia, H.; Boulmokh, Y.; Houache, O. HPLC Coupled to UV–Vis Detection for Quantitative Determination of Phenolic Compounds and Caffeine in Different Brands of Coffee in the Algerian Market. J. Taiwan. Inst. Chem. Eng. 2014, 45, 1314–1320. [Google Scholar] [CrossRef]
- Brito Cangussu, L.; Leão, D.P.; Oliveira, L.S.; Franca, A.S. Profile of Bioactive Compounds in Pequi (Caryocar Brasilense Camb.) Peel Flours. Food Chem. 2021, 350, 129221. [Google Scholar] [CrossRef] [PubMed]
- LAEMMLI, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Habitante, A.M.Q.B. Phase Transitions of Pigskin Gelatin. Food Hydrocoll. 2001, 15, 377–382. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018; 12p.
- Sartori, T.; Menegalli, F.C. Development and Characterization of Unripe Banana Starch Films Incorporated with Solid Lipid Microparticles Containing Ascorbic Acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- ASTM E96/E96M-24a; Standard Test Methods for Gravimetric Determination of Water Vapor Transmission. ASTM International: West Conshohocken, PA, USA, 2024; 16p.
- Leão, D.P.; Melo, J.C.S.; Franca, A.S.; Oliveira, L.S. Comparative Evaluation of Agricultural Residues in the Production of Dietary Fibers. In Proceedings of the 2nd International Conference on Nutrition and Food Sciences, Moscow, Russia, 27–28 July 2013. [Google Scholar]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Muzquiz, M.; García-Vallejo, M.C.; Burbano, C.; Hoyos, M.C.C.; Ayet, G.; Robredo, L.M. Determination of Caffeic and Chlorogenic Acids and Their Derivatives in Different Sunflower Seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- Venktesh, A.; Prakash, V. Low-Molecular-Weight Proteins from Sunflower (Helianthus annuus L.) Seed: Effect of Acidic Butanol Treatment on the Physicochemical Properties. J. Agric. Food Chem. 1993, 41, 193–198. [Google Scholar] [CrossRef]
- Sripad, G.; Rao, M.S.N. Effect of Methods to Remove Polyphenols from Sunflower Meal on the Physicochemical Properties of the Proteins. J. Agric. Food Chem. 1987, 35, 962–967. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative Activity and Functional Properties of Protein Hydrolysate of Yellow Stripe Trevally (Selaroides Leptolepis) as Influenced by the Degree of Hydrolysis and Enzyme Type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Improvement of Functional Properties of Sunflower Protein Isolates near Isoelectric Point: Application of Heat Treatment. LWT 2018, 98, 411–417. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High Intensity Ultrasound Treatment of Protein Isolate Extracted from Dephenolized Sunflower Meal: Effect on Physicochemical and Functional Properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Pierpoint, W.S. O-Quinones Formed in Plant Extracts. Their Reaction with Bovine Serum Albumin. Biochem. J. 1969, 112, 619–629. [Google Scholar] [CrossRef][Green Version]
- Sabir, M.A.; Sosulski, F.W.; Finlayson, A.J. Chlorogenic Acid-Protein Interactions in Sunflower. J. Agric. Food Chem. 1974, 22, 575–578. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Vereijken, J.M.; Merck, K.B.; Gruppen, H.; Voragen, A.G.J. Emulsion Properties of Sunflower (Helianthus annuus) Proteins. J. Agric. Food Chem. 2005, 53, 2261–2267. [Google Scholar] [CrossRef]
- Kitamura, Y.; Okawa, H.; Kato, T.; Sugawara, K. Effect of Ultrasound Intensity on the Size and Morphology of Synthesized Scorodite Particles. Adv. Powder Technol. 2016, 27, 891–897. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol Nanoemulsions Incorporated in Quinoa Protein/Chitosan Edible Films; Antifungal Effect in Cherry Tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Gökkaya Erdem, B.; Dıblan, S.; Kaya, S. Development and Structural Assessment of Whey Protein Isolate/Sunflower Seed Oil Biocomposite Film. Food Bioprod. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; Sobral, P.J.d.A. Gelatin-Based Films Reinforced with Montmorillonite and Activated with Nanoemulsion of Ginger Essential Oil for Food Packaging Applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Chen, Q.J.; You, N.; Liang, C.Y.; Xu, Y.N.; Wang, F.; Zhang, B.; Zhang, P. Effect of Cellulose Nanocrystals-Loaded Ginger Essential Oil Emulsions on the Physicochemical Properties of Mung Bean Starch Composite Film. Ind. Crops Prod. 2023, 191, 116003. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Oxygen Permeability and Mechanical Properties of Films from Hydrolyzed Whey Protein. J. Agric. Food Chem. 2000, 48, 3913–3916. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of Essential Oils and Homogenization Conditions on Properties of Chitosan-Based Films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Cunha, R.L. Impact of Oil Type and WPI/Tween 80 Ratio at the Oil-Water Interface: Adsorption, Interfacial Rheology and Emulsion Features. Colloids Surf. B Biointerfaces 2018, 164, 272–280. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An Overview on Preparation of Emulsion-Filled Gels and Emulsion Particulate Gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Cai, D.; Yan, X.; Zhou, S.; Meng, Y.; Chen, X.; Wang, G.; Ding, W. Cellulose Nanocrystals from Rice Bran as Excellent Emulsifiers for Independently Stabilizing Pickering Emulsions. Ind. Crops Prod. 2024, 222, 120098. [Google Scholar] [CrossRef]
- Chevalier, R.C.; Almeida, N.A.; de Oliveira Rocha, L.; Cunha, R.L. Antimicrobial Potential of Oregano Essential Oil Vehiculated in Pickering Cellulose Nanofibers-Stabilized Emulsions. Int. J. Biol. Macromol. 2024, 275, 133457. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Kahyaoglu, T. Characterization of a New Biodegradable Edible Film Made from Salep Glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of Active Edible Films Made from Banana Starch and Curcumin-Loaded Nanoemulsions. Food Chem. 2022, 371, 131121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and Antioxidant Properties of Hydroxypropyl Methylcellulose-Based Active Composite Films Incorporating Oregano Essential Oil Nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Tessaro, L.; Lourenço, R.V.; Martelli-Tosi, M.; Sobral, P.J.A. Gelatin/Chitosan Based Films Loaded with Nanocellulose from Soybean Straw and Activated with “Pitanga” (Eugenia Uniflora L.) Leaf Hydroethanolic Extract in W/O/W Emulsion. Int. J. Biol. Macromol. 2021, 186, 328–340. [Google Scholar] [CrossRef]
- Giménez, B.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Gómez-Estaca, J.; Montero, P. Role of Sepiolite in the Release of Active Compounds from Gelatin–Egg White Films. Food Hydrocoll. 2012, 27, 475–486. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of Dietary Polyphenols to Cellulose: Structural and Nutritional Aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of Polyphenols to Plant Cell Wall Analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]

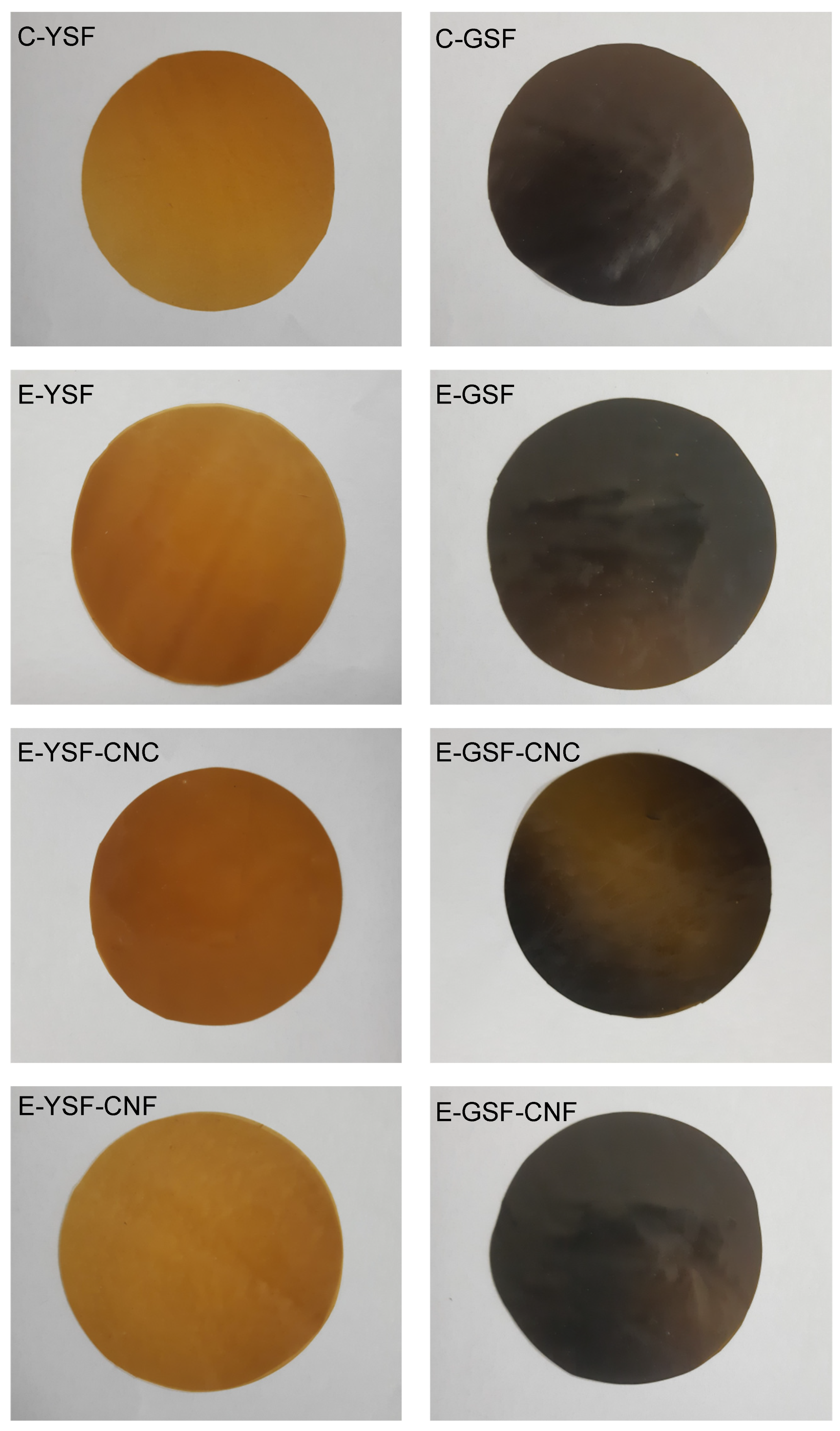

| Composition | YSF (5% w/w) | GSF (5% w/w) | Glycerol (1.5% w/w) | Sunflower Oil (3.5% w/w) | CNC (0.05% w/w) | CNF (0.05% w/w) |
|---|---|---|---|---|---|---|
| C-YSF | x | x | ||||
| C-GSF | x | x | ||||
| E-YSF | x | x | x | |||
| E-GSF | x | x | x | |||
| E-YSF-CNC | x | x | x | x | ||
| E-GSF-CNC | x | x | x | x | ||
| E-YSF-CNF | x | x | x | x | ||
| E-GSF-CNF | x | x | x | x |
| Composition | D32 (μm) | Span | Zeta Potential (mV) | k (Pa.sn) | n | η at 10 s−1 (mPa.s) |
|---|---|---|---|---|---|---|
| C-YSF | 3.52 ± 0.24 a | 1.79 ± 0.05 f | −32.87 ± 0.77 a | 0.01 ± <0.01 e | 0.87 ± <0.01 a | 21.0 ± 0.3 e |
| E-YSF | 0.90 ± 0.01 d | 3.39 ± 0.02 b | −35.70 ± 0.26 c | 0.03 ± <0.01 e | 0.79 ± 0.01 b | 29.6 ± 1.8 e |
| E-YSF-CNC | 0.93 ±< 0.01 d | 3.61 ± 0.01 a | −36.67 ± 0.67 d | 0.05 ± <0.01 e | 0.73 ± <0.01 c | 35.9 ± 0.6 e |
| E-YSF-CNF | 0.98 ± 0.01 d | 3.55 ± 0.04 a | −37.15 ± 0.44 d | 0.13 ± <0.01 d | 0.67 ± <0.01 e | 86.8 ± 8.1 d |
| C-GSF | 2.95 ± 0.05 b | 1.86 ± 0.01 e | −33.39 ± 0.95 ab | 0.11 ± 0.01 d | 0.69 ± <0.01 d | 67.5 ± 0.3 d |
| E-GSF | 1.34 ± 0.02 c | 2.67 ± 0.02 cd | −33.67 ± 0.71 ab | 0.36 ± 0.01 c | 0.59 ± <0.01 f | 135.2 ± 12.6 c |
| E-GSF-CNC | 1.31 ± 0.02 c | 2.73 ± 0.04 c | −34.73 ± 0.88 bc | 0.47 ± 0.02 b | 0.54 ± <0.01 g | 165.6 ± 9.1 b |
| E-GSF-CNF | 1.38 ± 0.01 c | 2.63 ± 0.02 d | −34.98 ± 0.97 c | 0.68 ± 0.01 a | 0.54 ± <0.01 g | 296.5 ± 7.6 a |
| Film Composition | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) Flexibility | Young’s Modulus (MPa) Rigidez | Water Vapor Permeability (g/m.s.Pa) |
|---|---|---|---|---|---|
| C-YSF | 0.10 ± <0.01 d | 6.0 ± 0.5 a | 100.5 ± 8.5 b | 0.80 ± 0.05 a | 1.15 ± 0.09 abc |
| E-YSF | 0.22 ± 0.02 a | 1.5 ± 0.2 de | 109.9 ± 5.4 bc | 0.14 ± 0.02 e | 0.94 ± 0.02 c |
| E-YSF-CNC | 0.16 ± 0.02 c | 1.5 ± 0.1 e | 92.6 ± 9.0 c | 0.15 ± 0.01 e | 1.39 ± 0.15 ab |
| E-YSF-CNF | 0.18 ± 0.02 b | 1.9 ± <0.1 cd | 65.6 ± 9.1 d | 0.18 ± 0.02 de | 1.47 ± 0.03 a |
| C-GSF | 0.13 ± 0.02 d | 4.4 ± 0.3 b | 149.7 ± 3.2 a | 0.43 ± 0.04 b | 1.42 ± 0.18 a |
| E-GSF | 0.19 ± 0.01 bc | 2.1 ± <0.1 c | 99.1 ± 3.8 bc | 0.19 ± 0.02 de | 0.99 ± 0.04 bc |
| E-GSF-CNC | 0.21 ± 0.04 a | 1.9 ± 0.2 c | 62.0 ± 9.4 de | 0.22 ± 0.06 cd | 1.36 ± 0.05 ab |
| E-GSF-CNF | 0.20 ± 0.01 ab | 2.2 ± 0.2 c | 52.8 ± 6.4 e | 0.25 ± 0.02 c | 1.33 ± 0.12 abc |
| Film Composition | L* | a* | b* | h (°) | C* |
|---|---|---|---|---|---|
| C-YSF | 10.4 ± 0.7 cd | 1.0 ± 0.2 d | 0.4 ± <0.1 e | 19.5 ± 3.7 e | 1.1 ± 0.2 e |
| E-YSF | 26.5 ± 0.9 a | 6.9 ± 0.5 a | 18.0 ± 0.2 a | 69.1 ± 1.4 bcd | 19.3 ± 0.1 a |
| E-YSF-CNC | 25.7 ± 0.6 a | 5.6 ± 0.9 b | 19.2 ± 1.2 a | 73.7 ± 2.8 abc | 20.0 ± 1.1 a |
| E-YSF-CNF | 26.9 ± 0.6 a | 3.6 ± 0.5 c | 15.7 ± 0.4 b | 77.2 ± 1.8 a | 16.1 ± 0.4 b |
| C-GSF | 19.6 ± 0.4 b | 2.0 ± 0.1 d | 6.3 ± 0.2 c | 74.2 ± 4.0 ab | 6.6 ± 0.2 c |
| E-GSF | 9.2 ± 0.2 d | 0.9 ± 0.1 d | 2.1 ± 0.1 d | 66.2 ± 3.0 cd | 2.3 ± 0.1 de |
| E-GSF-CNC | 11.5 ± 0.6 c | 1.2 ± 0.1 d | 3.2 ± 0.3 d | 69.0 ± 2.5 bcd | 3.5 ± 0.3 d |
| E-GSF-CNF | 10.7 ± 0.9 cd | 1.0 ± 0.1 d | 2.1 ± 0.2 d | 65.7 ± 2.2 d | 2.3 ± 0.2 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.; Cangussu, L.B.; Cunha, R.L.; Oliveira, L.S.d.; Franca, A.S.; Costa, A.L.R. Investigating the Impact of Chlorogenic Acid Content and Cellulose Nanoparticles on Sunflower Protein-Based Emulsions and Films. Foods 2025, 14, 824. https://doi.org/10.3390/foods14050824
Gomes A, Cangussu LB, Cunha RL, Oliveira LSd, Franca AS, Costa ALR. Investigating the Impact of Chlorogenic Acid Content and Cellulose Nanoparticles on Sunflower Protein-Based Emulsions and Films. Foods. 2025; 14(5):824. https://doi.org/10.3390/foods14050824
Chicago/Turabian StyleGomes, Andresa, Lais Brito Cangussu, Rosiane Lopes Cunha, Leandro Soares de Oliveira, Adriana Silva Franca, and Ana Letícia Rodrigues Costa. 2025. "Investigating the Impact of Chlorogenic Acid Content and Cellulose Nanoparticles on Sunflower Protein-Based Emulsions and Films" Foods 14, no. 5: 824. https://doi.org/10.3390/foods14050824
APA StyleGomes, A., Cangussu, L. B., Cunha, R. L., Oliveira, L. S. d., Franca, A. S., & Costa, A. L. R. (2025). Investigating the Impact of Chlorogenic Acid Content and Cellulose Nanoparticles on Sunflower Protein-Based Emulsions and Films. Foods, 14(5), 824. https://doi.org/10.3390/foods14050824








